Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SA Orthopaedic Journal
On-line version ISSN 2309-8309
Print version ISSN 1681-150X
SA orthop. j. vol.14 n.1 Centurion Feb./Mar. 2015
SHOULDER
Reverse total shoulder arthroplasty for complex proximal humeral fractures in the elderly: How to improve outcomes and avoid complications
P RyanI; RP DachsII; JP du PlessisII; B VrettosIII; S RocheIV
IMBChB(UCT), FC(Orth)(SA), MMed(Orth)UKZN; Department of Orthopaedic Surgery, University of Cape Town
IIMBChB(UCT), FCS(SA)Ortho, MMed(Orth)UCT; Department of Orthopaedic Surgery, University of Cape Town
IIIMBChB(Zim), FRCS(Eng), FCS(SA)Orth, MMed(Orth)UCT; Department of Orthopaedic Surgery, University of Cape Town
IVMBChB(UCT), LMMC, FCS(SA)Ortho; Department of Orthopaedic Surgery, University of Cape Town
ABSTRACT
The use of reverse total shoulder arthroplasty for the management of complex proximal humerus fractures has gained popularity in the last five to ten years. We present a concise review of conservative and surgical treatment of proximal humerus fractures and a more detailed review of published series of reverse shoulder arthroplasty for fracture treatment. We discuss ways of optimising results and avoiding complications.
Key words: reverse shoulder, shoulder replacement, proximal humerus fracture
Introduction
Proximal humeral fractures account for around 5% of humeral fractures.
They are the third most common non-vertebral fragility fracture after the hip and distal radius.1 The incidence in females over the age of 50 is estimated overall at around 2.2 per 1 000 per year, and there is an exponential increase between the ages of 65 and 80 years.2
Females are affected twice as frequently as males, and the severity of fracture is noted to increase with advancing age.3 As with other fragility fractures, there is an association with increased mortality rate in the post-fracture period, which remains elevated for up to a decade; however, this is most likely a consequence of underlying medical co-morbidities.4
While most simple, minimally or non-displaced fractures can be managed successfully with non-operative means, the more complex three- and four-part displaced fractures remain difficult to treat and have worse outcomes.
Patient factors including advancing age, increased number and severity of co-morbidities, pre-existing rotator cuff abnormalities, osteoporosis, and ability to engage in post-operative rehabilitation all influence the management decision and the ability to regain functional independency.
The complexity of these injuries is a function of fracture severity (and the associated risk of avascular necrosis), and the difficulty in attaining union of the greater and lesser tuberosities in anatomical position. Avascular necrosis (AVN) complicates between 21 and 75% of three- and four-part fractures, and is related to the initial injury, the fracture pattern, the integrity and size of the medial cortical hinge, and surgical dissection. There is debate and conflicting literature regarding the optimal management of this difficult group, whether it be non-operative or surgical, and if surgical, which surgery.
In order to evaluate the benefits and risks of the various surgical techniques, it is important first to understand the natural history of non-operatively managed fractures, and to know the outcomes of the different surgical options.
Conservative management
Edelson et al. performed a prospective observational study of 76 patients with complex proximal humerus fractures managed non-operatively. They concluded that although the range of motion was limited, pain was minimal, and that the majority of patients had an acceptable functional status. Patients were grouped according to a 3D classification of fracture pattern. The average forward flexion for the various groups ranged from 106 to 123°, external rotation 34 to 43°, and internal rotation from L3 to T9. Pain at rest and with activity averaged 0.4 and 2.8 respectively on a 1-10 analogue pain scale.5 These results should be borne in mind when evaluating functional outcome and pain after surgical interventions.
Locked plate fixation
Locked plate technology for fixation of fractures associated with poor bone quality has expanded in recent years. However, there is conflicting evidence regarding the outcomes when used for proximal humerus fractures.
