Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SA Orthopaedic Journal
versão On-line ISSN 2309-8309
versão impressa ISSN 1681-150X
SA orthop. j. vol.13 no.4 Centurion Dez. 2014
Retrospective analysis of adverse reactions to metal-on-metal lumbar disc arthroplasties in 378 consecutive patients
S BergI; N JansenII
IMD, PhD; Stockholm Spine Center, Löwenströmska Hospital, Stockholm, Sweden
IIM En; North-West University (Potchefstroom campus), South Africa
ABSTRACT
BACKGROUND: Spinal disc arthroplasty implants are primarily manufactured from metal/polymer materials. Biological reaction to wear debris ultimately requires clinical studies for assessment. Research into biological reaction of metal-on-polyethylene and metal-on-metal wear debris of knee and hip arthroplasties is well progressed as opposed to similar research on spinal arthroplasties.
MATERIALS AND METHOD: The Swedish Spine Register provides a resource for the evaluation of adverse events and clinical outcome to lumbar metal-on-metal total disc replacements. The resource will be used for a retrospective analysis of the cases in this study. The material reviewed consists of a total of 378 Swedish patients treated between October 2003 and May 2009 (181 male, 197 female); average age was 39.2 years. By means of a questionnaire, 94% of the patients were followed up after two years and 88% after five years
RESULTS: No reported cases were found of suspected or confirmed metal hypersensitivity or pseudotumors. This may be due to symptom-producing pseudotumors being extremely rare and the difficulty to form questions which would be able to indicate the presence of the adverse outcome
CONCLUSION: Based on the results from this study, it can be concluded that the results do not exclude the possibility that patients might have non-symptomatic pseudotumors, but being non-symptomatic, the authors doubt the importance and relevance of further investigating those isolated cases
Key words: spinal arthroplasty, metal-on-metal, pseudotumors, hypersensitivity, metallosis, SweSpine
Introduction
Neck or back pain associated with degenerative changes of the functional spinal unit is one of the leading causes of disability among adults. Chronic low back pain (CLBP) with a prevalence in excess of a one-year period is as high as 73%.1 Disc degeneration is a frequent cause of CLBP, and conservative treatment including physiotherapy and exercise is the first step in attempting to reduce pain and improve function. Should conservative treatment fail, surgical intervention, which includes fusion or disc replacement procedure, is considered as a last resort.2
The gold standard in surgical treatment of CLBP has been fusion. In the past decade, spinal disc arthroplasty and dynamic stabilisation devices have received growing acceptance as treatment for back and neck pain.3,4 Finite element studies have confirmed that unnatural disc kinematics result in higher stress imparted to adjacent discs.5,6 Unnatural kinematics may be caused by sclerotic, degenerated or fused intervertebral discs. Experience and knowledge gained from treatment of other joints with arthroplasty have led to the development of artificial discs (disc prostheses). The rationale for selecting total disc replacement (TDR) over fusion is a desire to preserve motion between vertebral bodies that might reduce adjacent segment disease.6,7
In all joint replacement surgery there are four main questions to be answered: What is 1) the correct indication for the procedure, 2) the best surgical technique to be used, 3) the most favourable design of the implant and 4) the most appropriate materials to use? This study focuses on the fourth questions, namely, the specific material frequently used.
