Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SA Orthopaedic Journal
On-line version ISSN 2309-8309
Print version ISSN 1681-150X
SA orthop. j. vol.14 n.1 Centurion Feb./Mar. 2015
SPINE
Gunshot face as a cause of hyperextension central cord syndrome in a young patient
L BomelaI; RN DunnII
IMBChB(UCT), FCOrth(SA); Spine Fellow: Spine Surgery Unit, Groote Schuur Hospital; From Groote Schuur Hospital and the Department of Orthopaedics, University of Cape Town, South Africa
IIMBChB(UCT), MMed(UCT)Ortho, FCOrth(SA) Consultant Spine and Orthopaedic Surgeon Professor and Head of the Department of Orthopaedics, University of Cape Town Head: Orthopaedic Spinal Services, Groote Schuur Hospital Spine Deformity Service, Red Cross Children's Hospital; From Groote Schuur Hospital and the Department of Orthopaedics, University of Cape Town, South Africa
ABSTRACT
Central cord syndrome (CCS) is a syndrome where the patient's upper limbs are more severely affected than the lower limbs in terms of neurological deficit. This is typically found in an elderly patient with pre-existing spondylosis and a minor hyperextension injury. This case report highlights an unusual cause of CCS in a young patient with pre-existing congenital cervical canal stenosis and acute hyperextension induced by a facial gunshot. The aetiology and management dilemmas are discussed.
Key words: central cord syndrome, gunshot, hyperextension, spinal cord injury
Introduction
Acute central cervical cord syndrome is commonly seen in elderly patients with underlying cervical spine stenosis following a fall and subjected to a hyperextension force.1 Patients present with an incomplete spinal cord injury with predominantly upper limb weakness and relatively spared lower limbs.2
We present an unusual case of a young man who was subjected to an acute hyperextension force during a gunshot to the face. In addition to the infrequently encountered aetiology, the management challenges will be discussed.
Case report
A 43-year-old male was admitted to our tertiary hospital Trauma Unit after having sustained a gunshot injury to the face.
He was fully conscious with features of neurogenic shock (BP 93/50 and heart rate 86). Anal tone was present but decreased.
The bullet had entered through the philtrum and exited above the right maxillary sinus. These facial injuries were cleaned and sutured.
His neurological examination confirmed a C4 incomplete lesion with motor weakness but sensory preservation. There was reduced anal tone and he required a urinary catheter due to retention.
On arrival at the Trauma Unit he was screened by low-dose digital X-ray whole body scan (Lodox) which excluded a skull fracture and confirmed that the bullet had not been retained. No cervical spine pathology was identified although these images were of poor quality.
Computerised tomography (CT) scan illustrated a linear undisplaced fracture of the anterior wall of the right maxillary antrum with extension into the alveolar process. There was haemorrhage into the right maxillary sinus.
Magnetic resonance imaging (MRI) demonstrated mild retrolisthesis of C3/C4 with increased signal in the pre-vertebral tissue as well as interspinous ligaments. Disc osteophyte complexes were present at C3/4 and C4/5 in a congenitally narrowed canal. There was cord compression and contusion from C3-C5 as evidenced by hyperintense cord signal on the T2 MRI sequence as revealed by high signal foci within the cord (Figures 1 and 2).
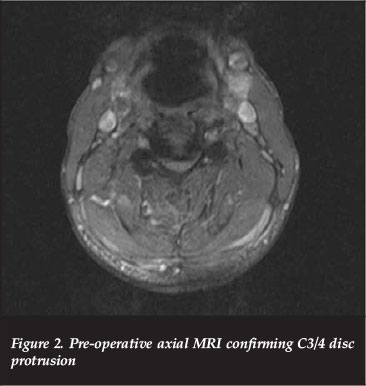
His neurological examination confirmed a C4 incomplete lesion with motor weakness but sensory preservation
The patient was stabilised physiologically and referred to the Acute Spinal Cord Injury (ASCI) Unit for supportive ventilation and treatment of atelectasis, bronchopneumonia and shock.