In a recent prospective randomised control trial (PRCT) of 50 patients comparing non-operative management and locked-plate fixation for complex displaced fractures, it was reported that at 12 months the functional scores (American Shoulder and Elbow Score ASES, and Constant Score CS) were similar, and that the only significant difference was better radiographs in the operated group.6
Complications of plate fixation, the most frequent relating to screw cut-out, are common. Spross et al. reported on their results at one year in a group of 293 patients managed with locked plates. They had a 28.2% incidence of complications, the majority of which required re-operation. Screw cut-out was seen in 11.2% and was related to secondary fracture displacement and AVN. Of note however, they report a median Constant-Murley score of 89/100 in patients who did not suffer a complication.7 In a systematic review (including 514 patients in 12 studies) of the use of locked plates for proximal humerus fractures, Sproul et al. reported an overall complication rate of 33% (excluding varus malunion as a complication), which included 10% AVN, 8% screw perforation, 6% subacromial impingement, and 4% infection.8
Hemiarthroplasty
In the early 1950s, Neer introduced hemiarthroplasty for the treatment of proximal humerus fractures at high risk of AVN. Although he and others have published good results with this, it is understood that elderly patients have worse outcomes with hemiarthroplasty than their younger counterparts. Clinical outcome is reliant upon the prosthesis height and version, and tuberosity position and union; however, in some studies up to 50% tuberosity malpositioning is seen, which can result in superior migration, stiffness, weakness, pain and lower functional scores (Figure 1).9, 10
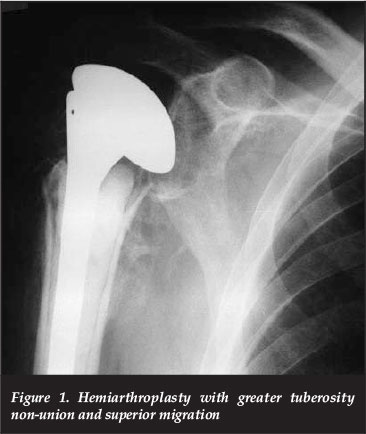
In some studies up to 50% tuberosity malpositioning is seen, which can result in superior migration, stiffness, weakness, pain and lower functional scores Factors associated with final tuberosity malposition are: prosthesis malposition, female sex, and age over 75 years.
Boons et al. in a recent PRCT comparing non-operative management with hemiarthroplasty in patients older than 65 years reported similar functional (CS, Simple shoulder test) and pain scores at 12 months, and that the non-operated group had better abduction strength at 3 and 12 months.11 Olerud et al.12in a PRCT of 55 patients evaluated at two years reported a statistically significant improvement in quality of life score (EQ-5D) for hemiarthroplasty over non-operative treatment, but no significant differences regarding the CS or range of motion. Although there was a trend towards improved DASH and pain scores in the hemi group, this was not statistically significant. The EQ-5D score includes patient responses regarding feelings of anxiety/depression, ambulatory ability, etc., and may have less significance than subjective patient scores which are specific to the anatomic region affected, such as the Oxford Shoulder Score (OSS).
Despite the above, there is general consensus that while hemiarthroplasty may be variable with regard to functional outcome, there is consistently good pain relief, with around 80% of patients experiencing no or minimal pain.13,14
Reverse total shoulder arthroplasty
The reverse total shoulder arthroplasty (RTSA) was introduced for the treatment of glenohumeral arthritis with an incompetent/irreparable rotator cuff. Initial designs met with unacceptably high complication and revision rates, and were abandoned by some. In the mid-1980s Grammont's work and design15 of a more medialised and lowered centre of rotation led to promising results when utilised for cuff deficient arthritic shoulders. Since then, the indications for use of this design have expanded to include fracture sequelae, acute fractures, revision and tumour surgery.
With better understanding and improvements in component design and biomechanics, as well as understanding of scapular morphology, some of the inherent problems with reverse shoulder arthroplasty are being addressed.
