Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SA Orthopaedic Journal
versión On-line ISSN 2309-8309
versión impresa ISSN 1681-150X
SA orthop. j. vol.14 no.2 Centurion jun. 2015
http://dx.doi.org/10.17159/2309-8309
KNEE/SEPSIS
Mycobacterium fortuitum as infectious agent in a septic total knee replacement: Case study and literature review
RG VenterI; C SolomonII; M BaartmanIII
IMBChB(Stell); Registrar, Orthopaedics, Tygerberg Hospital, Stellenbosch University
IIMBChB(Stell), FCS Ortho; Orthopaedic Surgeon, Department of Orthopaedic Surgery, Paarl Hospital
IIIMBChB, FCS Ortho; Head of Department, Department of Orthopaedic Surgery, Paarl Hospital
INTRODUCTION
Infection of prosthetic joints with non-tuberculous mycobacteria (NTM) is rare. The rapidly growing mycobacteria (RGM) are a subgroup of NTM. They are not very virulent organisms, found ubiquitously in the environment, and most infections in humans are due to direct inoculation of the organism into a joint or soft tissue. We describe a 70-year-old patient, who developed an infection with Mycobacterium fortuitum after primary knee arthroplasty, one of only a handful described in the literature. Peri-prosthetic infections with RGM are a challenge because there is a lack of data guiding management, and because the diagnosis is often delayed. Routine cultures of joint effusions or tissue are often discarded before the non-tuberculous mycobacteria have a chance to culture (in our case, 14 days). Principles of treatment include: making a diagnosis from tissue culture, staged revision surgery with aggressive surgical debridement of the joint and high dosages antibiotics (for at least six weeks, treating empirically initially until a sensitivity profile for the organism is available). The second stage of the revision should be delayed by 3-6 months. In our case the removed implant was autoclaved and re-implanted loosely with antibiotic-loaded cement as part of the first-stage revision.
Key words: total knee replacement, peri-prosthetic joint infection, septic arthritis, rapidly growing mycobacteria, non-tuberculous mycobacteria, Mycobacterium fortuitum, Mycobacterium abcessus, Mycobacterium chelonae, Mycobacterium smegmatis, Mycobacterium kansasii, Mycobacterium goodii
Case report
We present a 70-year-old, Caucasian, HIV-negative patient, from a rural district in the Western Cape, South Africa. She presented to an outreach clinic with a 10-year history of left knee pain. Clinically she had a 20 degrees valgus deformity (correctable) without fixed flexion. Radiographically there was marked osteopaenia, cysts and degenerative changes. A clinico-radiological diagnosis of rheumatoid arthritis was made (Figures 1 and 2). She had no previous medical history of note, and did not have any infiltrations or penetrating wounds of that knee. No other joints were affected.
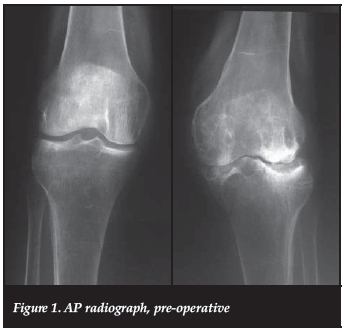

A total knee replacement was performed in April 2013 (Triathlon®, Stryker). The pre-operative blood investigations included a white cell count of 7.10 χ 109 and rheumatoid factor of 198.3 IU/ml. The erythrocyte sedimentation rate was not requested.
Intra-operative findings included large cysts in the medial tibial plateau, medial femoral condyle and a large concavity of the lateral plateau requiring bone graft, as well as marked distal femoral erosion with minimal erosion of the posterior aspect of the lateral femoral condyle. This atypical pattern of joint erosion prompted us to sample synovial tissue.
Post-operatively she did very well, her early recovery and rehabilitation was uneventful, and she was discharged a week later in good health. The post-operative blood investigations also suggested a diagnosis of rheumatoid arthritis. Anti-cyclic citrullinated peptide: more than 250 units, erythrocyte sedimentation rate: 85 mm/hr.
Histological investigation of the synovial biopsy showed proliferated synovial cell lining, infiltration of lymphocytes, plasma cells, but also neutrophils. The histology was therefore highly suggestive of rheumatoid arthritis, but the component of acute inflammation was unaccounted for. No microbiological examinations were ordered at the time. Figures 3 and 4 show component placement post-operatively.


