Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SA Orthopaedic Journal
versão On-line ISSN 2309-8309
versão impressa ISSN 1681-150X
SA orthop. j. vol.16 no.2 Centurion Mai./Jun. 2017
http://dx.doi.org/10.17159/2309-8309/2017/v16n2a1
PAEDIATRICS
Management of osteogenesis imperfecta at the Chris Hani Baragwanath Hospital
GO OduahI; GB FirthII; JM PettiforIII; K ThandrayenIV
IMBBS, FC Orth(SA), MMed(Orth)(Wits) Honorary Lecturer, Division of Orthopaedic Surgery. Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMBBCh, FCS Orth(SA), MMed(Orth)(Rand), Division of Orthopaedic Surgery, Chris Hani Baragwanath Academic Hospital, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIMBBCh, FC Paed(SA), PhD(Med) Department of Paediatrics and MRC/Wits Developmental Pathways for Health Research Unit, Chris Hani Baragwanath Academic Hospital. Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVMBBCh, FCPaed(SA), MMed, PhD, Certificate in Endocrinology and Metabolism (Paeds) Department of Paediatrics, Chris Hani Baragwanath Academic Hospital. Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Osteogenesis imperfecta (OI) is a genetically inherited metabolic bone disorder that results in multiple fractures and deformities in children. The treatment of OI has undergone tremendous improvement in the last two decades worldwide.
AIMS: To review the clinical presentation and management of fractures in children with OI.
METHODS: A retrospective audit of patients treated for OI at Chris Hani Baragwanath Academic Hospital.
(CHBAH), from January 2000 to December 2011 was performed.
RESULTS: Seventy-eight patients with OI were reviewed. The male to female ratio was 1:1.1. The median age at presentation was 20 months. The patients were classified according to the Sillence classification. Thirty-four patients were type III and 22 were type IV. Twenty patients (26%) had a first degree relative with OI
The majority of patients received bisphosphonate (88%) and of these patients, 69 (93%) received intravenous bisphosphonate therapy; the remaining 7% received oral bisphosphonates. The most common long bone fractures were of the femur (93 fractures) and tibia (60 fractures).
Sixty-six long bones (49 patients) received intramedullary rodding (IM). The mean age at time of surgery was 7 years. The indication for osteotomy and IM rodding was fracture of the long bones. Fifty-one long bones out of the 66 long bones rodded (77%) underwent revision surgery for complications - 49% (25/51) had rod migration, 39% (20/51) had peri-implant fractures and 12% (6/51) had rod breakage.
Of 27 patients with type III OI, 14 (52%) were walking at final follow-up - eight were walking with assistive devices and six (22%) were walking independently. Of 19 patients with type IV OI, 16 (84%) were walking at final follow-up - four were walking with an assistive device and 12 (63%) were walking independently.
CONCLUSION: An ongoing multidisciplinary approach to the management of children with OI is of paramount importance. There is an urgent need to improve the level of awareness of this rare condition among health professionals in order to facilitate prompt diagnosis and early referral.
Key words: osteogenesis imperfecta, South Africa, intramedullary rodding, fractures, mobility
Introduction
The first scientific description of osteogenesis imperfecta (OI) was provided by the Swedish army surgeon Olaus Jakob Ekman in 1788.1 In his doctoral thesis entitled 'Congenital osteomalacia', Ekman described hereditary bone fragility in three family generations.2 Vrolik in 1849 was the first to coin the term 'osteogenesis imperfecta' meaning abnormally formed bone.1,3 The presence of Wormian bone mosaic of the skull, abnormal teeth colourations and bowed legs in an Egyptian mummy from 1000 BC suggests that the disease has been with man since ancient times.3 OI has been described in every race and continent of the world. The prevalence of OI is approximately 16 cases per million index patients.3 The major clinical manifestations of OI are frequent, multilevel fractures that cause limb deformities. Frequent fractures resulting from bone fragility lead to malunion and bowing, which render the bone more prone to recurrent fractures. The bowing of bone may occur even in the absence of a fracture or in the presence of multiple micro-fractures. Musculoskeletal abnormalities are typically as a result of defects in types IA1 or IA2 collagen, which is the primary component of the protein matrix in bone, tissues and organs. Bone tissue anomalies are the most visible manifestation of OI.3 A recent study has shown the importance of the compromised metabolic pathway of collagen, thus highlighting the heterogeneity of the different types of OI described.1,4
The main objectives of the surgical treatment of OI are to reduce disability and correct deformity, to enable the child to achieve relative independence in activities of daily living, and to attain the greatest degree of mobility possible.3
The aims of this study were to assess the clinical presentation, fracture incidence, and medical and surgical management of these patients at the Chris Hani Baragwanath Academic Hospital (CHBAH), Soweto, South Africa.