Indications
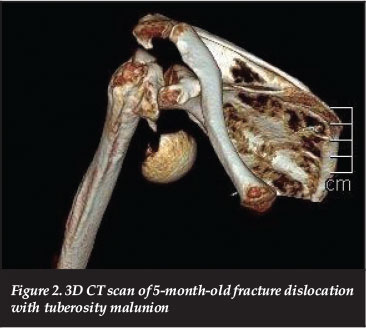
Currently the indication for RTSA in the trauma setting are: displaced complex fractures or fracture-dislocations in elderly patients (Figure 2), who have pre-existing cuff pathology, or those in whom anatomic tuberosity healing is unlikely to occur.16 A strong smoking history may be considered as a relative indication due to its negative effect on tissue (and tuberosity) healing.17
Outcomes
Results of RTSA used for fractures
Table I summarises some of the recent papers on the use of RTSA for acute fractures.18-22
Results of RTSA vs hemiarthroplasty, and/or ORIF for fractures
Sirveaux et al.,23 in a prospective multicentric study, report improved anterior active elevation with RTSA compared to hemiarthroplasty (mean of 113° vs 88°).
Gallinet et al.24 retrospectively reviewed their proximal humeral fractures managed with arthroplasty. In the initial study period they performed hemiarthroplasty, and in the latter RTSA. Age at time of surgery was similar for both groups (74 years). They report significantly better forward elevation in the RTSA group, but better internal and external rotation in the hemiarthroplasty patients. The RTSA patients had statistically significantly better Constant scores. Pain, mobility and activity favoured RTSA, while only strength was better in hemiarthroplasty. Within the RTSA group, 27 underwent tuberosity repair versus 14 without. The CS for those with tuberosity repair was statistically significantly greater for those with tuberosity repair (60.1 vs 51.7). They highlight the speed at which the RTSA group regained functional autonomy. Complication rates were similar between groups.
Contradicting this, Young et al.,25in their review of ten RTSAs and eight hemiarthroplasties used for fractures in patients with mean ages of 77 and 75 years respectively, could not demonstrate any improvement in outcome scores (ASES, OSS) or range of motion for RTSA, suggesting its use in fracture management is still unclear, while also alluding to cost implications of RTSA, and the ability to revise hemiarthroplasty should the need arise. This is however a very small series.
Garrigues et al.26retrospectively reviewed their hemiarthroplasty and RTSA results for acute fractures in an elderly population group. Despite the hemiarthroplasty group being younger (mean 69 years vs 80 years), they demonstrated statistically significant improvement in forward elevation (121° vs 91°) and functional shoulder scores (ASES 81 vs 47) in the RTSA group. The mean rotation was similar, which they attribute to tuberosity fixation undertaken in all cases of RTSA. Four of their 12 hemiarthro-plasties suffered complications (resulting in revision to RTSA in three cases), while only one of the RTSA was reported as having a complication (Sirveaux grade 1 notching).
In a review of the New Zealand Joint Registry, Boyle et al.27 showed improved patient reported outcomes at five-year follow-up (OSS 41.5 vs 32.3) in patients who had RTSA compared to hemiarthroplasty for acute fractures. This is despite the RTSA group being significantly older (mean age 79.6 vs 71.9 years). In their study, there was no significant difference in revision rate or one-year mortality. It is noted, however that the proportion of surgeries performed by 'high volume' shoulder surgeons was far greater in the RTSA than the hemiarthroplasty group (72.7 vs 31%).
More recently, Chalmers et al2Sperformed a retrospective cohort study in which they prospectively evaluated nine patients over the age of 65 years who underwent RTSA for acute fractures, and then retrospectively compared them to age- and gender-matched control groups who had undergone either Open Reduction Internal Fixation (ORIF) or hemiarthroplasty. At a minimum of one-year follow-up, there was no significant difference in their shoulder (SST and ASES) and Short-Form 12-item scores.
The RTSA group however achieved significantly better movement. All nine patients attained forward elevation greater than 90°, and significantly more reached external rotation of 30°. In their costs analysis (including prosthesis and physical therapy) they report similar expenditure for ORIF and RTSA, and greater cost for hemiarthroplasty. This they attribute to the RTSA group for the most part not needing a structured supervised physical therapy programme.
Although there are advantages to RTSA, including improvement in the range of motion, which may result in better shoulder scores, one should remain cognisant of the expense involved, and the increased complication rate inherent to RTSA.
