Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SA Orthopaedic Journal
versão On-line ISSN 2309-8309
versão impressa ISSN 1681-150X
SA orthop. j. vol.14 no.3 Centurion Ago./Set. 2015
http://dx.doi.org/10.17159/2309-8309/2015/V14N3A2
ANKLE AND FOOT
Achilles tendinopathy - Part 1: Aetiology, diagnosis and non-surgical management
A HornI; G McCollumII
IMBChB(Pret); Registrar
IIMBChB(UCT), FC Orth(SA); Consultant Orthopaedic Surgeon Department of Orthopaedic Surgery, Groote Schuur Hospital, Cape Town
ABSTRACT
Non-insertional Achilles tendinopathy is by far the most common condition affecting this strong tendon and the incidence is on the rise due to increasing participation in recreational sports worldwide. Although the exact aetiology of Achilles tendinopathy is poorly understood, recent advances in understanding the pathophysiology of this condition have aided clinicians in developing improved methods of preventing and treating this painful, ubiquitous problem. Several contributing factors have been identified but repetitive microtrauma and inadequate, pathological healing appear to be the main culprits. Diagnosis is clinical but can be aided by the judicious use of imaging in equivocal cases. Activity modification and non-surgical modalities form the mainstay of treatment, with eccentric exercise programmes showing the best outcomes in prospective series. Several other non-surgical modalities exist and many show promising preliminary results. Surgical treatment options are discussed in Part 2 of this review, which will be published in Vol 14 No 4 of this journal.
Key words: Achilles tendinopathy, tendon, sports, eccentric exercises
Introduction
The Achilles tendon is the strongest tendon in the human body and is able to transmit loads of up to 12 times body weight during running.1,2 Over the last few decades, the incidence of tendinopathy of the body of the Achilles tendon has increased dramatically. This is thought to be due to an increase in the number of recreational middle- and longdistance runners, as well as a general increase in the intensity and duration of training among athletes.3-5
The term 'tendinosis' was first adopted by Puddu in 1976.6 This refers to intratendinous degeneration,7 which can be mucoid, calcific, lipoid, fibrinoid or even bony.8 Tendinosis, or tendinopathy, presents histologically as the loss of normal collagen architecture with fibre disorientation and thinning and an increase in hypocellular mucinous material and interfibrillar glycosaminoglycans.9,10 Granulation tissue and inflammatory cells are largely absent, rendering the term 'tendinitis' inappropriate.6,9 Currently the term 'tendinopathy' is preferred to describe pathological conditions in and around tendons resulting largely from overuse.11
The exact aetiology and pathophysiology of this painful condition remain largely unknown.12 Management varies widely from centre to centre with variable results reported in the literature. This review will focus on tendinopathy of the body of the Achilles tendon, excluding insertional and acute inflammatory conditions, and will discuss recent advances in understanding the underlying pathology as well as various management options.
Basic science
Anatomy and histology
The Achilles tendon originates proximally as an extension of the gastrocnemius muscle and receives fibres from the soleus muscle along almost its entire length. It is on average 15 cm (11-26 cm) long, and its average width is 6.8 cm at its origin, 1.8.cm at its narrowest point and 3.4 cm at its insertion into the calcaneus.13 Microscopically the tendon consists of collagen fibrils (95% type I collagen) and elastin imbedded in a matrix a proteoglycans and water. Tenocytes and tenoblasts are arranged in vertical columns among the collagen fibres.7,14 Individual bundles of collagen fibrils are bound by endotenon, which carry neural, vascular and lymphatic supply to the tendon, and is continuous with the epitenon that surrounds the entire tendon. The epitenon, in turn, is in contact with the paratenon, a unique structure consisting of areolar tissue, which forms an elastic sleeve around the tendon, allowing free movement of the tendon within the surrounding tissues. The collagen fibres display varying degrees of spiralling, which leads to less buckling when the tendon is lax and less deformity under stress. This spiral configuration may, however, compromise blood flow within the tendon and paratendon. The blood supply to the tendon is poor and mainly supplies peritendinous tissues from a recurrent branch of the posterior tibial artery. There are also contributions from the peroneal artery and at its periphery from the muscle bellies and calcaneus respectively.15 A relatively avascular area, found approximately 2-6 cm proximal to the calcaneal insertion, has been described and is thought to be the reason that the majority of ruptures and pathology occur in this area.16 More recent physiological examination however, has suggested the blood flow is uniform along the whole tendon.17 Nerve supply is from attaching muscles and cutaneous nerves, especially the sural nerve.18 Numerous receptors for pain and other neurotransmitter actions, such as proprioception, are present within the tendon and surrounding structures.14
Biomechanics
Tendons have a high tensile strength and can withstand up to 4% strain before incurring microscopic structural damage, making them ideal for transmitting force from muscle to bone.7 Energy is stored within the muscle during stance, and released during heel off. This is most relevant during running and jumping. During walking most of the energy comes from the catapult action of the calf muscles, as opposed to recoil of the tendon.19 Using a 'buckle'-type transducer implanted in the body of the tendon, Komi et al. recorded peak forces of up to 9 kN (12.5 χ body weight) within the tendon during running, with much lower forces being transmitted during slow walking (2.6 kN) and cycling (1 kN).1,2 The collagen fibres maintain their normal wavy configuration up to 4% strain with microscopic failure occurring at 6% strain and macroscopic failure or rupture at 8-10% strain.1,2,7,20 In the ageing tendon, collagen turnover decreases, as do water and mucopolysaccharide content.