Materials and methods
A retrospective audit of patients treated for OI was done using an existing cohort of patients in the Paediatric Department (Metabolic Bone Clinic) at CHBAH, from January 2000 till December 2011.
All the information was retrieved from the hospital files and from the stored radiographs of each patient. The information that was gathered from the hospital file included demographic data, clinical presentation, age at initial fracture, total number of fractures, type of treatment received, and mobility status at final follow-up. The data collection sheet also included details of the complications of surgical interventions such as rod migration, re-fracture and re-operations. The diagnosis of OI was made based on clinical and radiological features assessed by consultants in the field of paediatric metabolic bone disease and confirmed by the paediatric orthopaedic surgical team.
Two patients with insufficient medical records were excluded from further data analysis.
Data was entered into Microsoft Excel spreadsheets and subsequently imported into Statistica statistical software version 10.0 (Statsoft, USA). Parametric, continuous variables were described using means and standard deviations. Medians and interquartile ranges were used for non-parametric data. Student-t tests or Mann-Whitney U tests were used to detect significant differences. Categorical variables were described using frequencies and percentages. P values were obtained using chi-square or Fisher's exact tests. The study was approved by the University of the Witwatersrand's Human Research Ethics Committee (ethics approval number: M120415).
Results
A total of 78 patients were seen within the period under review. The majority of patients were of black (90%) ethnicity followed by white (5%), mixed ancestry (4%) and Indian (1%). The median age at presentation was 20 months (IQR 0^8). The male to female ratio was 1:1.1 (37 males and 41 females). Twenty patients (26%) had a positive family history of a first degree relative with OI of which one of the patients was from a consanguineous marriage. The majority of the patients (59%) were from Gauteng. Three patients came from other Southern African countries.
Thirty-seven patients (48%) were referred by local and regional clinics, 14 (18%) by paediatricians and 11 (14%) by orthopaedic surgeons. The referring health care professionals were not documented in 16 (20%) of the patients' records.
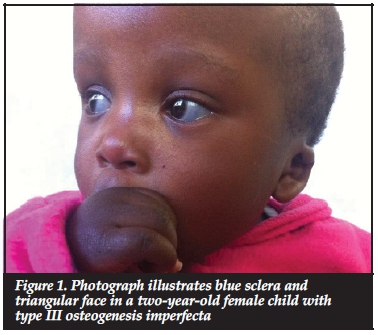
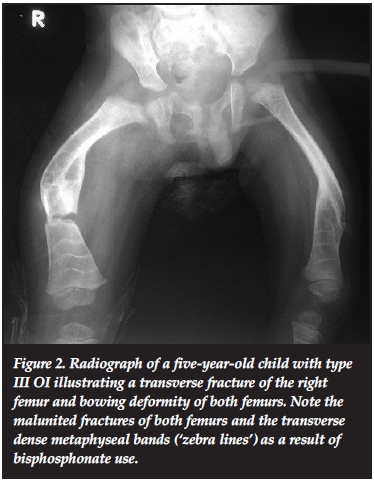
Most of the patients in our series presented with the typical features of OI. These included blue sclerae in 52.6% (41 patients). Figure 1 illustrates blue sclera in a two-year-old female child with type III OI. Dentinogenesis imperfecta was seen in 26.7% (21 patients). Triangular facies were seen in 50.0% (39 patients). The other features included recurrent fractures (Figure 2) and lower limb deformities in 66.7% (52 patients).