Avoiding complications/optimising results
The overall complication rate and long-term survival in RTSA is inferior to anatomic TSA with an intact rotator cuff.
Infection
The rate of infection with RTSA approximates that of total shoulder arthroplasty and is reported at between 0 and 3%.29 The rate seen in primary surgery is equivalent for various indications such as fractures, fracture sequelae and cuff tear arthropathy; however, the rate of infection is increased up to 8% when a reverse prosthesis is used for revision surgery.30
The most common infecting organisms post total shoulder arthroplasty are Gram-positive Staphylococcus aureus, Staphylococcus epidermidis and Proprionibacterium acnes (P acnes).31
Although initially considered a non-pathogenic bacterium and a contaminant of microbiological specimens, there is a growing body of evidence supporting the significance of P acnes post rotator cuff, instability and arthroplasty surgery of the shoulder.32-36
P acnes is a Gram-positive anaerobic bacillus which is found to colonise areas with abundant sebum-rich hair follicles. In a study comparing colonisation of various sites, Patel et al.37reported a greater prevalence around the shoulder compared to other sites such as the hip and knee. In this study, they demonstrate a similar prevalence of colonisation in the shoulder as S aureus. Men have a greater burden of colonisation than women due to increased hair and perspiration in the area, and are more frequently affected by infection due to P acnes.33, 37
Simple measures such as sealing off of the axilla with a sterile occlusive drape prior to skin incision, as well as the use of a suitable prophylactic antibiotic such as clindamycin 600-900 mg is advocated to reduce the risk of P acnes infection.
The rate of infection is increased up to 8% when a reverse prosthesis is used for revision surgery
If the humeral component is cemented, appropriate antibiotic-loaded cement should be used. In a retrospective review of over 500 RTSAs, Nowinski et al. demonstrated a 3% infection rate in the group who had no antibiotics in the cement, and no infections in the group who had either tobramycin, or vancomycin, or a combination of both added to the cement.29
There are also concerns regarding potential peri-prosthetic 'dead space' post RTSA, due to the morphology of the implanted components, and to the surgical procedure, which entails excision of part of the rotator cuff. Although there is no compelling evidence to support the use of post-operative low pressure closed drainage systems post shoulder arthroplasty, they are commonly used for short post-operative periods as post-operative haematoma formation may be complicated by positive cultures, and re-operation for haematoma is associated with poor clinical results.36 Boileau et al.38reported on frequent subacromial haematoma formation, and on one case in which the haematoma by means of a 'piston mechanism' became interposed between the components, and resulted in prosthetic instability. They recommend drain insertion as well as abduction bracing for three weeks to prevent this complication. Whether a drain is utilised or not, meticulous haemostasis is imperative.
Notching
Notching (Figure 3) refers to the loss of bone from the inferior pole of the scapular neck. The cause is a mechanical impingement of the medial aspect of the humeral component on this area when the arm is adducted.39 The mechanical impingement may cause polyethelene wear leading to focal osteolysis and notch progression.40
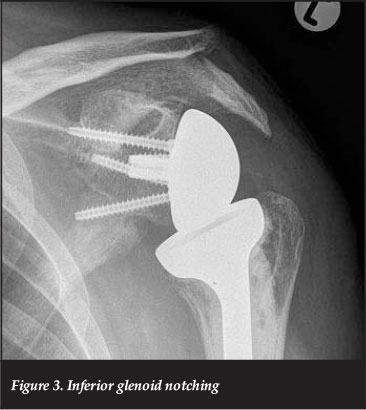
The incidence and severity of notching is seen to increase with advancing follow-up,39 and may progress to the extent that it causes glenoid component loosening and instability.