This leads to a less stiff, more compliant tendon with reduced recoil, resulting in more muscle work and heat generation during activity, and potentially more tendon damage. Resistance training increases the diameter and stiffness of the tendon and therefore decreases the risk of rupture.21
Epidemiology
Achilles tendinopathy is most commonly seen in middle-and long-distance runners.4,5,11 It was also found to be the most frequently reported injury among master runners in a recent series.22 It is common in athletes who participate in track and field, soccer, tennis and other ball games that involve a large amount of running.5,12 In a recent paper on the epidemiology of Achilles tendinopathy, Järvinen et al. reported an annual incidence of 7-9% in top-level runners. In this cohort, midsubstance disease dominated (55%-66%) followed by insertional conditions including retrocalcaneal bursitis (20%-25%).4 The condition is, however, not exclusive to athletes with 31% of patients in a recent series leading a sedentary lifestyle.23
Aetiology
While the exact aetiology of Achilles tendinitis remains unknown, repetitive tendon overload during vigorous training is generally regarded as the main culprit.3,11 Even within its physiological limits, repetitive strain can lead to microtrauma which, if not given sufficient time to heal, will lead to tendinopathy.11
Various predisposing factors have been identified. These can be classified either as intrinsic or extrinsic factors. A statistically significant association with the metabolic syndrome has been described, with almost 60% of patients with hyperlipidaemia or hypercholesterolaemia experiencing improvement in symptoms with lipid-lowering agents.24,25 The ABO blood groups A and O, use of the oral contraceptive pill and the post-menopausal state have also been implicated,24 and there is a well-known association with the use of fluoroquinolone antibiotics.26 Although the seronegative spondyloarthropathies are strongly associated with Achilles enthesopathy, it is seldom implicated (<1%) in midsubstance disease and rupture.27 Table I lists all the intrinsic and extrinsic factors.

Of the intrinsic factors, lower limb malalignment, and especially hyperpronation of the forefoot, is the most commonly implicated.29 Leg length discrepancy, muscle weakness and imbalance, and increased weight are also important contributing factors. Extrinsic predisposing factors include sudden change in intensity and duration of training, poor technique, inappropriate footwear and running on hard, slippery or slanting surfaces.3-5,12,14
In Achilles tendinitis, repetitive tendon overload during vigorous training is generally regarded as the main culprit
The aforementioned theories have, however, not been proven by any longitudinal hypotheses-based studies.3
Mechano-neuro-biological interactions
Pain is the most bothersome and persistent symptom in Achilles tendinopathy, yet the cause of the pain remains unknown. Repetitive mechanical loading leads to increased local production of prostaglandin E2 (PGE2) in experimental models30 and the histological picture of tendinopathy has been induced in rat tendons by intratendinous injection of PGE2.31 Levels of PGE2 and other inflammatory markers are, however, not elevated in patients with symptomatic Achilles tendinopathy when compared to controls;32 therefore, it is postulated that PGE2 plays a role in the development of tendinopathy but is absent during the chronic phase. High levels of glutamate and glutamate receptors were found in chronic painful tendons using microdialysis, suggesting hyperexcitability of neurons as another contributing factor to development of pain in Achilles tendinopathy.32
Tendon injury leads to increased expression of sensory neuropeptides such as substance P (SP) and its receptor neurokinin-1.33 SP has been demonstrated to play a role in recruitment of inflammatory mediators and growth factors after tendon injury, in addition to its role in nociception.34 This upregulation in the expression of SP has been posted as a possible cause of pain associated with Achilles tendinopathy. Another contributing factor is thought to be autonomic dysregulation within the diseased tendon.