Hip and knee replacements have been performed since the 1970s, providing knowledge that particulate wear debris may be associated with post-operative complications such as peri-implant osteolysis or pseudotumors characterised as an enlargement of benign tissue.8
Selection of the wear couple material is for the most part a compromise between the wear rate, robustness and toxicity. There seems to be no single hip bearing material combination which reduces all clinical complications.9
In most cases, hip bearing design includes metal-on-polymer (MoP), metal-on-metal (MoM), or ceramic-on-ceramic (CoC) wear couples. Soft bearing wear, such as polyethylene, in general is dominated by adhesive wear, while hard-on-hard bearings such as MoM or CoC are dominated by abrasive and surface fatigue wear.10
The use of MoM as a hip bearing has become popular in the last years due to the possibility to reduce the risk of joint luxation by larger femoral heads, but also perceived advantages of being more robust than MoP or CoC, as well as having a lower volumetric wear than MoP.11-13 Osteolysis associated with MoM bearing implants is seldom reported, and is considered as the prime motivation for a return to MoM wear couples.14,15
Recently a particular MoM hip arthroplasty has been found to have poor short-term results. Hexter et al. suggest that a substantial portion of the blood ion levels are as a result of corrosion at the taper interface between the hip stem and ball and not the intended wear couple.16 Bernthal et al. suggest that the design flaw lies not with the specific implant but rather with the ultra large monobloc cobalt-chromium-molybdenum (CCM) acetabular shells.17
Over the medium to long term, the mechanical advantages of MoM over MoP are potentially offset by a higher frequency of foreign-body tissue reaction due to smaller particle size of wear debris, as well as bioactive cobalt and chrome ion release.15,18
The most common cause of total hip arthroplasty (THA) failure is loosening of the acetabular cup or femoral part due to osteolysis.19-22 Case studies indicate that failure due to metallic debris infiltration have also been reported.23-25 It is reported that reaction to polyethylene particles is that a fibrotic granulation tissue typically develops.26
If the degree of metallosis (such as adjacent tissue staining in black or grey) is significant and probably a certain size of the particles is produced, a 'foreign body reaction' may take place because submicron and micron-sized wear particles are encapsulated by the host body.27 Fibroblast and inflammatory cells within the tissue are activated, and as a result foreign body granulation tissue develops. The tissue tends to be fibrotic, but may undergo additional changes such as necrosis, and heterotopic ossification.
Local inflammation is mostly dependent on three factors; particle load, shape and chemical reactivity. In general, a higher inflammatory response will be produced with a higher concentration of debris per tissue volume (particle load), elongated fibres and more chemically reactive compo-sition.20 Particle load is a function of the accuracy of device placement, as misalignment may lead to fouling between components near articulation extremes. The fixed centre of rotation spinal disc replacements is likely to increase the volume of wear debris if not accurately placed.28
The effects of pseudotumors have been found to be locally destructive, requiring revision surgery in a high proportion of patients.29 The extent of the encapsulation may be significant enough to appear on computed tomography scans.27
Potential risks associated with MoM hip implants are a biological, foreign body reaction. Engh et al. suggest that the process starts as an inflammatory response progressing to a necrotic tissue involving soft and/or hard tissue.30 The most appropriate blood fraction is controversial when studying effects of MoM implants.29 The acceptable limit of blood cobalt levels is therefore yet to be established.31,32
The potential for the release of metal ions was examined in a subset of Kineflex MoM Cervical Disc subjects from a US IDE clinical study, which included sample collection at all investigational sites participating in the study that were qualified and had the facilities to do the collection. These sites collected samples from 32 subjects for analysis. Metal ion analysis was evaluated by a core laboratory (Rush University).
Osteolysis associated with MoM bearing implants is seldom reported, and is considered as the prime motivation for a return to MoM wear couples
Mean serum cobalt and chromium serum levels ranged from 0.37 to 1.24 μg/L and 0.27 to 1.11 μg/L; respectively, at follow-up times ranging from 6 weeks to 72 months after implantation. As shown in Figure 1, data through extended follow-up with the metal ion cohort showed metal ion levels were stable or decreasing over time after 12 months post-operative through the 72-month visit.