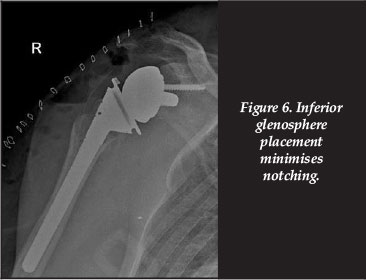
The anatomy of the scapula with relation to glenoid component positioning and the resultant scapular notching has been studied. In a computer-generated biomechanical model, de Wilde et al. evaluated the maximal adduction or notch angle (humeral component in conflict with the inferior scapular neck) for the following circumstances: a change in the humeral neck shaft inclination, change in the polyethylene cup depth, lateralisation of the centre of rotation, inferior glenoid inclination, increase in glenosphere radius, and increased inferior prosthetic overhang. Of these six scenarios, increasing the inferior overhang resulted in the most significant gain of notch angle.41
This is borne out in clinical studies where positioning of a glenoid component in the inferior aspect of the native glenoid, and increasing the inferior glenosphere overhang, reduces the incidence of notching.30
Other authors have investigated the effect of lateralisation of the centre of rotation on glenoid notching. Boileau et al.42 utilised a composite bone graft and component, while Valenti et al.43investigated a modified glenoid component that lateralised the centre of rotation without the need for bone grafting. While the latter showed neither notching nor glenoid loosening in 76 shoulders at a minimum of two-years' follow-up, there remain concerns regarding the resultant increase in torque and shear forces associated with lateralisation that may increase the rate of glenoid component loosening.
Instability
The incidence of component dislocation (Figure 4) post RTSA ranges from 0.01% to 28% for the various indications for surgery.43,44 Dislocation has been related to inadequate deltoid tensioning, tuberosity malunion (with resultant impingement) as well as tuberosity excision and inability to repair the tuberosities. It is more frequently seen in revision surgery. In a short- to mid-term analysis of their cases, Wellman et al. showed a four-fold increase in the dislocation rate for revision surgery compared to primary RTSA for cuff tear arthropathy.30
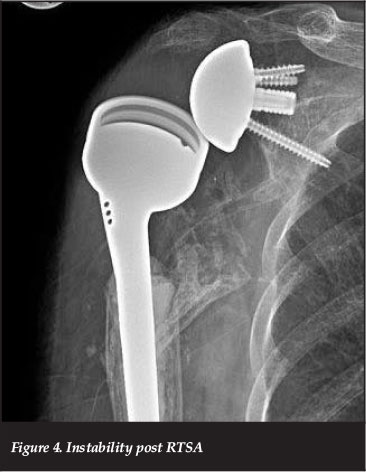
In a recent study on instability and infection rates, Trappey et al. report an instability rate of 12% in the patients with an irreparable subscapularis tendon, and a <1% instability when the subscapularis was repairable. Their highest rate of instability was seen in the fracture sequelae group with 28% instability. They attribute this to the difficulty in managing the subscapularis musculo-tendinous unit as the lesser tuberosity is often malunited, non-united or resorbed. The second most affected group in their series was those with a massive cuff tear with involvement of the subscapularis tendon.44 This association of subscapularis insufficiency and instability post RTSA has been confirmed by other authors.45
Acromial fractures
Acromial fractures (Figure 5) complicate between 1 and 7% of RTSA. They have been classified by Crosby and Hamilton46 into three groups depending on the anatomic location. Types 1 and 2 are lateral to the acromio-clavicular joint, and type 3 is medial. The type 3 fractures commonly involve the spine of the scapula and are related to screw position.
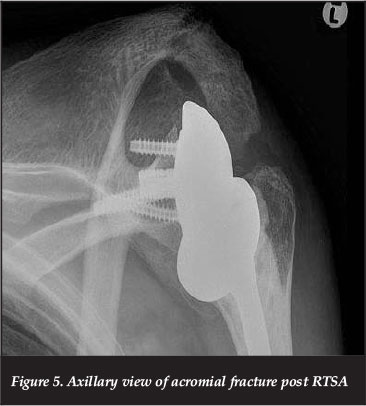
In a review of 52 acromial fractures post RTSA, Otto et al.47 identify osteoporosis as a significant risk factor for acromial fracture. While 14 of their 16 spine fractures (type 3 fractures) occurred from a screw tip, there was no difference in screw orientation and length between this and the other groups. They were unable to show a causal relationship between screw placement and scapular spine fractures. Their study highlights the usefulness of the radiographic features of a decreasing acromio-humeral distance, and increasing lateral acromial tilt on consecutive radiographs for the diagnosis of acromial fractures.