Repetitive mechanical loading, as well as injury, lead to increased sensory in-growth and increased nociception in the normally aneuronal tendon.33,35 Under normal circumstances this is followed by autonomic nerve ingrowth with subsequent decreased nociception and nerve retraction. This process is dysregulated in tendinopathy with increased sensory nerve sprouting and deficient autonomic regulation.35
Diagnosis
Pain, whether at rest or during activity, is always the presenting complaint with Achilles tendinopathy. A thorough history should be obtained and should include presence of predisposing factors, onset of symptoms, duration of symptoms, associated activities and relieving factors. In the early stages of the disease pain is usually experienced at the onset and after training, but as the disease progresses pain can be experienced even at rest, restricting activity and sports.5,14
Mid-portion and insertional tendinopathy are distinguished based on location of tenderness, and the presence of paratendinopathy should be noted as these conditions often co-exist.4 With midsubstance disease, the pain is located 2-6 cm from the insertion. With insertional tendinopathy the pain is often associated with walking uphill or on sand, and with retrocalcaneal bursitis pain can be localised to the lateral or medial aspect of the heel.36
On examination, swelling, redness, pain and warmth are the most common findings, and identifying the exact location of the problem is important (Figure 1a and b). Swelling can be diffuse or nodular and there may be associated crepitus with movement indicating paratenon involvement. Two clinical tests are described. The Royal London Hospital test involves the presence of a tender nodule within the tendon substance with the ankle in neutral. This nodule then becomes non-tender or disappears with active plantar- or dorsiflexion. The painful arc sign describes a tender or painful nodule or swelling that moves up or down with ankle plantar- and dorsiflexion.
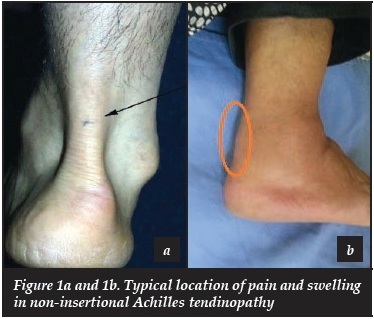
This distinguishes involvement of the body of the tendon from paratenon involvement.5,14 Maffuli et al. evaluated the clinical significance of these two tests and found them moderately sensitive but very specific and concluded that they should not be used in isolation as diagnostic tools.37
The physical evaluation should always include gait analysis, alignment of the heel and forefoot, as well as ankle and subtalar range of motion.
Imaging
Standard radiographs are of little value in the diagnosis and management of Achilles tendinopathy; however, it is frequently requested as the first investigation to exclude other causes of heel pain. Occasionally areas of calcification can be identified on plain radiographs, supporting the diagnosis of Achilles tendinopathy.5 The two pertinent imaging modalities are MRI and ultrasound.
Ultrasound has been reported by Khan et al. to have a positive and negative predictive value of 0.65 and 0.68 respectively, and a sensitivity and specificity of 0.80 and 0.49 respectively, when compared to clinical examination.38 Abnormal findings on ultrasound include tendon thickening, midsubstance tears, hyper- or hypo-echoic lesions, peritendinous fluid and thickening of the paratenon.14 Hypo-echoic areas correlate well with histological abnormalities39 and are associated with less favourable outcomes. Ultrasound did not perform well in identifying insertional pathology.38 Power Doppler ultrasound has been used to detect neovascularisation in Achilles tendinopathy. A positive correlation between neovascularisation and pain has been demonstrated, but it does not predict a poor prognosis, whereas inhomo-geneity does. We therefore advise caution when requesting and interpreting the results of this investigation.40
MRI is less operator-dependent than US, but far more expensive and less accessible. Ultrasound also has the added benefit of being interactive and therefore enabling the operator to focus on areas of pathology. In their prospective study, Khan et al. found for MRI a positive and negative predictive value of 0.56 and 0.94 respectively, and a sensitivity and specificity of 0.95 and 0.50 respectively, when compared to clinical examination.38 These values do not differ greatly from those achieved by ultrasound. Typical abnormal findings in MRI include thickening of the tendon and paratenon as well as lesions with increased signal intensity (Figure 2);14 however, Soila et al. recently demonstrated that normal anatomy of the Achilles tendon can be quite variable, leading to diagnostic misinterpretation when using MRI.41

Imaging appears to add little value to the diagnosis and management of AT in the hands of experienced clinicians. It may, however, be of value to those less experienced clinicians who are uncertain of their diagnosis and clinical findings.