The patients were classified clinically using the original Sillence classification, and the number and percentages of each type of OI are shown in Table I. The majority of patients in this study were classified as type III and type IV: 38 (48.7%) and 23 (29.5%) patients respectively. Thus, the more severe types of OI were seen at the Metabolic Bone Clinic. There were no patients with type II OI as the patients were collected from the Metabolic Bone Clinic and did not include patient records of those seen as inpatients in the neonatal wards.
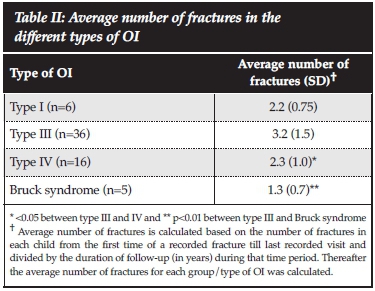
At presentation 49% of the patients had achieved age-appropriate milestones. Of the different types of OI, only type III had a smaller number of patients (33%) that had achieved age-appropriate milestones (p=0.03) while 50%, 52% and 100% of patients with Bruck syndrome, type IV and type I OI respectively had achieved age-appropriate milestones.
The mean duration of follow-up was lower in type I (1.8 years [SD ±1.2]) and type III (2.6 years [SD ±1.8]) OI patients compared to type IV (4.2 years [SD ±2.4]) and patients with Bruck syndrome (4.9 years [SD ± 2.7], p<0.05 between type I and IV; p<0.01 between type I and Bruck syndrome). Patients with Bruck syndrome had the longest duration of follow-up and this was significantly greater compared to type I (p<0.01) and type III (p<0.01) OI patients.
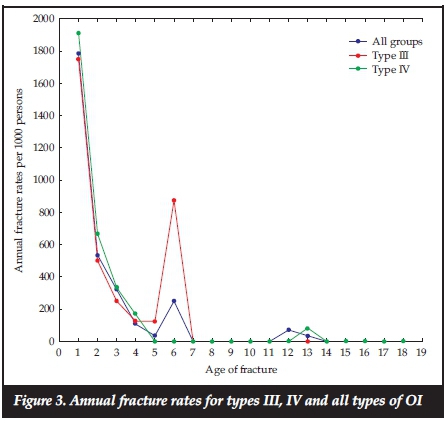
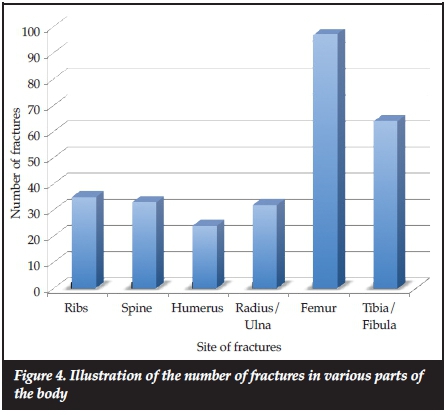
The total number of fractures from first reported fracture to the last clinic visit assessed by the researchers, was available for 65 patients. The average number of fractures from the first reported fracture till the last clinic visit differed according to the type of OI (Table I), and the duration of follow-up for individual patients also varied. The average fracture rate over the first 18 years of life for all types of OI patients was 174.6 fractures/year per 1 000 persons and for types III and IV, the rates were 201.4 and 175.9 fractures/year per 1 000 persons. Figure 3 shows the fracture rates per year for all types of OI and type III and type IV OI only. Fracture rates were highest in the first 6 years of life. As shown in Table II, type III OI patients had the highest average number of fractures compared to the other types of OI, and this was significantly greater compared to type IV and Bruck syndrome (p<0.05 and p<0.01 respectively). The greatest number of fractures occurred in the long bones of the lower extremities. The femur was the most frequent site of fracture followed by the tibia/fibula, then the upper extremities combined, and thereafter the ribs and lastly the spine (Figure 4).