As proximal humeral fractures are fragility fractures, it is incumbent upon the treating physician that these patients be investigated for osteoporosis, and managed accordingly. This should not only reduce their risk of acromial fracture, but also that of other fragility fractures, with their associated morbidity and mortality.
Range of movement
Inherent in Gramont's RTSA design is infero-medialisation of the centre of rotation of the new shoulder. This has the advantage of recruitment of a larger (more medial) proportion of the deltoid muscle, as well as placing the deltoid under sufficient tension to enable optimal abduction and forward elevation. However, this has secondary effects on the anterior and posterior cuff musculature as the humerus is also medialised,38 effectively reducing the medio-lateral spinatii excursion and altering the force vectors of the muscles.
Although abduction and flexion are reasonably reproducible with RTSA, as seen in Tables I and II, the results for rotation are less consistent. This is due in part to the evolving surgical technique, and differing management of the greater and lesser tuberosities.
Gallinet et al. highlight the importance of anatomic tuberosity restoration in order to maximise rotation in RTSA when used for acute fractures.48 Comparing a group of patients whose tuberosities united in anatomic position with a group without repair or who had mal- or non-union of the tuberosities, they showed improved external rotation (49° vs 10°) as well as improved outcomes (CS 56 vs 50 and DASH 30 vs 40). Of significance, they allude to the fact that tuberosity mal- or non-union is not as detrimental in RTSA as it is in hemiarthroplasty. This improvement in rotation was confirmed by Valenti et al. who showed a difference of 8.6° vs 16.7° with greater tuberosity reinsertion. Interestingly, in their study, patients who did not have lesser tuberosity re-insertion also had statistically better external rotation (17.9° vs 9.1°) compared to those that did. Although they give no explanation for this, it is possibly due to the loss of the restriction of the subscapularis musculotendinous unit.16
Obtaining union of the greater and lesser tuberosities in optimal position may be challenging in the setting of high grade comminuted fractures. In a small series of seven patients, Levy et al.19describe a novel technique in which they fashion a horseshoe-shaped graft from the discarded humeral head. This is then placed around the humeral prosthesis, and the tuberosities fastened around this. They obtained tuberosity union in six of the seven cases. At a minimum of 12 months follow-up, the external rotation averaged 19° (0-30°) and manual muscle strength testing revealed 5/5 power for both internal and external rotation.
The significance of the remaining posterior cuff and specifically the teres minor in facilitating external rotation in RTSA for cuff-deficient shoulders has been emphasised in a number of publications.
In order to attain optimal results, one should pay special attention to:
1. Ensuring proper patient selection and indication for use of RTSA
2. Minimising the infection risk: use appropriate prophylactic antibiotics and antibiotic-loaded cement; maintain an aseptic operating field (with occlusion of the axilla)
3. Minimising the notching risk: ensure ideal placement of the glenoid base-plate (Figure 6), and thus inferior glenosphere overhang
4. Enhancing stability: use the correct humeral insert for the tension of the deltoid; avoid bony impingement; repair tuberosities
5. Optimising range of motion: anatomic repair of the greater and lesser tuberosities
6. Minimising the risk of acromial fractures: appropriate investigation and management of osteoporosis.
Summary
Complex proximal humeral fractures in elderly patients remain challenging to manage. Pre-fracture shoulder function is never entirely regained, no matter what treatment modality is utilised.
One should consider RTSA for complex proximal humeral fractures/fracture-dislocations in patients over the age of 70 years with pre-existing rotator cuff abnormalities, extensive osteoporosis, high likelihood of final tuberosity displacement, and the inability to participate in an extended rehabilitation programme.
The content of the article is the sole work of the authors. No benefits of any form have been or are to be received from a commercial party related directly or indirectly to the subject of the article.