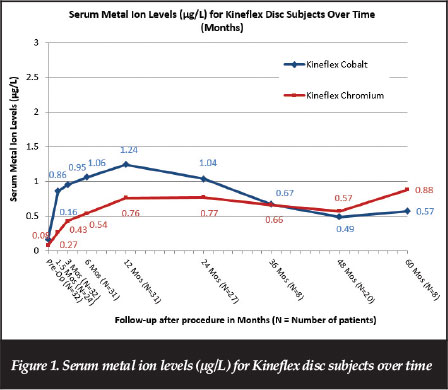
Metal ion data through extended follow-up with the metal ion cohort showed metal ion levels levelling off and decreasing over time after approximately 12 months postsurgery. Maximum serum metal ion levels associated with Kineflex implants were substantially lower than those found with other metal-on-metal implants, and substantially lower than threshold values that have been proposed as indicative of metallosis in literature for orthopaedic implants. Longterm follow-up data showed the metal ion levels associated with the Kineflex disc to be approximately five to 26 times lower than the 7 μg/L threshold of concern set by the British MHRA Medical Alert for MoM hip replacements issued on 22 April 2010.33 Further, MoM hips and MoM lumbar discs are substantially different in implant design, joint size and environment (synovial vs non-synovial), loads and range of motion, and in vitro and in vivo wear.
Hypersensitivity to metal ions may either occur within minutes (initiated by anti-body or formation of antibody-antigen complexes) or days (by a cell-mediated response).34 35 On the contrary, metallosis is a well-known phenomenon, being the infiltration of metal, predominantly oxidised, in the tissues in contact with the implant. The only known effect of metallosis is that the periprosthetic tissue is stained black, with microscopic necrosis.27
Hypersensitivity to metal ions may either occur within minutes or days
Materials commonly used for the manufacture of total disc replacements
Spinal disc arthroplasty devices mostly feature MoM and MoP bearings in either fixed or mobile centre of rotation configurations. The most popular metal and polymer used by TDR device wear surfaces are cobalt-chromium-molyb-denum (CCM), and ultra-high molecular weight polyethylene (UHMWPE). Polyether ether ketone (PEEK) is a polymer which has a high degree of biocompatibility, and is under investigation as a MoP wear couple.36
Materials presently used for lumbar and cervical spinal disc replacement devices, which are US Food and Drug Administration (FDA) approved, are listed in Tables I and II respectively. Although comprehensive laboratory tests as well as animal studies are performed prior to clinical trials, novel uses of biomaterials lead to difficulty in predicting in vivo performance.26,37
Retrieval analyses of motion preserving spinal devices have to a large extent validated the high mechanical strength and low wear advantages of MoM over MoP. UHMWPE wear surfaces have displayed the potential to crack or plastically deform in both hip and spinal applications.26,27,46 Ooij et al.2 report on high wear rates of the polyethylene component of the SB Charité III lumbar disc replacement, suggesting that the device would have a lifespan of less than 40 years.2
The nature of wear particles obtained by in vitro wear simulation tests of hip, knee, and spinal wear are of a similar morphology.20 Over the short term, the increase of ion levels in blood after a single level MoM lumbar disc replacement (Maverick™) was found to be of the same order of magnitude as that of a well-functioning MoM hip arthro-plasty.47 Subsequent to this publication it has been reported that a specific MoM hip arthroplasty has been shown to exhibit much higher wear or metal ion release, but in this instance, it is believed to be related to the design of this specific implant.48
Hallab reviewed published data on concentrations of metal in body fluids, reporting that Co ion levels in blood serum increased from < 0.2-0.6 to 1.9-4.8 parts per billion (ppb) for TDR and 0.6-7.9 ppb for MoM total hip arthroplasties.20 Cr-ion levels were found to be higher in THA than TDR, 9.1 and 2.4 ppb respectively. It is therefore noted that in lumbar TDR, Co and Cr ion levels are lower than after THA.20,47
Complications such as peri-implant osteolysis and metal hypersensitivity are rare and make for a challenging diagnosis.30,49 Biological reaction to wear debris depends on volume as well as morphology of wear debris generated.50
Recently a few cases of suspected 'pseudotumors' after cervical or lumbar TDR were reported.51,52 The purposes of this study were to analyse in what patient material MoM TDRs were used and to report on the clinical symptomatic incidence of pseudotumors or metal hypersensitivity brought on by MoM wear couples in lumbar spinal disc arthroplasties.