Intra-operative findings included large cysts in the medial tibial plateau, medial femoral condyle and a large concavity of the lateral plateau requiring bone graft, as well as marked distal femoral erosion with minimal erosion of the posterior aspect of the lateral femoral condyle
Three weeks post-operatively she presented to her local district hospital with symptoms of knee pain and swelling, vomiting and dysuria. She was diagnosed with acute gastroenteritis and a urinary tract infection. However, her left knee pain and swelling was not addressed at the time.
The patient became lost to arthroplasty follow-up and visits the clinic for the first time 2 months after the surgery. At this stage her knee was painful, tender and warm, and had developed two draining sinuses. The inflammatory markers were raised. White cell count: 10.8 χ 109, erythrocyte sedimentation rate: 100 mm/hr, C-reactive protein: 82.3 mg/L.
She underwent debridement of the knee as an emergency and tissue samples were taken for diagnostic purposes. Intravenous ampicillin, 2 g, 6 hrly and cloxacillin 2 g, 6 hrly, was started empirically. Routine cultures were negative; however, TB investigations were also ordered, and although direct microscopy was negative, the cultures revealed acid-fast bacilli at day five and day 12, respectively, but polymerase chain reaction test for MTB complex was negative. The organism was later identified as Mycobacterium fortuitum. Histological examination revealed features of acute and chronic inflammation, with granulation tissue, hemosidero-phages, fibrosis and giant cell formation.
We decided on empiric therapy of the rapidly growing mycobacteria based on available literature:1 meropenem, 1 g, 8 hrly, IV and ciprofloxacin, 750 mg 12 hrly, PO.
Her second debridement took place three weeks after the first debridement. The implants and cement were removed completely. A radical synovectomy was performed. The removed femoral and tibial implants were cleaned and sterilised in an autoclave. The sterilised components were loosely re-implanted as a spacer with cement loaded with 5 g of vancomycin and 3.6 g of tobramycin per 40 g of Simplex Cement. A conventional UHMWPE spacer was also inserted.
Response to therapy was excellent - reduced pain and swelling and active flexion to 90° early post-operatively. The infection markers returned to almost normal levels within three weeks. White cell count: 7.0 χ 109, erythrocyte sedimentation rate: 46 mm in 1hr and C-reactive protein: 11.8 mg/L.
The patient remained in hospital for four weeks, receiving meropenem intravenously. The ciprofloxacin was stopped a week after the debridement, and replaced with amikacin, 600 mg dly and cefuroxime, 1.5 g, 6 hrly. At the end of four weeks of total intravenous therapy she was discharged home with a course of ciprofloxacin (500 mg, 12 hrly, orally) and cotrimoxazole (160/800 mg, 12 hrly, orally) for six weeks. The second stage revision of the joint three months after completion of the antibiotic course was planned if she remained clinically aseptic.
Response to therapy was excellent - reduced pain and swelling and active flexion to 90° early post-operatively
At the most recent follow-up visit in January 2014 (9 months after the primary total knee replacement) she was very satisfied with regard to function and pain. She had a full range of motion of the left knee, was fully weightbearing and all the inflammatory markers were within normal limits. She refused the second stage of revision. Figures 5 and 6 show the left knee at last follow-up visit.

Literature review
A literature search revealed 22 previous cases of prosthetic knee joint infections with rapidly growing mycobacteria (RGM).2-15
Infections of other prosthetic joints and infections where the infectious agent was one of the other non-tuberculous mycobacteria (NTM) were not included.
The most prevalent organisms were M. fortuitum (11 patients), M. chelonae (four patients), and M. goodii (three patients). By far the most were immunocompetent and not receiving any immunosuppressive medication.
The average time to onset of symptoms from prosthesis implantation was 136.6 weeks, in the 20 patients of whom this information was stated in the case reports. The shortest was 1 week,2 and the longest was 16 years.10
At presentation the mean erythrocyte sedimentation rate (ESR) was 38.2 mm/hr (reported in eight patients), the highest measured was 96 mm/hr.3 The time for RGM to appear in culture was reported in seven patients and the average time was 6.8 days. The longest was 11 days.13
In most cases the patients received at least one debridement in theatre with removal of the implants and insertion of an antibiotic-loaded cement spacer. The average time to successful revision arthroplasty, from the removal of the implant, was 21.5 weeks (reported in 12 cases). The shortest time to successful re-implantation was 12 weeks.9
One case of relapse was reported in 1987, after the revision arthroplasty was performed only seven weeks after resection arthroplasty. The infection was treated with doxycycline as suppressive therapy, until a superinfection with yet another organism prompted resection and arthrodesis.6
In four cases, for various reasons, the prosthesis was retained and the infection controlled with long-term suppressive therapy.2, 10, 15
Table I summarises the sensitivity profiles for the reported cases.