The patient was stabilised with regard to the neurogenic shock, and the bronchopneumonia treated with broad-spectrum antibiotics. Supportive ventilation was required. The neck was initially managed in a Philadelphia collar.
Based on the extensive nature of cord compression from C3-C5, and underlying congenital stenosis, a posterior-based procedure was chosen. A laminoplasty was performed rather than a laminectomy due to his young age.
However, despite an adequate canal enlargement intra-operatively, the patient had no neurological recovery in the subsequent two weeks. Thus a second stage anterior C3/4 disc osteophyte complex decompression was performed via a Smith-Robinson approach (Figure 3).
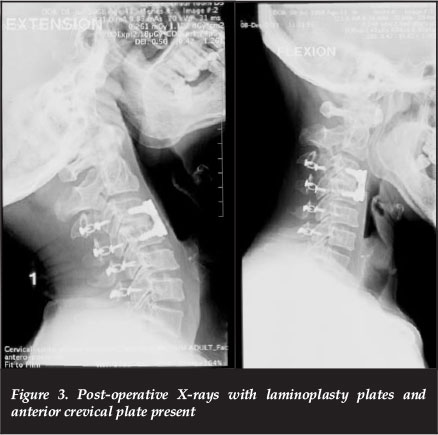
Following this anterior procedure there was an immediate neurological gain of at least an MRC grade, more so in the lower than upper limbs.
Three weeks later the patient was transferred to the spinal rehabilitation centre.
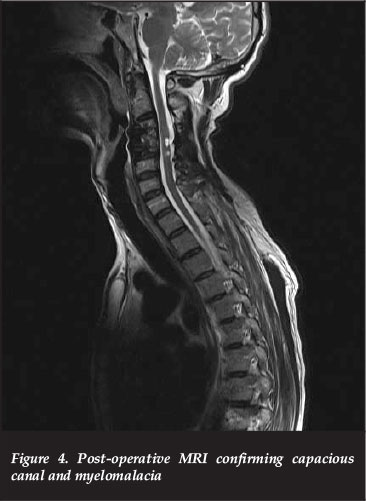
At the six-month post-operative visit, his lower limbs had improved from 1/5 to 3 and 4s but due to the severe spasticity he remained non-ambulatory, thus ASIA B to C. His upper limbs remained at 2/5 power. A follow-up MRI confirmed adequate canal decompression with myelomalacia of the cord (Figure 4).
Discussion
Acute traumatic central cervical cord syndrome was defined by Schneider in 1954 as an incomplete spinal cord injury with the upper extremities illustrating a significantly greater motor impairment than the lower extremities with variable bladder dysfunction and sensory abnormality below the affected level.2 However, Sir Thornburn was the first to describe cervical cord syndrome in literature in 1887 as 'a case of concussion of the spine'.3 It is caused by a variety of mechanisms but the most common is a hyperextension force resulting in cord compression and injury to the central part of the spinal cord with some sparing of the peripheral pathways.2,4
Three main mechanisms have been postulated:
1. Young patients sustaining a high velocity injury, e.g. motor vehicle accident, diving accident or fall from height
2. Older patients (>50 years) due to a hyperextension force in an already degenerate spine
3. Low velocity trauma in a patient with an acute central disc herniation4-9
Hyperextension of the cervical spine can cause damage to the spinal cord via buckling of the ligamentum flavum or impaction of the posterior elements with rupture of the posterior longitudinal ligament.2, 4, 8-10 Hyperextension can be caused by a contact or non-contact force. Direct frontal impact to the head can also cause anterior distraction and posterior compression of the spinal cord, a mechanism reported with the deployment of airbags.11,12
In this case, the young patient had underlying stenosis due to premature C3/4 degenerative stenosis. Despite the bullet not contacting the spine, it is likely to have induced an acute hyperextension force due to its trajectory across the face in an inferior-to-superior direction.