Material and methods
This is a retrospective study on consecutive patients who had received MoM TDR between 8 October 2003 and 13 May 2009 due to chronic low back pain, where pain and dysfunction were not reduced after prolonged conservative treatment over at least one year. All surgeries were carried out at Stockholm Spine Center in Sweden; 88 per cent of the procedures were performed by one surgeon (SB).
Follow-up on patients was performed two and five years after surgery with The Swedish Spine Register, but for this study, medical record data were also examined together with a follow-up visit with clinical examination when more than two years had passed since treatment. The Swedish Spine Register (SweSpine) is a non-profit organisation owned and administered by the Swedish Society of Spinal Surgeons. SweSpine was founded in 1993, and is currently a national registry.53
One of the aims of the registry is to provide outcome-based feedback from patients after surgical intervention, and thereby provide continuous improvement of spinal treatments. Adverse medical conditions are recorded within the register, which therefore serves as a rigorous report of patient feedback for the recorded period.
The SweSpine database was used to select only lumbar arthroplasties featuring MoM wear couples. The prostheses utilised were Maverick™ (Medtronic, Memphis, TE, USA), Kineflex™ (Southern Medical, Centurion, SA) and FlexiCore™ (Stryker, Kalamazoo, MI, USA) (Figures 2-4).



All surgeries were carried out at Stockholm Spine Center in Sweden; 88 per cent by one surgeon
To evaluate the outcome and quality of spinal treatment, patients answer questionnaires at one, two, five and ten year intervals after treatment. The questionnaires are sent to the patients with an attached pre-paid return envelope. All returned questionnaires are registered at one place by a single secretary. The questionnaires include present work status and medication, but also re-operations, EuroQol (EQ-5D), visual analogue scale (VAS) for back and leg pain, SF-36, Global Assessment (GA) of back pain and the original Oswestry Disability Index (ODI). At time of surgery, the surgeon records diagnosis, type of surgery, treated segments, implants, dates of in-hospital stay and immediate complications.
Re-operations are recorded in the register, including patients treated with MoM disc prostheses. Since more than 95% of TDR surgery in Sweden is performed in the clinic from which these results are obtained, a re-operation would most likely also be performed there. Thirty-seven per cent of the patients in this study were examined by MRI after more than two years had passed since surgery. The reason for the examination was most frequently because the patients were part of other studies, but in some cases they were performed due to recurrent or persistent LBP. These MRI scans were investigated for the occurrence of pseudotumors.
Results
The analysis of the treated patients showed that a total of 378 Swedish patients (181 male, 197 female), with average age and weight of 39.2 years and 77.1 kg respectively were treated. A total of 111 patients had previous spinal operations; 297 were active in sports, 45 were smokers. All patients were treated with a total of 642 MoM total lumbar disc replacements at one, two or three segments. Follow-ups were performed with 94 per cent of the patients after two years (356 / 378). In April 2013 317 of the patients had answered their five-year questionnaires (the 88% that had passed five years). The average time of follow-up was just over six years (4-10). TDRs were performed utilising Maverick™ in 227 cases, Kineflex™ in 140 cases and FlexiCore™ in 11 cases.
No reported cases of pseudotumors were found in the material, neither presented with clinical symptoms in questionnaires or by words at clinical visit, nor identified on MRI in the individual cases that had that examination late post-operatively.
No case of suspected or verified metal hypersensitivity was found.
Three patients were re-operated due to misplaced disc prostheses, all within a week after index surgery. No metallosis or inflammation was observed at this early stage after initial surgical treatment. In one patient who underwent a late decompression and fusion at a segment that three years previously had been treated with a MoM prosthesis, a slight metallosis was observed at one side posteriorly in the annulus fibrosus, but without any local reaction.