The majority (88%) of the patients received bisphospho-nates. The indications for bisphosphonates were at least two or more long bone fractures and/or at least one vertebral fracture. Sixty-nine patients (93%) received intravenous bisphosphonate therapy and only five patients received oral bisphosphonates (alendronate or Fosamax).
Aredia or pamidronate was the intravenous bisphos-phonate therapy available for patients from 2005 until 2007 and thereafter zoledronic acid or Zometa was readily available. There was only one adverse event reported in one patient of severe myalgia that occurred post intravenous administration of Zometa.
All the patients received non-operative treatment (plaster of Paris [POP], longitudinal skin traction, and Gallows' traction) at some stage during their fracture management, either temporarily pre-operatively or definitively if surgery was contraindicated. Sixteen patients received only non-operative treatment (Figure 5).
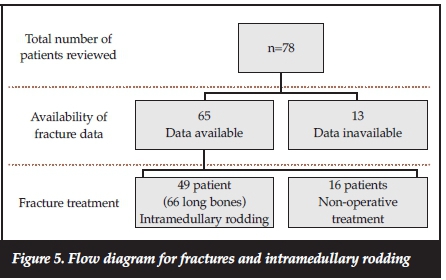
Sixty-six long bones (49 patients) received intramedullary rodding. The mean age at time of surgery was 7 (SD ±2.6) years. The youngest age at rodding was 3 years and the oldest child was aged 14 years. The indications for IM rodding were repeated fractures of the same long bone and osseous deformities or a combination of osseous deformities and acute fractures.
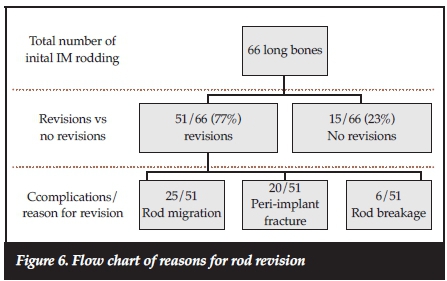
The complications of IM rodding seen in our series included rod migration (proximal and distal migration with or without tenting of the skin), rod breakage (with or without associated fractures) and peri-implant fractures. There were no infections.
A total of 51 long bones out of the 66 long bones rodded underwent single or repeated revisions for different indications. In 25 (49%) of these long bones, surgery had to be repeated because of rod migration. Another 20 (39%) of these long bones had peri-implants fractures and in six (12%) rod breakage occurred. A summary of rod revision is shown in Figure 6.
Further analysis on a subset of these patients was performed to identify specific factors associated with the final mobility status of the patients. Fifty-eight patients were analysed for mobility. The following factors were recorded: whether they were walking (assisted or independent) or not, whether surgery was performed, and the type of OI. There were only three patients with type I OI and they were all walking independently at final
follow-up. Of 27 patients with type III OI, 14 (52%) were walking at final follow-up - eight were walking with assistive devices and six (22%) were walking independently. Of 19 patients with type IV OI, 16 (84%) were walking at final follow-up - four were walking with an assistive device and 12 (63%) were walking independently.
Of seven patients with Bruck syndrome, only one (14%) was walking with an assistive device at final follow-up. There was no statistically significant association between all types of OI and walking whether surgery was performed or not. The age at first fracture was also not found to be significant regarding the final ability to walk or not as most patients were type III or IV. Finally, the number of total fractures was not associated with whether the patient was able to walk or not at final follow-up.
Discussion
To our knowledge, this study is the first to investigate the clinical presentation and surgical management of children with OI in South Africa. This study has one of the largest numbers of patients in a single series comprising a total of 78 patients. The majority of patients (59%) were from Gauteng where the study was conducted while three patients came from the neighbouring countries of Malawi and Zimbabwe, highlighting the scarcity of specialist clinics treating OI. The health care sectors in these countries are plagued with lack of geographic and financial accessibility and in some cases non-availability of quality health care.5
The male to female ratio of OI was 1:1.1 (37 male and 41 female), indicating no gender predilection. Lin et al. reported a male to female ratio of 1:2 (15 male and 33 female).6 Patel et al.7and Plotkin et al" showed similar sex ratios to the current study with 1:1.3 and a 1:0.9 male to female ratio respectively.