In order for the patient to be classified as a traumatic CCS, Pouw et at.13recommended that the upper limb ASIA motor score should be a minimum of 10 points lower than the lower limbs.13,14 In our case the differential was 30 points with an initial ASIA B improving to a C.
Radiological features of CCS vary. X-rays may be normal if there is no pre-existing pathology. Underlying congenital narrowing can be assessed with the Pavlov or Torg ratio. This is a ratio of canal size to anterior-to-posterior vertebral body dimension on the lateral X-ray. This should be >0.82 but in our patient was 0.5-0.7 from C3-C5.15
These patients are best investigated with an MRI where disc and ligament disruption, spinal canal compromise and degree of spinal cord injury can be assessed. The MRI may indicate cord oedema, cyst formation or, on rare occasions, a haematomyelia.2, 16-19
With regard to the case study, the MRI was an essential tool in identifying the multilevel cord compression and preexisting spondylosis.
The treatment of CCS is controversial. Aarabi et at.20state that management recommendations in an extensive literature review (1966-2011) is limited to Class III medical evidence.
He separates the treatment of all acute central CCS according to the presenting pathology:
1. Patients with MRI evidence of spinal cord signal change but no radiological abnormality can be treated medically.
2. Patients with skeletal pathology such as fracture must undergo surgery for stabilisation and decompression.
3. Patients with no bony abnormality but who have concomitant spinal stenosis have the option of either surgical or medical treatment.20
Timing of surgery in CCS remains controversial. The question remains as to whether there is a role for urgent decompression in order to enhance neurological recovery in patients with no instability. A systemic review by Lenehan et at.21reviewed whether there was a need to urgently decompress patients within 24 hours or stage the surgery.
Direct frontal impact to the head can also cause anterior distraction and posterior compression of the spinal cord
The conclusion was that patients who had ASIA C and below and persistent cord compression benefited from early intervention, but those with ASIA D deficit could be observed and potentially treated surgically later if there was no improvement.21 Other studies have supported the notion that surgically treated patients for acute cervical syndrome have better outcomes neuro-logically compared to those receiving only medical treatment.22-25 A trend towards decreased length of hospital stay and fewer complications has been illustrated in patients who are treated surgically compared to non-surgical groups.26-35
There is Class III evidence to support improved patient outcome in acute CCS by aggressive medical support to allow perfusion of the spinal cord.19, 31, 36, 37 Conservative treatment of patients with acute traumatic cervical spine syndrome may predispose the patient to persistent neuropathic pain and spasticity. The spasticity can be so severe as to hinder recovery, decrease the functional motor grade, prevent a patient from achieving potential ambulation and be the main cause of patient dissatisfaction. Physiotherapy and certain drugs such as baclofen, dantrolene and gabapentin may assist in the reduction of these symptoms.15-32-38-41 In this case study the patient was unable to achieve full ambulation due to the severe spasticity he developed. He underwent physical therapy and medical treatment to control the spasticity, to no avail.
Surgical options vary with regard to the pathology. Most patients present with multilevel pathology due to pre-existing spinal stenosis. Anterior decompression is favoured for focal pathology. This may include discectomy or corpectomy. Zhu et at.42performed meta-analysis comparing the anterior versus posterior approach for multilevel cervical spondylotic myelopathy. They found that anterior surgery provided better short-term neurological outcome but higher complication and re-operation rates compared with posterior surgery.42 Posterior surgery includes laminectomy, laminoplasty, foraminectomy and posterior cervical fusion. Posterior surgery has been associated with increased neck pain and disability,43-44 but this was disputed by a study by Seng et at.45 whereby in a two-year follow-up the study showed no increased neck instability or neck pain post laminoplasty in 52 patients. To date there is no proven superior approach with regard to treating multilevel disease.42,46,47
Our case highlights the dilemma of both pre-existing multilevel stenosis due to congenital narrow canal and premature spondylosis with a focal disc extrusion. To address this all anteriorly would necessitate multilevel corpectomies with prolonged theatre time and approach-related risks. Posterior decompression allows technically easier multilevel decompression with the laminectomy technique or slightly more demanding laminoplasty. As long as there is lordosis, the thecal sac will migrate posteriorly.19,48,49 However, this posterior migration is kept in check by the nerve roots which run antero-laterally.