Discussion
Information from other joint replacements (THA and TDR) has been discouraging regarding the use of UHMWPE. This is due to the concern of high wear rates, early plastic deformation or component failure, and local reactions such as osteolysis that may be caused by wear-particles. When TDR-implants are compared with repeated movements under loaded conditions, the volumetric wear of MoM is half of that of MoP per million cycles.56 These factors have induced the development of TDR-implants that do not use UHMWPE. At this stage of disc development, other materials now used for bearing couples in lumbar TDR are CCM and PEEK.
Metallosis is the black or grey stain colouring of soft tissue that is often seen when implants are removed. This is seen even after fusion, when mobility-induced wear is not present or limited. When discussing adverse events resulting from the implantation of metal components into humans, confusion prevails. In the authors' opinion this is because metallosis is not considered a complication, as it is not linked to any symptoms, but rather as a normal occurrence due to surface corrosion or deposition of wear debris.
It is possible that there is an occurrence of true hypersensitivity to any of the metals in the implant. This is however almost unreported and it might occur whether or not the implant also consists of polyethylene. The frequency of hypersensitivity reactions is not well known.15 Due to the rarity of metal hypersensitivity, as well as low accuracy of allergy tests, it is debatable whether screening should be performed prior to surgery.34
The formation of foreign body granulomas that can develop into pseudotumors can be considered as a physiological phenomenon. This is similar to what is observed if, for example, a needle tip is left in human tissue and there is no allergic reaction.
In recent reports there is a high frequency of foreign body reactions leading to the formation of pseudotumors after MoM total hip replacements. This is concerning as a large portion of TDRs performed today in Sweden are MoM.
The proposed mechanism for the development of pseudotumors is that metal wear particles start a 'foreign-body reaction' in the host that encapsulates the wear particles. This encapsulation is what forms the pseudotumor. It is also reasonable to believe that the total size of the encapsulating tissue is dependent on the amount of wear particles.
The total weight-strain on a hip prosthesis is assumed to be within the same range as that on a TDR implant. The loading pattern and total joint mobility is, however, far larger in a hip than in an artificial disc. The authors hypothesise that wear debris in a MoM artificial joint is, besides design and placement differences, dependent on load and actual 'travelled distance' between joint surfaces per time unit. If this hypothesis is relevant, fewer wear particles would be expected in a TDR than in a THA and therefore there is a lower likelihood of adverse reactions on wear products emerging.
In the case series of Swedish patients, no reports were seen on 'symptoms producing' pseudotumors. This could be due to the difficulty of forming questions that are directed to finding patients who had developed pseudotumors. Therefore the study has clear limitations. Despite this, the result states that none of the patients had developed pseudotumors that caused neurological symptoms or local pain. The material is from Scandinavia's largest spine clinic, where until now more than 95% of lumbar TDRs in Sweden were performed; therefore, these results can be considered to be valid.
The Swedish Spine Register (SweSpine) covers not only index surgery but also re-operations. Furthermore, the patients treated at Stockholm Spine Center are all referred from general practitioners or other orthopaedic clinics, where the receiving surgeons are expected to deal with any complications or need of re-operations that might occur. As a consequence of this study there has been a continuous and highly frequent use of MoM prostheses after April 2009 at the clinic, still without any discovered cases of 'pseudotumors'.
Conclusion
Based on the results from this study, it can be concluded that symptom-producing pseudotumors after TDR with MoM implants are extremely rare. The results do not exclude the possibility that patients might have non-symptomatic pseudotumors, but being non-symptomatic, the authors doubt the importance and relevance of further investigating those isolated cases.