Management
Non-surgical management
Achilles tendinopathy can frequently be adequately managed by activity modification and, in the case of ankle or lower limb malalignment, the use of orthotics. Muscle weakness and imbalance can be improved with appropriate training regimens and physiotherapy, but often active non-operative interventions may be more effective and more acceptable to the patient than rest alone.
Non-steroidal anti-inflammatory drugs (NSAIDs) are often used in the management of tendon injuries and they have been shown to be more effective than placebo in the short term.42 It is thought, however, that their efficacy is largely due to their analgesic rather than their anti-inflammatory properties,43 as numerous histological studies have failed to show the presence of inflammatory changes in Achilles tendinopathy.3-5,9,32 Ånström and Westlin44 failed to show any benefit of NSAIDs over placebo at 28 days in their randomised control trial, and there is evidence that these drugs might even hinder recovery or contribute to the development of Achilles tendinopathy.30,45
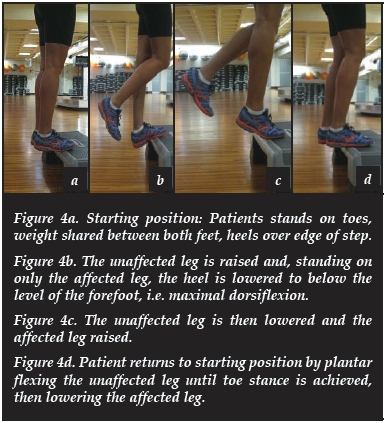
Eccentric exercises (Appendix 1 )
Eccentric exercise regimens were found to be the most effective non-operative intervention for Achilles tendinopathy in a recent systematic review.46 This treatment method was first described by Stanish47 in the mid-1980s but only became popular following a randomised control trial by Alfredson showing excellent results with the use of this method.48 The exercise programme they described is the one most commonly used today and involves exercises being performed twice daily, for 12 weeks.
Eccentric exercise regimens were found to be the most effective non-operative intervention for Achilles tendinopathyin a recent systematic review
The patient's forefoot is placed on a step and the heel is then lowered below the level of the forefoot, thus loading the tendon eccentrically. This is done with the knees straight and bent in three sets of 15 repetitions each. No concentric work is performed as the opposite leg is used to get back up to the starting position. Initially only the patient's body weight is used, but more weight is added by means of a heavy backpack as the exercises become better tolerated. This exercise programme has been shown to be significantly more effective than a concentric exercise programme (84% versus 36% return to normal activities after 12 weeks) in a randomised control trial by the same author in 2001.49 Few attempts have been made to elucidate the mechanism behind eccentric loading's effectiveness. A marked reduction in neovascularisation has been demonstrated following eccentric training, which correlated well with decreased pain,50 and Rees and colleagues demonstrated a sinusoidal pattern of loading and unloading during eccentric contraction that was not seen with concentric contraction. This oscillating mechanical loading is thought to act as a stimulus for tendon remodelling, similar to that found in bone.51 Combining eccentric exercises with repetitive low-energy shockwave therapy lead to even better outcomes in a recent randomised control trial, with significantly better pain scores at 4 months in the combined therapy group when compared to those who did eccentric exercises only.52
Shockwave therapy
Originally developed for the treatment of renal calculi, extracorporeal shockwave therapy (ESWT) has since been successfully employed for the treatment of various soft tissue conditions, more notably enthesopathies and calcific tendinopathies (Figure 3). ESWT is thought to improve tendon pain and healing by selectively defunctioning unmyelinated pain fibres, either directly or by the release of neuropeptides,53 and/or by causing interstitial and extracellular interface disruption leading to improved healing.54 The results of ESWT for mid-portion Achilles tendinopathy in the literature are conflicting. In a recent RCT comparing ESWT to placebo, Costa et al. failed to demonstrate a statistically significant benefit at 3 months.55 Rasmussen et al.56 showed improved hindfoot scores and comparable VAS in their treatment group who received ESWT in addition to an eccentric exercise programme, and Rompe et al.51demonstrated better outcomes with ESWT when compared to no intervention, but not when compared to eccentric exercises alone.

