Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SA Orthopaedic Journal
versión On-line ISSN 2309-8309
versión impresa ISSN 1681-150X
SA orthop. j. vol.13 no.4 Centurion dic. 2014
Hepatitis C virus and the risk to the healthcare worker
P GreylingI; Prof TLB le RouxII; L BothaIII
IMBChB(Pret), MMed(Orth)(Pret), FCS(Orth)SA; Consultant, Orthopaedic Surgery, Steve Biko Academic Hospital
IIMBChB(Pret), MMed(Orth)(Pret), FCS(Orth)SA; Head of Department Orthopaedic Surgery, 1 Military Hospital, Department of Orthopaedic Surgery, Steve Biko Academic Hospital, University of Pretoria, Faculty of Health Sciences
IIIMBChB(Pret); Orthopaedic Registrar, 1 Military Hospital
ABSTRACT
The risk of hepatitis C virus (HCV) transmission is six times higher per needle-stick exposure than is the risk of HIV infection (1.8% vs 0.3%). The prevalence of HCV in South Africa is not known but estimated to be between 0.1 and 0.7%. Genotype 5 is predominantly found in South Africa. Currently there is no protocol in place for HCV exposure. The treatment cost of HCV is expensive. Currently none of the insurance companies covers post-HCV exposure.
Key words: hepatitis C virus, transmission risk, post-exposure protocol
Introduction
South African surgeons need to familiarise themselves with the risk of acquiring hepatitis C virus (HCV) via sharps injury (SI). Any of the 60 blood-borne pathogens (BBP) may be transferred by SI, the three foremost being the human immunodeficiency virus (HIV), hepatitis B virus (HBV) and HCV.1 In South Africa (SA) there is little prevalence of HBV (5-7%) and the greatest risk is from HIV (17.9%) for which there are documented protocols and insurance cover.2,3
Little has been written of HCV risk to SA surgeons; there is no protocol in place for HCV exposure, little guidance for surgeons, and insurance companies do not cover HCV exposure claims.
A Canadian surgeon recently acquired HCV from an SI and was banned from practice for 6 months - testimony of the importance of HCV as a disease risk to surgeons.4
This paper outlines the prevalence of HCV in SA, the incubation and outcomes of HCV infection, the tests and medications available, and the protective strategies available to minimise HCV exposure.
Epidemiology
Hepatitis C virus was discovered in 1989; it is a small 50 nm, single-stranded RNA virus that belongs to the Flaviviridae family.5,6 There are six major HCV genotypes, with five more having recently been added (7-11), and more than 50 subtypes have been described. Genotype 5 is predominantly found in SA (40% of all HCV genotypes).7,8 The virus is able to mutate which results in changes that allow the virus to replicate and escape immune surveillance. The result is that HCV infecting humans are remarkably heterogeneous, with only a 70% similarity among all known isolates.9
Prevalence and risk of HCV transmission
The prevalence of HCV in SA is not known but estimated to be between 0.1 and 0.7% (WHO up to 10% in some areas).27 Three per cent of the world's population is estimated to be infected with HCV (130-170 million people).2,10 The World Health Organisation (WHO) has calculated that unsafe medical devices account for 2.3 million new HCV infections per year and 200 000 premature HCV-related deaths. The risk of HCV transmission is six times higher per needle-stick exposure than is the risk of HIV infection (1.8% vs 0.3%).5,11
Burden of disease
HCV is responsible for more than 350 000 deaths annually. Unsafe injections in healthcare settings result in 2 million new HCV infections yearly. Up to 30% of people infected with HIV are also infected with HCV. Unless the disease is contained, the death rate from HCV will rise to a level greater than that of AIDS.10,15
The cost of treating HCV is enormous. A 24-week treatment course is estimated at $10 200, but if either telaprevir or boceprevir is added, costs can go up to $70 000.4,17
Hepatitis C specific diagnostic tests
Diagnostic tests can be divided into two general categories:5,7,9,18
1. Serological assays:
Detect antibodies to HCV and are used as screening tests for prior exposure to HCV.2. Molecular assays:
Detect, quantify, and or characterise HCV RNA genomes within an infected patient.Categorised as:
- Qualitative
- Quantitative (quantitative PCR and branched DNA assay)
- Genotyping
Molecular test is necessary to confirm the diagnosis.
Treatment of acute hepatitis C
Early treatment of acute HCV improves rates of viral clearance. Treatment should be started within 3 months after testing positive for HCV.7
The current suggested treatment regimen consists of a combination of chemotherapy drugs for all types of hepatitis C:1,7,11,19,20,21
- Interferon alpha (pegingerferon alpha-2a) - 180 pg per week subcutaneously
- Ribavirin - 800 mg per day
- Boceprevir and telaprevir are recommended by the National Institute for Health and Clinical Excellence (NICE) as an option for the treatment of people with genotype 1.