References
1. Rumboldt Z. Degenerative disorders of the spine. Semin. Roentg. 2006; 41:327-62. [ Links ]
2. Ooij A van, Oner FC, Verbout AJ. Complications of artificial disc replacement: a report of 27 patients with the SB Charité disc. J Spinal Disord Tech. 2003; 16:369-83. [ Links ]
3. Billi F, Benya P, Ebramzadeh E, et al. Metal wear particles: What we know, what we do not know, and why. SAS Journal 2009; 3:133-42. [ Links ]
4. Schmoelz W, Huber JF, Nydegger T, et al. Dynamic stabilization of the lumbar spine and its effects on adjacent segments: an in vitro experiment. J Spinal Disord Tech. 2003; 16:418-23. [ Links ]
5. Ruberté LM, Natarajan RN, Andersson GB. Influence of single-level lumbar degenerative disc disease on the behavior of the adjacent segments - a finite element model study. J Biomech. 2009; 42:341-48. [ Links ]
6. Castellvi A, Huang H, Vestgaarden T, et al. Stress reduction in adjacent level discs via dynamic instrumentation: a finite element analysis. SAS Journal 2007; 1:74-81. [ Links ]
7. Huang RC, Tropiano P, Marnay T, et al. Range of motion and adjacent level degeneration after lumbar total disc replacement. Spine J. 2006; 6:242-47. [ Links ]
8. Dumbleton J, Manley M. Metal-on-metal total hip replacement: What Does the literature say? J Arthroplasty. 2005; 20:174-88. [ Links ]
9. Passuti N, Philippeau J, Gouin F. Friction couples in total hip replacement. Orthop Traumatol Surg Res. 2009; 95:S27-34. [ Links ]
10. Harper ML, Dooris A, Paré PE. The fundamentals of biotri-bology and its application to spine arthroplasty. SAS Journal 2009; 3:125-32. [ Links ]
11. Schiopu D, Girard J, Soenen M, et al. Metal ions levels measurements for early total hip replacement malfunction diagnosis with 'plasma-sprayed ceramic' bearings couple. Orthop Traumatol Surg Res. 2010; 96:75-79. [ Links ]
12. Beldame J, Carreras F, Oger P, et al. Cementless cups do not increase osteolysis risk in metal-on-metal total hip arthroplasty. Orthop Traumatol Surg Res. 2009; 95:478-90. [ Links ]
13. Crawford R, Ranawat CS, Rothman RH. Metal on metal: is it worth the risk? J Arthroplasty. 2010; 25:1-2. [ Links ]
14. Macpherson GJ, Breusch SJ. Metal-on-metal hip resurfacing: a critical review. Arch Orthop Trauma Surg. 2011; 131:101-10. [ Links ]
15. Delaunay C, Petit I, Learmonth ID, et al. Metal-on-metal bearings total hip arthroplasty: The cobalt and chromium ions release concern. Orthop Traumatol Surg Res. 2010; 96:894-904. [ Links ]
16. Adam Hexter, Anna Panagiotidou, et al. Wear and corrosion at the taper interface in retrieved ASR XL metal-on-metal total hip arthroplasty. Int J Surg. 2012; 12:S53-S109. [ Links ]
17. Nicholas M. Bernthal, Paul C. Celestre, et al. Disappointing short-term results with the depuy ASR XL metal-on-metal total hip arthroplasty. J Arthroplasty. 2012; 27:539-44. [ Links ]
18. Williams PA, Clarke IC. Understanding polyethylene wear mechanisms by modeling of debris size distributions. Wear. 2009; 267:646-52. [ Links ]
19. Murali R, Bonar SF, Kirsh G, et al. Osteolysis in third-generation alumina ceramic-on-ceramic hip bearings with severe impingement and titanium metallosis. J Arthroplasty. 2008; 23:1240.e13-1240.e19. [ Links ]
20. Hallab NJ. A review of the biologic effects of spine implant debris: Fact from fiction. SAS Journal 2009; 3:143-60. [ Links ]
21. Catelas I. Semi-quantitative analysis of cytokines in MM THR tissues and their relationship to metal particles. Biomaterials. 2003; 24:4785-97. [ Links ]