The mechanism of action of ESWT is poorly understood; theories include selective disruption of unmyelinated sensory nerve fibres,53 changes in the dorsal root ganglion and the stimulation of a local inflammatory response leading to improved healing.57
Nitric oxide
There is currently limited scientific evidence supporting the use of glyceryl trinitrate (GTN) in the treatment of Achilles tendinopathy. One randomised control trial by Paoloni58 in 2004 demonstrated improved outcomes at 6 months and also at late follow up of 3 years in the treatment group. A subsequent randomised trial failed to show any difference, either clinically or histologically, between patients treated with topical GTN and those treated with placebo.59 The mechanism of action and potential hazardous effects of the treatment modality is also unknown.
Injection therapies
Autologous blood and platelet-rich plasma
There are no good studies supporting the use of autologous blood injections for Achilles tendinopathy. There is often severe, sometimes disabling, pain following the injection, and the mechanism of action is poorly understood. The timing, frequency and quantity of blood to be used have also not been optimally determined.60
The use of platelet-rich plasma (PRP) for the treatment of various soft tissue conditions has gained popularity recently, the rationale being that the alpha-granules in the platelets contain a variety of growth factors and cytokines that initiate wound healing. The mechanism of action here is also poorly understood. It has been shown, in vitro, that the platelets release their growth factors within 10 minutes, and that all of these have dissipated by one hour following injection. It has also been shown that near physiological levels of platelets induce the most favourable response in osteoblasts and fibroblasts, and that higher dosages may in fact be detrimental to bone and tendon healing.61 In a recent prospective study, De Vos et al. compared the use of PRP to isotonic saline in combination with an eccentric exercise programme in patients with Achilles tendinopathy. There was no significant difference between the groups, and though a single randomised trial is far from providing conclusive evidence, it does raise questions regarding the validity of PRP for the use of this condition.62
High volume injections
This method is not widely practised and only one non-comparative study has been published.63 The method entails injection of up to 50 ml of saline around the Achilles tendon, the rationale being to disrupt and destroy the neovascularisation and accompanying neuronal ingrowth that have been implicated as a pain generator in Achilles tendinopathy. The authors reported reduced pain and improved function among subjects; they did, however, also inject 25 mg hydrocortisone in each patient which may have affected their outcomes.
Sclerosing therapy
The premise for this treatment modality is, again, that pain in Achilles tendinopathy is caused by neovascularisation around the tendon. Polidocanol, a sclerosing agent, is injected into the area of neovascularisation around the tendon under ultrasound guidance. Evidence for this treatment modality is limited to small comparative studies and retrospective reports, almost all of which were published by the same Swedish group.64-66 Reported outcomes are favourable and comparable to other established treatment modalities such as eccentric exercise and open revision surgery. In these studies, pain-free tendons consistently showed an absence of neovascularisation. Seeing that it has been proven that eccentric exercises also decrease neovascularisation, the use of this agent is not recommended as first-line therapy, but may be of value in those patients for whom eccentric exercises failed or are not an option.
Conclusion
Achilles tendinopathy is a common condition, and is becoming progressively more so. This condition is easily diagnosed clinically and responds well to conservative measures, of which a supervised eccentric exercise programme is the most effective in leading to resolution of symptoms. Alternative non-surgical treatments such as extracorporeal shockwave therapy and platelet-rich plasma injections also show promise, especially when used in conjunction with an exercise programme. Understanding the aetiology and pathophysiology of this condition is essential for diagnosing and successfully treating Achilles tendinopathy, as well as aiding future research. For those patients in whom conservative management fails, several surgical options are available, which will be discussed in Part 2 of this review. An eccentric exercise regimen is outlined in Appendix 1 of this section.
The content of this article is the original work of the authors. No benefits of any form have been or are to be received from a commercial party related directly or indirectly to the subject of this article.
Part 2 of this article will be published in the next edition of this journal: SAO], Spring 2015, Vol 14, No 4.