Twenty patients (26%) in the current study had a positive family history of a first degree relative with OI. Other studies have reported a higher percentage of a positive family history (40.3% and 46%).7,9 The majority of patients in this study were type III compared to the other studies where the majority of patients were type IV (9) and type I (7).
The median age at presentation was 20 months and less than 11% of cases were diagnosed in the first year of life. Greeley et al. reported that 25% of infants were diagnosed before one year of age at the Shriners Children's Hospital in Canada, an international orthopaedic referral centre which is a large referral centre for large parts of Canada, US and Mexico; thus, they are likely to be referred the severe forms presenting early.9
All the patients in this study were diagnosed and classified according to the Sillence classification by the presence of clinical characteristics. Greeley et al. in 2013 diagnosed 72% of their patients from clinical characteristics9 and suggested that special tests such as genetic and fibroblast testing are not necessary for the diagnosis of the majority of patients.9 In South Africa, genetic testing is not readily available and the diagnosis of OI is based on the family history, clinical presentation and radiological findings.
The majority of the patients in the current series were type III (48.7%) and type IV (29.5%). In the study done by Greeley et al., the majority of their patients were type I (34%) and type IV (35%),9 similar to findings in Taiwanese children with OI (type I [40%] and type IV [40%]).6 A recent cross-sectional multi-centre study of OI revealed that type I was the most prevalent type (49%), followed by types IV (27%) and III (18%).7 It is possible that the smaller number of patients with type I OI presenting to CHBAH reflect the fact that the diagnosis is missed in many of these individuals who are mildly affected and are more likely to be seen at primary and secondary health care facilities in South Africa where an inadequate medical and family history is obtained without referral to the specialist OI clinic.
More fractures occurred in the long bones of the lower extremities. The femur and tibia comprised more than 60% (93 femur fractures and 60 tibia fractures) of the total fractures. This is expected in the ambulant OI child, as these are the major weight-bearing bones of the body. Greeley et al. noted that the most common fractures at diagnosis were extremity fractures seen in 32% of patients.9
The average number of fractures was highest in type III OI patients, which was significantly higher than in type IV (p<0.05) and Bruck syndrome (p<0.01) patients. A meaningful comparison could not be made between types I and III due to the small number of patients in the type I group of patients. These findings are similar to the cross-sectional multicentre study of OI in North America.7
Since all the patients were on bisphosphonate therapy, it was not possible to demonstrate the efficacy of bisphos-phonates in this study, as there was no control group for comparisons.
Sixty-six long bones (49 patients) received intramedullary rodding. Of these, the majority (96%) were William's rods and the remaining 4% were Fassier-Duval (FD) telescoping rods. The smaller number is due to the recent introduction of FD rods for selected cases of femur fractures in children with good growth potential who were walking prior to the fracture. Zionts et al. reported that elongating nails in the tibia were associated with a higher incidence of major complications compared with those of the femur,10 thus elongating rods at CHBAH are the choice of IM rod for femurs only. In this study, the mean age at time of IM rodding surgery was 7 years. Long bone fractures in children younger than two years of age were treated non-operatively because of the technical difficulties associated with the procedure. The incidence of complications has been shown to be higher by Zionts et al. in children less than five years of age when IM rods are implanted.10 In the current study, only 16% of patients were under five years of age at the time of surgery and they have all had at least a single revision at the time of this report.