References
1. Hirzinger C, Tauber M, Resch H. [Proximal humerus fracture: new aspects in epidemiology, fracture morphology, and diagnostics]. Unfallchirurg, 2011;114(12): 1051-58. [ Links ]
2. Pages-Castella A, et al. Burden of osteoporotic fractures in primary health care in Catalonia (Spain): a population-based study. BMC Musculoskelet Disord, 2012;13:79. [ Links ]
3. Roux A, et al. Epidemiology of proximal humerus fractures managed in a trauma center. Orthop Traumatol Surg Res, 2012;98(6):715-19. [ Links ]
4. Melton LJ, 3rd, et al. Long-term mortality following fractures at different skeletal sites: a population-based cohort study. Osteoporos Int, 2013;24(5):1689-96. [ Links ]
5. Edelson G, et al. Natural history of complex fractures of the proximal humerus using a three-dimensional classification system. J Shoulder Elbow Surg, 2008;17(3):399-409. [ Links ]
6. Fjalestad T, et al. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma, 2012;26(2):98-106. [ Links ]
7. Spross C, et al. The PHILOS plate for proximal humeral fractures-risk factors for complications at one year. J Trauma Acute Care Surg, 2012;72(3):783-92. [ Links ]
8. Sproul RC, et al. A systematic review of locking plate fixation of proximal humerus fractures. Injury, 2011;42(4):408-13. [ Links ]
9. Boileau P, et al. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg, 2002;11(5):401-12. [ Links ]
10. Liu J, et al. Outcomes, and factors affecting outcomes, following shoulder hemiarthroplasty for proximal humeral fracture repair. J Orthop Sci, 2011;16(5):565-72. [ Links ]
11. Boons HW, et al. Hemiarthroplasty for humeral four-part fractures for patients 65 years and older: a randomized controlled trial. Clin Orthop Relat Res, 2012;470(12):3483-91. [ Links ]
12. Olerud P, et al. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg, 2011;20(7):1025-33. [ Links ]
13. Tanner MW, Cofield RH. Prosthetic arthroplasty for fractures and fracture-dislocations of the proximal humerus. Clin Orthop Relat Res, 1983;179:116-28. [ Links ]
14. Anjum SN, Butt MS. Treatment of comminuted proximal humerus fractures with shoulder hemiarthroplasty in elderly patients. Acta Orthop Belg, 2005;71(4):388-95. [ Links ]
15. Grammont PTP, Laffay J, Deries X. Concept study and realization of a new total shoulder prosthesis. Rhumatologie, 1987;39: 407-18. [ Links ]
16. Valenti P, et al. Mid-term outcome of reverse shoulder prostheses in complex proximal humeral fractures. Acta Orthop Belg, 2012;78(4):442-49. [ Links ]
17. Lenarz C, et al. Is reverse shoulder arthroplasty appropriate for the treatment of fractures in the older patient? Early observations. Clin Orthop Relat Res, 2011;469(12):3324-31. [ Links ]
18. Reitman RD, Kerzhner, Reverse shoulder arthoplasty as treatment for comminuted proximal humeral fractures in elderly patients. Am J Orthop (Belle Mead NJ), 2011;40(9):458-61. [ Links ]
19. Levy JC, Badman B. Reverse shoulder prosthesis for acute four-part fracture: tuberosity fixation using a horseshoe graft. J Orthop Trauma, 2011;25(5):318-24. [ Links ]
20. Klein M, et al. Treatment of comminuted fractures of the proximal humerus in elderly patients with the Delta III reverse shoulder prosthesis. J Orthop Trauma, 2008;22(10):698-704. [ Links ]
21. Bufquin T, et al. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br, 2007;89(4):516-20. [ Links ]
22. Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br, 2010;92(4):535-39. [ Links ]
23. Sirveaux F, Navez G, Favard L. Reverse prosthesis for acute proximal humerus fracture, the multicentric study. in Reverse Shoulder Arthroplasty. Clinical Results, Complications, Revision, ed. F. Sirveaux. 2006, Montpellier: Sauramps Medical. [ Links ]
24. Gallinet D, et al. Three or four parts complex proximal humerus fractures: hemiarthroplasty versus reverse prosthesis: a comparative study of 40 cases. Orthop Traumatol Surg Res, 2009;95(1):48-55. [ Links ]