22. Alhassan S, Goswami T. Wear rate model for UHMWPE in total joint applications. Wear. 2008; 265:8-13. [ Links ]
23. Milosev L, Antolic V, Minovic A, et al. Extensive metallosis and necrosis in failed prostheses with cemented titanium-alloy stems and ceramic heads. J Bone Joint Surg Br. 2000; 82:352-57. [ Links ]
24. Chang J-D, Lee S-S, Hur M, et al. Revision total hip arthroplasty in hip joints with metallosis: a single-center experience with 31 cases. J Arthroplasty. 2005; 20:568-73. [ Links ]
25. Oldenburg M, Wegner R, Baur X. Severe cobalt intoxication due to prosthesis wear in repeated total hip arthroplasty. J Arthroplasty. 2009; 24:825.e15-20. [ Links ]
26. Kurtz SM, Steinbeck M, Ianuzzi A, et al. Retrieval analysis of motion preserving spinal devices and periprosthetic tissues. SAS Journal 2009; 3:161-77. [ Links ]
27. Khan RJK, Wimhurst J, Foroughi S, et al. The natural history of metallosis from catastrophic failure of a polyethylene liner in a total hip. J Arthroplasty. 2009; 24:2-5. [ Links ]
28. Moumene M, Geisler FH. Comparison of biomechanical function at ideal and varied surgical placement for two lumbar artificial disc implant designs: mobile-core versus fixed-core. Spine J. 2007; 32:1840-51. [ Links ]
29. Kwon Y-M, Ostlere SJ, McLardy-Smith P, et al. 'Asymptomatic' pseudotumors after metal-on-metal hip resurfacing arthroplasty: Prevalence and metal ion study. J Arthroplasty. 2011; 26:511-18. [ Links ]
30. Engh Jr CA, Ho H, Engh CA, et al. Metal-on-metal total hip arthroplasty adverse local tissue reaction. Sem Arthroplasty. 2010; 21(1):19-23. [ Links ]
31. Walter LR, Marel E, Harbury R, et al. Distribution of chromium and cobalt ions in various blood fractions after resurfacing hip arthroplasty. J Arthroplasty. 2008; 23:814-21. [ Links ]
32. Medical Device Alert, Ref: MDA/2012/036 Issued: 25 June 2012 at 11:00. [ Links ]
33. Macdonald S. Can a safe level for metal ions in patients with metal-on-metal total hip arthroplasties be determined? J Arthroplasty. 2004; 19:71-77. [ Links ]
34. Niki Y, Matsumoto H, Otani T, et al. Screening for symptomatic metal sensitivity: a prospective study of 92 patients undergoing total knee arthroplasty. Biomaterials. 2005; 26:1019-26. [ Links ]
35. Hallab N, Jacobs JJ, Black J. Hypersensitivity to metallic biomaterials: a review of leukocyte migration inhibition assays. Biomaterials. 2000; 21:1301-14. [ Links ]
36. Austin H. 2008. Wear of PEEK all-polymer articulations for cervical spinal disc arthroplasty. Master's dissertation, University of Waterloo. GB. [ Links ]
37. Chang B-S, Brown PR, Sieber A, et al. Evaluation of the biological response of wear debris. Spine J. 2004; 4:239S-44S. [ Links ]
38. US Food and Drug Administration, Department of Health and Human Services. P040006 Charité™ Artificial Disc approval letter, Oct 26, 2004. Retrieved August 30, 2013, from http://www.accessdata.fda.gov/cdrh_docs/pdf4/p040006a.pdf [ Links ]
39. US Food and Drug Administration, Department of Health and Human Services. P050010 ProDics™-L Total Disc Replacement approval letter, Aug 14, 2006. Retrieved August 30, 2013, from www.accessdata.fda.gov/cdrh_docs/pdf5/p050010a.pdf [ Links ]
40. US Food and Drug Administration, Department of Health and Human Services. P060023 BRYAN® Cervical Disc approval letter, May 12, 2009. Retrieved August 27, 2013, from www.accessdata.fda.gov/cdrh_docs/pdf6/P060023a.pdf [ Links ]