Thus large anterior compression such as the C3/4 disc in this case may cause ongoing compression.50 Therefore an additional focal anterior decompression was performed when there was no initial neurological improvement. Of course, it will never be known whether the subsequent neurological recovery was directly due to the anterior approach or coincidental with delayed recovery from the posterior decompression.
The conclusion was that patients who had ASIA C and below and persistent cord compression benefited from early intervention, but those with ASIA D deficit could be observed and potentially treated surgically later if there was no improvement
Conclusion
This case reports an unusual cause of CCS in a young patient, via facial gunshot-induced hyperextension with indirect injury to the spinal cord. The management dilemma of anterior focal versus posterior multilevel decompression remains, and the decision is left to the surgeon on a case-by-case basis. In retrospect, with significant disc extrusion, an initial anterior decompression and fusion procedure is probably indicated.
The content of the article is the sole work of the authors. No benefits of any form have been or are to be received from a commercial party related directly or indirectly to the subject of the article.
As this is a case report, our Ethics committee does not require approval.
References
1. Peterson DI, Altman K. Central cervical spinal cord syndrome due to minor hyperextension injury. West J Med Jun, 1989;150:691-94. [ Links ]
2. Schneider RC, Thompson JM, Bebin J. The syndrome of acute central cervical spinal cord injury. J Neurol Neurosug Psychiat 1958;21(3):216-27. [ Links ]
3. Thornburn W. Cases on injury to the cervical region of the spinal cord. Brain 1887;9:510-43. [ Links ]
4. Harrop JS, Sharon A, Ratliff J. Central cord injury: pathophysiology, management and outcomes. The Spine Journal 2006;6:198S-206S. [ Links ]
5. Ishida Y, Tominaga T. Predictors of neurological recovery in acute cervical cord injury with only upper limb extremity impairment. Spine 2002;27:1652-57. [ Links ]
6. Dai L, Jia L. Central cord injury complicating acute disc herniation in trauma. Spine 2000;25:331-36. [ Links ]
7. Hayes KC, Askes HK, Kakulas BA. Retropulsion of inter-vertebral disc associated with traumatic hyperextension of the cervical spine and absence of vertebral fracture: an uncommon mechanism of spinal cord injury. Spinal Cord 2002;40:544-47. [ Links ]
8. Taylor AR, Blackwood W. Paraplegia in hyperextension cervical injuries with normal radiological appearances. J Bone Surg 1948;30B:245-48. [ Links ]
9. Schneider RC, Cherry G, Pantek H. The syndrome of acute central cervical spinal cord injury, with special reference to the mechanisms involved in hyperextension injuries of the cervical spine. J Neurosurg 1954;11:546-77. [ Links ]
10. Blacksin MF. Patterns of fracture after air bag deployment. J Trauma 1993;35:840-43. [ Links ]
11. Maxeiner H, Hahn M. Airbag-induced lethal cervical trauma. J Trauma 1997;42:1148-51. [ Links ]
12. Lenchan B, Street J, O'Toole P, Siddiqui A, Poynton A. Central cord syndrome in Ireland: the effect of age on clinical outcome. Eur Spine J 2009;18:1458-63. [ Links ]
13. Pouw MH, Van Middendorp JJ, Van Kampen A, Hirschfield S, Veth RPH, Curt A et al. Diagnostic criteria of traumatic central cord syndrome. Part 1: A systematic review of clinical descriptors and scores. Spinal Cord 2010;48(9): 652-56. [ Links ]