The complications of IM rodding seen in the current series was 77%. If followed up for long enough non-lengthening rods in growing children will result in complications in most if not all patients that will necessitate revision surgery. Most of the complications of IM rodding documented in the literature are on extensible rods therefore a direct comparative analysis with our study is not possible as only 4% of patients in the current study received Fassier-Duval rods. Nonetheless the documented complication rates for these extensible rods remain equally high. Zionts et al. documented a 100% complication rate with Bailey-Dubow extensible rods.10 Lang-Stevenson and Sharrard11 and Gamble et al.12reported complication rates of 64% and 69% respectively with use of Bailey-Dubow extensible rods. Recent reports by Lee K et al. highlight the risk of proximal rod migration in 14% of 50 cases using a telescopic rod13 and, in another paper, Lee RJ et al. highlight the problem of telescopic rod bending being an early sign of impending rod failure in 33% of 51 telescopic rods.14 Despite the high rate of revision surgery, long-term benefits of IM rodding outweigh the risks. It is difficult to evaluate the apparent benefits as it is considered unethical to deny patients IM rodding if surgically and medically indicated and thus no comparative group was available.
Mobility in OI children after treatment is not very well documented in the literature. In 1985 Shapiro showed that the age at initial fracture determined final mobility status in these children for all types of OI.15 In the current study, we found no difference in age at initial fracture and the final mobility status. This reflects the current cohort consisting of a homogenous group of mainly types III and IV. Ruck et al. showed that the use of the Fassier-Duval rod combined with bisphosphonates in a group of 60 children with OI improved ambulation, gross motor function, self-care and mobility at one year follow-up.16 Hoyer-Khun et al. showed that a specialised rehabilitation approach, which included whole body vibration training, physiotherapy, resistance and treadmill training, improved mobility in these children.17 Montpetit et al. used the Pediatric Evaluation of Disability Inventory to highlight the importance of improving the functional status of patients with type III OI who were often dependent for all areas at final follow-up in comparison to patients with type IV who were more often independent.18 This study was unable to show significant differences in independent walking between the different types of OI patients whom were operated on or not as the majority of these patients were type III and IV OI patients presenting with more severe disease. The importance of quality of life (QOL) in OI patients was highlighted by Dahan-Oliel et al. who reviewed ten studies and found that mental and social QOL was unaffected in these populations but that QOL was affected by pain, scoliosis activity limitations and participation restrictions.19 All of these studies highlight the importance of achieving maximal mobility at final follow-up by means of a multi-disciplinary approach.
The main limitation of this study was that it was a retrospective cohort study with the associated inherent problems of a retrospective study. The strength of the study is the large number of patients that were studied from a large geographic region, treated with the same protocol.
Conclusion
This paper consists of a group of more severe OI patients, namely type III (48.7%) and type IV (29.5%) who received bisphosphonates and surgery. Despite the severity of the patients in this cohort, 52% of patients with OI type III and 84% of patients with OI type IV were walking at final follow-up.
There is an urgent need to improve the level of awareness of this rare condition among health professionals in order to facilitate prompt diagnosis and early referral.
Acknowledgements
We wish to acknowledge and thank Professor AJF Robertson, Head of Paediatric Orthopaedics, Charlotte Maxeke Johannesburg Academic Hospital, for his ongoing surgical input and intellectual support in the management of these OI patients, and valuable comments to the final draft of this paper. In addition, we are very thankful to the staff of the Paediatric Metabolic Bone Unit of Chris Hani Baragwanath Academic Hospital for their continuous support and management of these patients with OI.
Compliance with ethics guidelines
The study was approved by the University of the Witwatersrand's Human Research Ethics Committee (ethics approval number: M120415).
No benefits of any form have been received from a commercial party related directly or indirectly to the subject of this article.