References
1. Komi PV, Salonen M, Järvinen M, Kokko O. In vivo registration of Achilles tendon forces in Man. I. Methodological development. Int ] Sports Med 1987;08:S3-S8. [ Links ]
2. Komi PV, Fukashiro S, Järvinen M. Biomechanical loading of Achilles tendon during normal locomotion. Clin Sports Med 1992 Jul;11(3):521-31. [ Links ]
3. Kader D, Saxena A, Movin T, et al. Achilles tendinopathy: some aspects of basic science and clinical management. Br ] Sports Med 2002;36:239-49. [ Links ]
4. Järvinen TAH, Kannus P, Maffuli N, Khan KM. Achilles Tendon Disorders: Etiology and Epidemiology. Foot Ankle Clin N Am 2005;10:255-66. [ Links ]
5. Maffuli N, Kader D. Tendinopathy of Tendo Achilles. J Bone Joint Surg [Br] 2002; [ Links ]84-B:1-8.
6. Puddu G, Ippolito E, Postacchini F. A classification of Achilles tendon disease. Am J Sports Med 1976;4(4):145-50. [ Links ]
7. Jozsa L, Kannus P. Human tendon: anatomy, physiology and pathology. Champaign: Human Kinetics, 1997. [ Links ]
8. Longo UG, Ronga M, Maffulli N. Achilles Tendinopathy. Sports Med Arthrosc Rev. 2009;17:112-26. [ Links ]
9. Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies: Update and implications for clinical management. Sports Med 1999;27:393-408. [ Links ]
10. Leadbetter WB. Cell matrix response to tendon injury. Clin Sports Med 1992;11:533-78. [ Links ]
11. Maffuli M, Khan KM, Puddu G. Overuse tendon condition: Time to change a confusing terminology. Arthroscopy 1998;14:840-43. [ Links ]
12. Järvinen TAH, Kannus P, Paavola M et al. Achilles tendon injuries. Curr Opin Rheumatol. 2001;13:150-55. [ Links ]
13. Doral MN, Bozkurt M, Turhan E, et al. Functional anatomy of the Achilles tendon. In: Achilles Tendinopathy: Current Concepts. Calder J, Karlsson J, Maffulli N et al., editors. 1st edition. United Kingdom,DJO publications, 2010. pp9-14. [ Links ]
14. Paavola M, Kannus P, Jàrvinen TAH et al. Achilles Tendinopathy: Current concepts review. J Bone Join Surg [Am]2002;84-A(11):2062-75.
15. Chen TM, Rozen WM, Pan W-R et al. The arterial anatomy of the Achilles tendon: Anatomical study and clinical implications. Clinical Anatomy 2009;22:377-85. [ Links ]
16. Carr AJ, Norris SH. The blood supply of the calcaneal tendon. J Bone Joint Surg [Br] 1989;71-B:100-101.
17. Åström M, Westlin N. Blood flow in the human Achilles tendon assessed by laser Doppler flowmetry. J Orthop Res 1994;12:246-52. [ Links ]
18. Stilwell DL. The innervations of tendons and aponeurosis. Am J Anat. 1957;100:289-317. [ Links ]
19. Maganaris CN, Paul JP. Tensile properties of the in vivo gastrocnemicus tendon. Journ Biomech 2002;35:1639-46. [ Links ]
20. O'Brien M. Functional anatomy and physiology of tendons. Clin Sports Med 1992;111:141-51. [ Links ]
21. Magnusson SP, Narici MV, Maganaris CN, et al. Human tendon behaviour and adaptation, in vivo. Journ Physiol 2008;586(1):71-81. [ Links ]
22. Knobloch K, Yoon U, Vogt PM. Acute and overuse Injuries correlated to hours of training in mater running athletes. Foot Ankle Int 2008;29:671-76. [ Links ]
23. Rolf C, Movin T. Etiology, Histopathology and outcome of surgery in Achillodynia. Foot Ankle Int 1997;18(9):565-69. [ Links ]
24. Holmes GB, Lin J. Etiologic factors associated with symptomatic Achilles tendinopathy. Foot Ankle Int 2006;27(11):952-59. [ Links ]
25. Klemp P, Halland AM, Majoos FL, et al. Musculoskeletal manifestations in hyperlipidaemia: acontrolled study. Ann Rheum Dis 1993;52:44-48. [ Links ]
26. Huston KA. Achilles tendinitis and tendon rupture due to fluoroquinolone antibiotics. N Engl J Med 1994;331:748. [ Links ]
27. Järvinen TAH, Kannus P, Maffulli N et al. Achilles tendon disorders: Etiology and epidemiology. Foot Ankle Clin N Am. 2005:10:255-66. [ Links ]
28. Kannus P. Etiology and pathophysiology of chronic tendon disorders in sports. Scand J Med Sci Sports. 1997;7:78-85. [ Links ]
29. Clement DB, Taunton JE, Smart GW. Achilles tendinitis and peritendinitis: Etiology and treatment. Am J Sports Med 1984;12(3):179-84. [ Links ]