Interferon alpha and ribavirin are on the South African national essential medicines list for the treatment of HCV; however, there are no guidelines available suggesting who is eligible for treatment.2
Duration of treatment depends on the genotype. In patients with genotype 2 or 3, a 24-week course is effective, while a 48-week course is suggested for genotype 1. Genotype 5 responds similarly to type 2 and 3, and a 24-week course is recommended.5,7,16,21
If no response (drop in viral load) is noted within 24 weeks of combination therapy, treatment should be stopped as no benefit has been proven with continued treatment.7,16
Follow-up monitoring after completion of treatment should include serum aminotransferase levels and testing for HCV RNA at 6 months.
Interferon alpha and ribavirin are on the South African national essential medicines list for the treatment of HCV; however, there are no guidelines available suggesting who is eligible for treatment
Prevention of hepatitis C
Hepatitis C vaccination
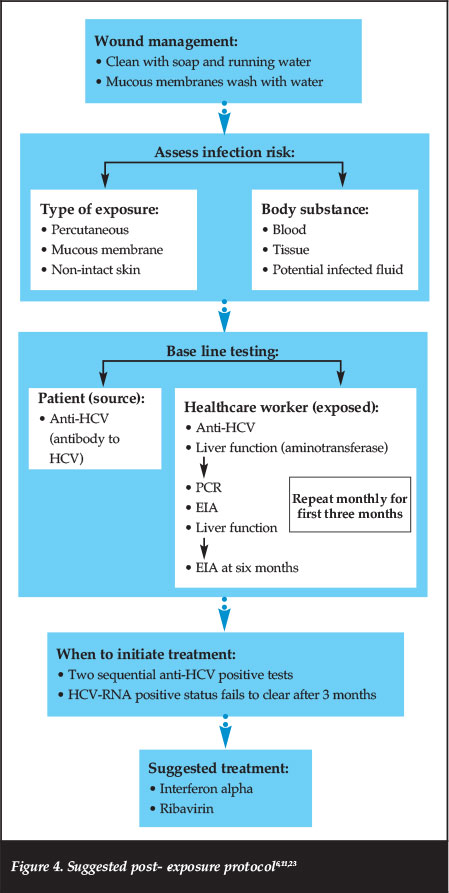
There is no reliable vaccine due to the unique characteristics of HCV, which include high replication rate and multiple mutation possibilities. Neutralising antibodies emerge too late to prevent chronic infection.16,22 Refer to Figure 4 for the suggested post-exposure protocol.
Should HCV positive surgeons refrain from operating? (surgeon risk to patient)
Current guidelines allow surgeons who are antibody-positive for HCV to continue performing procedures. If however it has been shown that they have transmitted HCV to a patient they are not allowed to continue operating.6,11,24
Currently in South Africa there are no guidelines advising infected surgeons on the reporting, assessment, and management of any incidents in which patients appear to have been exposed to a healthcare worker's blood.23
There is controversy in identifying infected healthcare workers. At present there is no legislation on this subject.25
Risk to surgeon
Healthcare workers sustain 0.5-4.7 sharps-related injuries per year. The risk of virus transmission after percutaneous exposure is 1.8% (six times higher than the risk for HIV).11,23
Perception of surgeons regarding hepatitis C
In a questionnaire survey by the Royal College of Surgeons of England it was reported that 67% of orthopaedic surgeons do not routinely report sharps injuries or eye contamination.
The reason given is the complex process for reporting an injury on duty.3
Medical insurance and post-exposure hepatitis C
The treatment cost of hepatitis C is expensive. Currently none of the insurance companies covers post-HCV exposure for healthcare workers.
Should HCV not be receiving the same insurance coverage as HIV?