41. US Food and Drug Administration, Department of Health and Human Services. P060018 PRESTIGE® Cervical Disc System approval letter, Jul 16, 2007. Retrieved August 27, 2013, from www.accessdata.fda.gov/cdrh_docs/pdf6/P060018A.pdf [ Links ]
42. US Food and Drug Administration, Department of Health and Human Services. P070001 ProDisc™-C Total Disc Replacement approval letter, Dec 17, 2007. Retrieved August 27, 2013, from www.accessdata.fda.gov/cdrh_docs/pdf7/P070001A.pdf [ Links ]
43. US Food and Drug Administration, Department of Health and Human Services. P100003 Secure-C Artificial Cervical Disc approval letter, Sep 28, 2012. Retrieved August 27, 2013, from www.accessdata.fda.gov/cdrh_docs/pdf10/P100003a.pdf [ Links ]
44. US Food and Drug Administration, Department of Health and Human Services. P100012 PCM® Cervical Disc System approval letter, Oct 26, 2012. Retrieved August 27, 2013, from www.accessdata.fda.gov/cdrh_docs/pdf10/P100012A.pdf [ Links ]
45. US Food and Drug Administration, Department of Health and Human Services. P110002 Mobi-C® Cervical Disc Prosthesis approval letter, Aug 07, 2013. Retrieved August 30, 2013, from http://www.accessdata.fda.gov/cdrh_docs/pdf11/P110002a.pdf [ Links ]
46. Kurtz SM, Peloza J, Siskey R, et al. Analysis of a retrieved polyethylene total disc replacement component. Spine J. 2005; 5:344-50. [ Links ]
47. Gornet MF, Burkus K, Skipor AK, et al. Prospective study of serum metal ion levels in patients with cobalt-alloy metal-on-metal lumbar disc replacements. Spine J. 2007; 7:74S-74S. [ Links ]
48. Hexter A, et al. Wear and corrosion at the taper interface in retrieved asr xl metal-on-metal total hip arthroplasty. Int. J. Surg. 2012; 10:8;55. [ Links ]
49. Anand A, McGlynn F, Jiranek W. Metal Hypersensitivity: Can it mimic infection? J Arthroplasty. 2009; 24:826.e25-826.e28. [ Links ]
50. Kowandy C, Mazouz H, Richard C. Isolation and analysis of articular joints wear debris generated in vitro. Wear. 2006; 261:966-70. [ Links ]
51. Cavanaugh D, Nunley P, Kerr E, et al. Delayed hyperreactivity to metal ions after cervical disc arthroplasty. A case report and literature review. Spine, 2009; 34:E262-5. [ Links ]
52. Guyer R, Shellock J, MacLennan B, et al. Early failure of metal-on-metal artificial disc prostheses associated with lymphocytic reaction. Diagnosis and treatment experience in four cases. Spine 2011;36:E492-97. [ Links ]
53. Strömqvist B, Jönsson B, Fritzell P, et al. The Swedish National Register for Lumbar Spine Surgery. Swedish Society for Spinal Surgery. Acta Orthop 2001; 72: 99-106. [ Links ]
54. Singh K, Vaccaro AR, et al. Assessing the potential impact of total disc arthroplasty on surgeon practice patterns in North America. Spine J., 2004; 4: S195-S201. [ Links ]
55. Hähnle UR, Weinberg IR, et al. Kineflex (Centurion) lumbar disc prosthesis: insertion technique and 2-year clinical results in 100 patients. SAS Journal 2007; 1:28-35. [ Links ]
56. Bushelow M, Walker J, Coppes J, et al. Comparison of wear rates: metal/UHMWPE and metal-on-metal total disc arthroplasty. Spine J. 2007; 7:97S. [ Links ]
 Correspondence:
Correspondence:
Dr Svante Berg
Stockholm Spine Center
Löwenströmska Hospital
19489 Upplands Väsby
SE-195 89 Stockholm
Sweden
Email: svante.berg@spinecenter.se
This article is also available online on the SAOA website (www.saoa.org.za) and the SciELO website (www.scielo.org.za). Follow the directions on the Contents page of this journal to access it.