30. Li Z. Inflammatory response of human tendon fibroblasts to cyclic mechanical stretching. Am J Sports Med 2004;32(2):435-40. [ Links ]
31. Khan MH, Li Z, Wang JH. Repeated exposure of tendon to prostaglandin-E2 leads to localized tendon degeneration. Clin J Sports Med 2005;15:27-33. [ Links ]
32. Alfredson H, Thorsen K, Lorentson R. In situ micro-dialysis in tendon tissue: high levels of glutamate but not PGE2 in chronic Achilles tendon pain. Knee Surg Sports Traumatol Arthrosc 1999;7:378-81. [ Links ]
33. Schubert T, Weidler C, Lerch K, et al. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis 2005;64:1083-86. [ Links ]
34. Carlsson O, Schizas N, Li J, et al. Substance P injections enhance tissue proliferation and regulate nerve in-growth in rat tendon repair. Scand J Med Sci Sports 2011;21(4):562-69. [ Links ]
35. Ackerman PW, Li J, Lundeberg T, et al. Neuronal plasticity in relation to nociception and healing of rat Achilles tendon. J Orthop Research 2003;21:432-41. [ Links ]
36. Silbernagel K, van Sterkenburg MN, Karlsson J. Diagnosis. In: Achilles Tendinopathy: Current Concepts. Calder J, Karlsson J, Maffulli N et al., editors. 1st edition. United Kingdom,DJO publications, 2010. pp9-14. [ Links ]
37. Maffuli N, Kenward MG, Testa V, et al. Clinical diagnosis of Achilles tendinopathy with tendinosis. Clin J Sport Med 2003;13(1):11-15. [ Links ]
38. Khan KM, Forster BB, Robinson J et al. Are ultrasound and magnetic resonance imaging of value in assessment of Achilles tendon disorders? A two year prospective study. Br J Sports Med 2003;37:149-53. [ Links ]
39. Astrom M, Gentz CF, Nilsson P, et al. Imaging in chronic Achilles tendinopathy: a comparison of ultrasonography, magnetic resonance imaging and surgical findings in 27 histologically verified cases. Skeletal Radiol 1996;25:615-20. [ Links ]
40. Zanetti M, Metxdorf A, Kundert H, et al. Achilles tendons: clinical relevance of neovascularisation diagnosed with power Doppler US. Radiology. 2003;227(2):556-60. [ Links ]
41. Soila K, Karjalainen PT, Aronen HJ, et al. High resolution MR imaging in the asymptomatic Achilles tendon: new observations. Am J Roentgenol. 1999;173:323-28. [ Links ]
42. Ginsberg F, Famaey JP. Double blind, randomized crossover study of the percutaneous efficacy and tolera-bility of a topical indomethacin spray versus placebo in the treatment of tendinitis. The J Int Med Res.1991;19:131-36. [ Links ]
43. Rees J, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med 2009;37:1855-67. [ Links ]
44. Ånström M, Westlin N. No effext of piroxicam on Achilles tendinopathy. A randomised study of 70 patients. Acta Orth Scand 1992;63:631-34. [ Links ]
45. Tsai WC, Hsu CC, Chou SW, et al. Effects of celecoxib on migration, proliferation and collagen expressionof tendon cells. Connective tissue research.2007;48:46-51. [ Links ]
46. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of mid-portion Achilles tendinopathy: A systematic review. Clin J Sport Med 2009;19:54-64. [ Links ]
47. Stanish WD, Rubinovich RM, Curwin S. Eccentric exercise in chronic tendinitis. Clin Orthop Relat Res. 1986;208:65-68. [ Links ]
48. Alfredson H, Pietila T, Jonsson T. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sport Med 1998;26:360-66. [ Links ]
49. Mafi N, Lorentzon R, Alfredson H. Superior short-term results with eccentric calf muscle training compared to concentric training in a randomized prospective multi-centre study on patients with chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc 2001;9:42-47. [ Links ]
50. Ohberg L, Alfredson H. Effects of neovascularisation behind the good results with eccentric training in chronic mid-portion Achilles tendinosis? Knee Surg Sports Traumatol Arthrosc 2004;12:465-70. [ Links ]
51. Rees JD, Lichtwark GA, Wolman RL, et al. The mechanism of efficacu of eccentric loading in Achilles tendon injury: An in vivo study in humans. Rheumatology (Oxford, England) 2008;47:1493-97. [ Links ]