Discussion
Infection of a prosthetic joint can be a potentially devastating complication following knee arthroplasty and a critical part of treatment is identification of the causative organism, the most common being Staphylococcus.1
Infections with RGM can be difficult to diagnose for a variety of reasons. They are rarely the causative organism and therefore not suspected. Acid-fast staining is often not positive and although quicker than M. tuberculosis, they take longer to culture than other organisms (1-2 weeks in liquid cultures),17 and by that time routine cultures might have been discarded.
The RGM are divided into three groups, based on differentiation according to antimicrobial susceptibility testing: fortuitum group, with wider antimicrobial sensitivity, chelonae/abcessus group and smegmatis group that incorporates M. goodii and M. woliskyi.1'
These organisms are pervasive in the environment and have been isolated from water, soil, and in hospitals.19 Infections in humans have been well described, and occur mostly secondary to direct inoculation20 or contaminations of wounds. In case reports these often occurred in clusters, and on more than one occasion contaminated hospital fresh water supply was suspected.21,22
Cornelius et al. described a cluster of three M. fortuitum prosthetic joint infections (two knees and one hip), postulating intra-operative contamination.9 Ahmad describes a case of peri-prosthetic infection, most probably acquired during physical therapy in a whirlpool bath.12 However, in most cases of peri-prosthetic infection with NTM the source of infection is never identified.
Predisposing factors have been proposed, like rheumatoid arthritis, other comorbidities and pre-operative intra-articular steroid depot injections.3 Whether this implies a possible direct inoculation of the organisms into the joint or the immune depressing effects of the steroid is unclear. Furthermore, NTM infection of native joints, even one without violation of the joint (in an HIV-positive patient), has been described, implying that purely haematogenous spread also occurs.23,24
Infections with RGM can be difficult to diagnose
This family of organisms grow in biofilm,25 possibly explaining why there is a delay in presentation, but more importantly, explaining why aggressive debridement, removal of the implants, and prolonged courses of antibiotics seem to offer the only hope of cure.
Conclusion
Prosthetic joint infection with non-tuberculous mycobacteria can be considered as rare. We report a case of a prosthetic knee infection with one of the rapidly growing mycobac-teria, M. fortuitum and review the previous case reports in the literature. Considering the outcomes of previously reported cases, delay in identification of the causative organism appears to be a significant cause of morbidity, emphasising the importance of a high index of suspicion, especially if initial microbial cultures are negative.
The most effective way to treat these infections seems to be removal of the implant and debridement combined with antimicrobial therapy for at least six months, guided by sensitivity testing, before re-implantation is attempted. Based on documented sensitivity profiles of previous cases, we recommend empiric meropenem, IV and ciprofloxacin, IV while sensitivity profiles are pending, and the use of antibiotic-impregnated cement.
For patients that are poor candidates for surgery, however, chronic suppressive therapy in a poor surgical candidates has also been shown to be effective.2, 10, 15
The content of this article is the original work of the author. No benefits of any form have been or are to be received from a commercial party related directly or indirectly from this article.