References
1. Burnei G, Vlad C, Georgescu I, Gavriliu TS, Dan D. Osteogenesis imperfecta: diagnosis and treatment. J Am Acad Orthop Surg 2008;16(6):356-66. [ Links ]
2. Peltier LF. The classic: congenital osteomalacia. Ekman OJ. Clin Orthop Rel Res 1981;159:3-5. [ Links ]
3. Kocher MS, Shapiro F. Osteogenesis imperfecta. J Am Acad Orthop Surg 1998;6(4):225-36. [ Links ]
4. Forlino A, Marini JC. Osteogenesis imperfecta. Lancet, 2016. 387(10028):1657-71. [ Links ]
5. Peters DH, Garg A, Bloom G, Walker DG, Brieger WR, Rahman MH. Poverty and access to health care in developing countries. Ann N Y Acad Sci 2008;1136:161-71. [ Links ]
6. Lin HY, Lin SP, Chuang CK, Chen MR, Chang CY, Niu DM. Clinical features of osteogenesis imperfecta in Taiwan. J Formos Med Assoc 2009;108(7):570-76. [ Links ]
7. Patel RM, Nagamani SC, Cuthbertson D, Campeau PM, Krischer JP, Shapiro JR, et al. A cross-sectional multicenter study of osteogenesis imperfecta in North America - results from the linked clinical research centers. Clin Genet 2015;87(2):133-40. [ Links ]
8. Plotkin H, Rauch F, Bishop NJ, Montpetit K, Ruck-Gibis J, Travers R, et al. Pamidronate treatment of severe osteoge- nesis imperfecta in children under 3 years of age. J Clin Endocrinol Metab 2000;85(5):1846-50. [ Links ]
9. Greeley CS, Donaruma-Kwoh M, Vettimattam M, Lobo C, Williard C, Mazur L. Fractures at diagnosis in infants and children with osteogenesis imperfecta. J Pediatr Orthop 2013;33(1):32-36. [ Links ]
10. Zionts LE, Ebramzadeh E, Stott NS. Complications in the use of the Bailey-Dubow extensible nail. Clin Orthop Rel Res 1998;(348):186-95. [ Links ]
11. Lang-Stevenson AI, Sharrard WJ. Intramedullary rodding with Bailey-Dubow extensible rods in osteogenesis imperfecta. An interim report of results and complications. J Bone Joint Surg Br 1984;66(2):227-32. [ Links ]
12. Gamble JG, Strudwick WJ, Rinsky LA, Bleck EE. Complications of intramedullary rods in osteogenesis imperfecta: Bailey-Dubow rods versus nonelongating rods. J Pediatr Orthop 1988;8(6):645-49. [ Links ]
13. Lee K, Park MS, Yoo WJ, Chung CY, Choi IH, Cho TJ. Proximal migration of femoral telescopic rod in children with osteogenesis imperfecta. J Pediatr Orthop 2015;35(2):178-84. [ Links ]
14. Lee RJ, Paloski MD, Sponseller PD, Leet AI. Bent telescopic rods in patients with osteogenesis imperfecta. J Pediatr Orthop 2015 [Epub ahead of print]. [ Links ]
15. Shapiro F. Consequences of an osteogenesis imperfecta diagnosis for survival and ambulation. J Pediatr Orthop. 1985 Jul-Aug;5(4):456-62. [ Links ]
16. Ruck J, Dahan-Oliel N, Montpetit K, Rauch F, Fassier F. Fassier-Duval femoral rodding in children with osteogenesis imperfecta receiving bisphosphonates: functional outcomes at one year. J Child Orthop. 2011 Jun;5(3):217-24. doi: 10.1007/s11832-011-0341-7. Epub 2011 May 8. [ Links ]
17. Hoyer-Kuhn H, Semler O, Stark C, Struebing N, Goebel O, Schoenau E. A specialized rehabilitation approach improves mobility in children with osteogenesis imperfecta. J Musculoskelet Neuronal Interact 2014;14(4):445-53. [ Links ]
18. Montpetit K, Palomo T, Glorieux F, Fassier F, Rauch F. Multidisciplinary treatment of severe osteogenesis imperfecta: functional outcomes at skeletal maturity. Arch Phys Med Rehabil. 2015;96(10):1834-39. [ Links ]
19. Dahan-Oliel N, Oliel S, Tsimicalis A, Montpetit K, Rauch F and Dogba MJ. Quality of life in osteogenesis imperfecta: a mixed-methods systematic review. Am J Med Genet A. 2016;170A(1):62-76. [ Links ]
 Correspondence:
Correspondence:
Dr GO Oduah
PO Box 30728 2017
Braamfontein Johannesburg South Africa
Tel: +27 834 700 912
Email: george.oduah@gmail.com