52. Rompe JD, Furia J, Maffulli N. Eccentric loading versus eccentric loading plus shock-wave treatment for mid-portion Achilles tendinopathy: A randomised control trial. Am J Sports Med 2009;37:463-70. [ Links ]
53. Rompe JD, Furia JP, Maffulli N. Mid-portion Achilles tendinopathy-current options for treatment. Disabil and Rehab.2008;30:1666-76 [ Links ]
54. Rompe JD, Nafe B, Furia JP, et al. Eccentric loading, shockwave treatment, or wait-and-see policy for tendinopathy of the main body of tendo Achilles: a randomized control trial. Am J Sports Med 2007;35:374-83. [ Links ]
55. Costa ML, Shepstone l, Donnell ST, et al. Shock wave therapy for chronic Achilles tendon pain. Clin Orthop Relat Res.2005;440:199-204. [ Links ]
56. Rasmussen S, Christensen M, Mathiesen I, et al. Shockwave therapy for chronic Achilles tendinopathy: a double-blind, randomized clinical trial of efficacy. Acta Orthopaedica 2008;79:249-56. [ Links ]
57. Ogden Ja, Toth-Kischkat A, Schultheiss R. Principles of shock wave therapy. Clin Orthop Relat Res. 2001;348:8-17 [ Links ]
58. Paoloni JA, Appleyard RC, Nelson J, et al. Topical glycerol trinitrate treatment of chronic noninsertional Achilles tendinopathy: A randomized, double blind, placebo controlled trial. J Bone Joint Surg (Am).2004;86:916-22. [ Links ]
59. Kane TP, Ismail M, Calder JD. Topical glyceryl trinitrate and noninsertional Achilles tendinopathy: a clinical and cellular investigation. Am J Sport Med 2008;36:1160-63. [ Links ]
60. Kampa RJ, Connel DA. Autologous blood injections: Whole blood and platelet-rich plasma. In: Achilles Tendinopathy: Current Concepts. Calder J, Karlsson J, Maffulli N et al., editors. 1st edition. United Kingdom,DJO publications, 2010. pp131-42. [ Links ]
61. Graziani F, Ivanovski S, Cei S et al. The in vitro effect of different PRP concentrations on osteoblasts and fibrob-lasts. Clin Oral Implants Res 2006:17;212-19. [ Links ]
62. De Vos RJ, Weir A, van Schie H et al. Platelet-rich plasma injection for chronic Achilles tendinopathy. A randomised controlled trial. JAMA 2010;303:144-49. [ Links ]
63. Chan O, O'Dowd D, Padhiar N et al. High volume image guided injection in chronic Achilles tendinopathy. High volume image guided injections in chronic Achilles tendinopathy. Disability & Rehabilitation. 2008;30(20-22);1697-708. [ Links ]
64. Alfredson H, Öhberg L. Sclerosing injections to areas of neo-vasculariation reduce pain in chronic Achilles tendinopathy: a double-blind randomised controlled trial. Knee Surg Sports Traumatol Arthrosc 2005;13:338-44. [ Links ]
65. Lind B, Ohberg L, Alfredson H. Sclerosing polidocanol injections in mid-portion Achilles tendinosis: remaining good clinical results and decreased tendon thickness at 2-year follow-up. Knee Surg Sports Traumatol Arthrosc 2006;14:1327-32. [ Links ]
66. Öhberg L, Alfredson H. Ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis: a pilot study of a new treatment. Br J Sports Med 2002;36:173-75. [ Links ]
 Correspondence:
Correspondence:
Dr A Horn
Postnet Suite 342
Private Bag X18
Rondebosch 7701
Cape Town
Cell: 071 679 4228
Work: 021 404 5108
Email: anriahorn@gmail.com
Eccentric exercise programme
(Alfredson H et al. Am J Sports Med. 1998;26:360-66)
- Exercises are to be performed with knee straight to eccentrically load gastrocnemicus, and knee bent to load soleus.
- Patients should aim to perform three sets of 15 exercises twice a day, every day, for 6 weeks. If too painful initially, patients can start with one set of 10 exercises and then gradually increase frequency.
- As patients become more comfortable with the exercises, load can be added by means of a backpack. Load can be increased by increments of 5 kg.
- Exercises are to be continued as long as the patient remains symptomatic, after which a maintenance regimen can be instituted.
- Exercises are illustrated and explained step-wise in Figures 4a-d.