References
1. Brown-Elliott B a, Nash K a, Wallace RJ. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev. 2012 Jul;25(3):545-82. [ Links ]
2. Eid A, Berbari E. Prosthetic joint infection due to rapidly growing mycobacteria: report of 8 cases and review of the literature. Clin Infect Dis. 2007 Sep 15;45(6):687-94. [ Links ]
3. Wang S, Yang C, Chen Y, Lay C, Tsai C. Septic arthritis caused by Mycobacterium fortuitum and Mycobacterium abscessus in a prosthetic knee joint: case report and review of literature. Intern Med.2011;50(19):2227-32. [ Links ]
4. Cheung I, Wilson A. Mycobacterium fortuitum infection following total knee arthroplasty: A case report and literature review. Knee 2008 Jan;15(1):61-63. [ Links ]
5. Saccente M. Mycobacterium fortuitum group periprosthetic joint infection. Scand J Infect Dis. 2006 Jan;38(8):737-39. [ Links ]
6. Herold RC, Lotke PA, MacGregor RR. Prosthetic joint infections secondary to rapidly growing Mycobacterium fortuitum. Clin Orthop Relat Res. 1987 Mar;(216):183-86. [ Links ]
7. Booth J, Jacobson J. Infection of prosthetic arthroplasty by Mycobacterium fortuitum. Two case reports. J Bone Joint Surg Am. 1979 Mar;61(2):300-302. [ Links ]
8. Pring M, Eckhoff D. Mycobacterium chelonae infection following a total knee arthroplasty. J Arthroplasty. 1996;11(1):115-16. [ Links ]
9. Cornelius L, Reddix R, Burchett C, Bond G, Fader R. Cluster of Mycobacterium fortuitum prosthetic joint infections. J Surg Orthop Adv. 2007 Jan;16(4):19<^98. [ Links ]
10. Porat M, Austin M. Bilateral knee periprosthetic infection with Mycobacterium fortuitum. J Arthroplasty. 2008 Aug;23(5):787-89. [ Links ]
11. Yung Y, Li P, Lee Q Wong Y, Wai Y. Treatment of Mycobacterium fortuitum infection of total knee arthroplasty: A case report. J Orthop. 2012 Dec;16(2):82-85. [ Links ]
12. Ahmad S, Khakoo R a. Left knee prosthesis-related Mycobacterium goodii infection. Int J Infect Dis. 2010 Dec;14(12):e1115-16. [ Links ]
13. Ferguson DD, Gershman K, Jensen B, Arduino MJ, Yakrus M a, Cooksey RC, et al. Mycobacterium goodii infections associated with surgical implants at Colorado hospital. Emerg Infect Dis. 2004 Oct;10(10):1868-71. [ Links ]
14. Jeong JH, Seo Y-H, Kim K-H, Ahn J-Y, Park P-H, Park Y-K. Mycobacterium wolinskyi infection confirmed by rpoB gene sequencing. J Clin Lab Anal. 2012 Sep;26(5):325-27. [ Links ]
15. Tompkins JC, Harrison MS, Witzig RS. Mycobacterium goodii infection of a total knee prosthesis. Infect Med. 2008;25(11):522-25. [ Links ]
16. Sia IG, Berbari EF, Karchmer AW. Prosthetic joint infections. Infect Dis Clin North Am. 2005 Dec;19(4):885-914. [ Links ]
17. Brown-Elliott B. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontu-berculous mycobacteria. Clin Microbiol. 2012 Jul;25(3):545-82. [ Links ]
18. Kothavade RJ, Dhurat RS, Mishra SN, Kothavade UR. Clinical and laboratory aspects of the diagnosis and management of cutaneous and subcutaneous infections caused by rapidly growing mycobacteria. Eur J Clin Microbiol Infect Dis. 2013 Feb;32(2):161-88. [ Links ]
19. Brown T. The rapidly growing mycobacteria: Mycobacterium fortuitum and Mycobacterium chelonei. Infect Control. 1985;6(7):283-88. [ Links ]
20. Miron D, El A, Zuker M. Mycobacterium fortuitum osteomyelitis of the cuboid after nail puncture wound. Pediatr. 2000;19(5):475-88. [ Links ]
21. Hoffman P, Fraser D. Two outbreaks of sternal wound infections due to organisms of the Mycobacterium fortuitum complex. J Infect Dis. 1981;143(4):533-42. [ Links ]
22. Meyers H, Brown-Elliott B a, Moore D, Curry J, Truong C, Zhang Y, et al. An outbreak of Mycobacterium chelonae infection following liposuction. Clin Infect Dis . 2002 Jun 1;34(11):1500-507. [ Links ]
23. Butt AA, Janney A. Arthritis due to Mycobacterium fortuitum. Scand J Infect Dis. 1998;30:525-27. [ Links ]
24. Bernard L, Vincent V, Lortholary O, Raskine L, Vettier C, Colaitis D, et al. Mycobacterium kansasii septic arthritis: French retrospective study of 5 years and review. Clin Infect Dis. 1999 Dec;29(6):1455-60. [ Links ]
25. Chen JM, German GJ, Alexander DC, Ren H, Tan T, Liu J. Roles of Lsr2 in colony morphology and biofilm formation of Mycobacterium smegmatis. J Bacteriol. 2006;188(2):633-41. [ Links ]
 Correspondence:
Correspondence:
Dr RG Venter
22 Minaret Close
Welgevonden Estate
Stellenbosch 7600
Email: rgventer@gmail.com