14. Van Middendorp JJ, Pouw MH, Hayes KC, Williams R, Chhabra HS, Putz C et al. Diagnostic criteria of traumatic central cord syndrome. Part 2: A questionnaire survey among spine specialists. Spinal Cord 2010;48:657-63. [ Links ]
15. Pavlov H, Torg JS, Robie B, Jahre C. Cervical spinal stenosis: determination with vertebral body ratio method. Radiology Sep 1987;164(3):771-75. [ Links ]
16. Goldberg A, Kershan S. Advances in imaging of vertebral and spinal cord injury. J Spinal Cord Med 2009;33(2):105-116. [ Links ]
17. Song J, Mizuno J, Inoue T, Nakagawa H. Clinical evaluation of traumatic central cord syndrome: emphasis on clinical significance of hyper intensity, cord compression and intramedullary high signal intensity on magnetic resonance imaging. Surgical Neurology 2006;65:117-23. [ Links ]
18. Collingnon F, Martin D, Lenelle J, Stevenhart A. Acute traumatic central cord syndrome magnetic resonance imaging and clinical observations. J Neurosurg (Spine 1) 2002;96:29-33. [ Links ]
19. Molliqaja G, Payer M, Schallera K, Tessitore E. Acute traumatic central cord syndrome: A comprehensive review. Neurochirurgie 2014;60:5-11. [ Links ]
20. Aarabi B, Alexander M, Mirvis SE, Shanmuganathan K, Chesler D, Maulucci C, et al. Predictors of outcome in acute traumatic central cord syndrome due to spinal stenosis. J Neurosurg Spine. 2011;4(1):122-30. [ Links ]
21. Lenehan B, Fisher CG, Vaccaro A, Fehlings M, Aarabi B, Dvorak MF. The urgency of surgical decompression in acute central cord injuries with spondylosis and without instability. Spine (Phila Pa 1976) 2010;35(21 suppl): S180-186. [ Links ]
22. Fehlings MG, Vaccaro A, Wilson JR, Singh A, Cadotte DW, Harrop JS, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One 2012;7(2):e32037. [ Links ]
23. Chen TY, Dickman CA, Eleraky M, Sonntag VK. The role of decompression for acute incomplete cervical spinal cord injury in cervical spondylosis. Spine 1998;23(22):2398-403. [ Links ]
24. Chen TY, Lee ST, Lui TN, Wong CW, Yeh YS, Tzaan WC, et al. Efficacy of surgical treatment in traumatic central cord syndrome. Surg Neurol 1997;48(5):435-40. [ Links ]
25. Brodkey JS, Miller Jr CF, Harmody RM. The syndrome of acute central cervical spinal cord injury revisited. Surg Neurol 1980;14(4):251-57. [ Links ]
26. Furlan JC. Noonan V, Cadotte DW, Fehlings MG. Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: Examination of pre-clinical and clinical studies. J Neurotraum 2011;28:1371-99. [ Links ]
27. Cadotte DW, Fehlings MG. Spinal cord injury: a systematic review of current treatment options. Clin Orthop Relat Res 2011;469:732-41. [ Links ]
28. Fehlings MG, Perrin RG. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine 2006;31:28-35. [ Links ]
29. Kim I. 2011. Acute traumatic central cord syndrome: early decompression or not? Korean J Spine 2011;8(1):45-51. [ Links ]
30. La Rosa GCA, Cardali S, Cacciola F, Tomasello F. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord 2004; 42(9):503-12. [ Links ]
31. Aarabi B, Hadley MN, Dhall SS, Gelb DE, Hurlbert RJ, Rozzelle CJ et al. Management of acute traumatic central cord syndrome (ATCCS). Neurosurgery 2013;72(Suppl. 2):195-204. [ Links ]
32. Chen L, Yang H, Yang T, Xu Y, Bao Z, Tang T. Effectiveness of surgical treatment for traumatic central cord syndrome. J Neurosurg Spine 2009;10:3-8. [ Links ]
33. Stevens EA, Powers AK, Branch CL. The role of surgery in traumatic central cord syndrome. Neurosurgery Quarterly 2009;19(4):222-27. [ Links ]