Healthcare workers sustain 0.5-4.7 sharps-related injuries per year. The risk of virus transmission after percutaneous exposure is 1.8% (six times higher than the risk for HIV)
Conclusion
There is no cure for hepatitis C at the present, and no postexposure prophylaxis is recommended. Immunoglobulins are not effective, interferon-alpha does not prevent transmission, antiviral agents have not been adequately assessed and there is no vaccine.22,25
Although surgeons fear transmission of HIV, it is hepatitis C which poses the greater risk.26
We would like to acknowledge the positive information and advice from Terry Grimmond from Hamilton New Zealand. The content of the article is the sole work of the authors. No benefits of any form have been or are to be received from a commercial party related directly or indirectly to the subject of the article
References
1. Waljee JF, Malay S, Chung KC. Sharps injuries: The risks and relevance to plastic surgeons. PRSJ 2013;131:784-91. [ Links ]
2. Lazarus JV, Safreed-Harmon K, Sperle I. Global policy report on the prevention and control of viral hepatitis in WHO member states. WHO report 21 May 2012:1-220. [ Links ]
3. Global aids response progress report 2012. [ Links ]
4. Jeanes D. The Butterfly Effect how a needle-stick grew into a big deal. COA Bulletin ACO. Summer 2012:11-13. [ Links ]
5. Maheshwari A, Ray S, Thuluvath P. Acute Hepatitis C. Lancet 2008;372:321-32. [ Links ]
6. Stevens AB, Coyle PV. Hepatitis C virus: an important occupational hazard. Occup. Med. 2000;50:377-82. [ Links ]
7. Botha JF, Kassianides C, Schneider HR, Song E, Spearman W, van der Merwe SW. South African Hepatitis C Management Guidelines 2012. The South African Gastroenterology Review 2010:20-25. [ Links ]
8. Abuelhassan W. Hepatitis C virus infection in 2012 and beyond. South Afr J Epidemiol Infect 2012;27(3):93-97. [ Links ]
9. Bonkovsky HL, Metha S. Hepatitis C: A review and update. J Am Acad Dermatol 2001:159-79. [ Links ]
10. Averhoff FM, Glass N, Holtzman D. Global Burden of Hepatitis G: Considerations for Health Providers in the United States. CID2022 ;55(Suppl 1):S10-S15. [ Links ]
11. Asthana S, Kneteman N. Operating on a patient with hepatitis C. Can J Surg 2009;52:337-42. [ Links ]
12. Ryoo SM, Kim WY, Kim W, Lim KS, Lee CC, Woo JH. Transmission of hepatitis C virus by occupational percutaneous injuries in South Korea. JFMA 2012;111:113-17. [ Links ]
13. Adams D. Needlestick and sharps injuries: practice update. Nursing Standard 2012;26:49-57. [ Links ]
14. Madhava V, Burgess C, Drucker E. Epidemiology of chronic hepatitis C virus infection in sub-Saharan Africa. The Lancet Infectious Diseases 2002;2:293-302. [ Links ]
15. Rhoads J. Natural History and Epidemiology of Hepatitis C. JANAC 2003;14:18S-25S. [ Links ]
16. Liang, TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Annal of Internal Medicine 200;132:296-305. [ Links ]
17. Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med 2013;368:1859-61. [ Links ]
18. Gretch DR. Diagnostic tests for hepatitis C. Hepatology 1997;26:43S-47S. [ Links ]
19. McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling M-H, Cort S, Albrechgt JK. Interferon Alfa-2b alone or in combination with Ribavirin as initial treatment for chronic Hepatitis C. N Engl J Med 1998;339:1485-92. [ Links ]
20. NIH. National Institutes of Health Consensus Development Conference Panel Statement: Management of Hepatitis C. Hepatology 1997;26:2S-10S. [ Links ]
21. Qureshi H, Agha F. Should HBV or HCV positive surgeons refrain from operating? J Pak Med Assoc 2011;61:843-45. [ Links ]
22. Jawaid A, Khuwaja AK. Treatment and vaccination for Hepatitis C: Present and future. J Ayub Med Coll Abbottabad 2008;20:129-33. [ Links ]
23. Ramsay ME. Guidance on the investigation and management of occupational exposure to hepatitis C. Communicable Disease and Public Health 1999;2:258-62. [ Links ]
24. Crockcroft A. Surgeons who test positive for hepatitis C should not be transferred to low risk duties. Rev Med Virol 2000;10:79-82. [ Links ]
25. Viral Hepatitis Prevention Board. Viral hepatitis. VHPB 2005;14:1-16. [ Links ]
26. Wallis GC, KIM WY, Chaudhary BR, Henderson JJ. Perceptions of orthopaedic surgeons regarding hepatitis C viral transmission: a questionnaire survey. Ann R Coll Surg Engl 2007;89:276-80. [ Links ]
 Correspondence:
Correspondence:
Dr P Greyling
Department of Orthopaedic Surgery
Steve Biko Academic Hospital
Pretoria South Africa
Tel: 012 354 2851
Email: pgrey@live.com
This article is also available online on the SAOA website (www.saoa.org.za) and the SciELO website (www.scielo.org.za). Follow the directions on the Contents page of this journal to access it.