25. Young SW, et al. Comparison of functional outcomes of reverse shoulder arthroplasty versus hemiarthroplasty in the primary treatment of acute proximal humerus fracture. ANZ J Surg, 2010;80(11):789-93. [ Links ]
26. Garrigues GE, et al. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics, 2012;35(5):e703-8. [ Links ]
27. Boyle MJ, et al. Functional outcomes of reverse shoulder arthro-plasty compared with hemiarthroplasty for acute proximal humeral fractures. J Shoulder Elbow Surg, 2013;22(1):32-37. [ Links ]
28. Chalmers PN, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg, 2014;23(2):197-204. [ Links ]
29. Nowinski RJ, et al. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg, 2012;21(3):324-28. [ Links ]
30. Wellmann M, et al. Short and midterm results of reverse shoulder arthroplasty according to the preoperative etiology. Arch Orthop Trauma Surg, 2013;133(4):463-71. [ Links ]
31. Sperling JW, et al. Infection after shoulder arthroplasty. Clin Orthop Relat Res, 2001;382:206-16. [ Links ]
32. Millett PJ, et al. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res, 2011;469(10):2824-30. [ Links ]
33. Levy PY, et al. Propionibacterium acnes postoperative shoulder arthritis: an emerging clinical entity. Clin Infect Dis, 2008;46(12):1884-86. [ Links ]
34. Athwal GS, et al. Deep infection after rotator cuff repair. J Shoulder Elbow Surg, 2007;16(3):306-11. [ Links ]
35. Sperling JW, et al. Infection after shoulder instability surgery. Clin Orthop Relat Res, 2003;414):61-64. [ Links ]
36. Cheung EV, Sperling JW, Cofield RH. Infection associated with hematoma formation after shoulder arthroplasty. Clin Orthop Relat Res, 2008;466(6):1363-67. [ Links ]
37. Patel A, et al. Propionibacterium acnes colonization of the human shoulder. J Shoulder Elbow Surg, 2009;18(6):897-902. [ Links ]
38. Boileau P, et al. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg, 2005;14(1 Suppl S):147S-161S. [ Links ]
39. Valenti P. Delta 3 reversed prosthesis for arthritis with massive rotator cuff tear: long term results (> 5 years). 2001, Saurups Medical Montpellier: 2000 Shoulder Prosthesis... two to ten year follow-up (eds G Walch , P Boileau , Mole D). p. 253-59. [ Links ]
40. Vanhove B, Beugnies A. Grammont's reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg, 2004;70(3):219-25. [ Links ]
41. de Wilde LF, et al. Prosthetic overhang is the most effective way to prevent scapular conflict in a reverse total shoulder prosthesis. Acta Orthop, 2010;81(6):719-26. [ Links ]
42. Boileau P, et al. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res, 2011;469(9):2558-67. [ Links ]
43. Valenti P, et al. Do less medialized reverse shoulder prostheses increase motion and reduce notching? Clin Orthop Relat Res, 2011;469(9):2550-57. [ Links ]
44. Trappey GJt, O'Connor DP, Edwards TB. What are the instability and infection rates after reverse shoulder arthroplasty? Clin Orthop Relat Res, 2011;469(9):2505-11. [ Links ]
45. Edwards TB, et al. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg, 2009;18(6):892-96. [ Links ]
46. Crosby LA, Hamilton A, Twiss T. Scapula fractures after reverse total shoulder arthroplasty: classification and treatment. Clin Orthop Relat Res, 2011;469(9):2544-49. [ Links ]
47. Otto RJ, et al. Scapular fractures after reverse shoulder arthro-plasty: evaluation of risk factors and the reliability of a proposed classification. J Shoulder Elbow Surg, 2013. [ Links ]
48. Gallinet D, et al. Improvement in shoulder rotation in complex shoulder fractures treated by reverse shoulder arthroplasty. J Shoulder Elbow Surg, 2013;22(1):38-44. [ Links ]
 Correspondence:
Correspondence:
Dr P Ryan
Email: paullisa.ryan@gmail.com