34. Stevens EA, Marsh R, Wilson JA, Sweasey TA, Branch CL Jr, Powers AK. A review of surgical intervention in the setting of traumatic central cord syndrome. Spine J. Oct 2010;10(10):874-80. [ Links ]
35. Van Middendorp JJ, Hosman AJF, Doi ARS. The effects of the timing of spinal surgery after traumatic spinal cord injury: A systematic review and meta-analysis. Journal of Neurotrauma Nov 2013;30:1781-94. [ Links ]
36. Smith HE, Albert TJ. Management of central cord syndrome. Essentials of spinal cord injury: basic research to clinical practice. New York: Thieme; 2012;329-36. [ Links ]
37. Vale FL, Burns J, Jackson AB, Hadley MN. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg 1997;87(2):239-46. [ Links ]
38. Nicholson BD. Evaluation and treatment of central pain syndromes. Neurology 2004;62(5 Suppl. 2):S30-6. [ Links ]
39. Dvorak MF, Fisher CG, Hoekama J, Boyd M, Noonan V, Wing PC et al. Factors predicting motor recovery and functional outcome after traumatic central cord syndrome. Spine 2005;30:2303-11. [ Links ]
40. Aito S, D'andrea M, Werhagen L, Farsetti L, Capelli S, Bandini B et al. Neurological and functional outcome in traumatic central cord syndrome. Spinal Cord 2007;45:292-97. [ Links ]
41. Tow AP, Kong KH. Central cord syndrome: functional outcome after rehabilitation. Spinal Cord 1998;36:156-60. [ Links ]
42. Zhu B, Xu Y, Liu X, Liu Z, Dang G. Anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy: a systemic review and meta-analysis. Eur Spine J. Jul 2013;22(7):1583-93. [ Links ]
43. Hosono N, Yonenobu K, Ono K. Neck and shoulder pain after laminoplasty. A noticeable complication. Spine (Phila Pa 1976) 1996;21(17):1969-73. [ Links ]
44. Wada E, Suzuki S, Kanazawa A, Matsuoka T, Miyamoto S, Yonenobu K. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: a long-term follow-up study over 10 years. Spine 2001;26:1443-48. [ Links ]
45. Seng C, Tow BP, Siddiqui MA, Srivastava A, Wang L, Yew AK et al. Surgical treated cervical myelopathy: a functional outcome comparison study between multilevel anterior cervical decompression fusion with instrumentation and posterior laminoplasty. Spine J. Jul 2013;13(7):723-31. [ Links ]
46. Lawrence BD, Jacobs WB, Norvell DC, Hermsmeyer JT, Chapman JR, Brodke DS. Anterior versus posterior approach for treatment of cervical myelopathy:a systemic review. Spine (Phila Pa 1976) Oct 2013;15;38(22 Suppl 1): S173-82. [ Links ]
47. Liu T, Xu W, Cheng T, Yang HL. Anterior versus posterior surgery for multilevel cervical myelopathy, which one is better? A systemic review. Eur Spine J 2011;20:224-35. [ Links ]
48. Uribe J, Green BA, Vanni S. Moza K, Guest JD, Levi AD. Acute traumatic central cord syndrome - experience using surgical decompression with open-door expansile laminoplasty. Surg Neurol 2005;63:505-10. [ Links ]
49. Kim SW, Hai DM, Sundaram S, Kim YC, Park MS, Paik SH et al. Is cervical lordosis relevant in laminoplasty? The Spine Journal 2013;13:914-21. [ Links ]
50. Hirai T, Okawa A, Arai Y, Takahashi M, Kawabata S,Kato T et al. Middle-erm results of a prospective comparative study of anterior decompression with fusion and posterior decompression with laminoplasty for the treatment of cervical spondylotic myelopathy. Spine (Phila Pa 1976) Nov 2011;36(23):1940-47. [ Links ]
 Correspondence:
Correspondence:
Prof Robert Dunn
Email: robert.dunn@uct.ac.za














