Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SA Orthopaedic Journal
versión On-line ISSN 2309-8309
versión impresa ISSN 1681-150X
SA orthop. j. vol.13 no.3 Centurion sep. 2014
TUMOUR AND INFECTION
The management of chronic osteomyelitis: Part II - Principles of post-infective reconstruction and antibiotic therapy
LC MaraisI; N FerreiraII; C AldousIII; TLB le RouxIV
IMBChB, FCS Orth(SA), MMed(Ortho); Tumour, Sepsis and Reconstruction Unit, Department of Orthopaedics, Grey's Hospital, University of KwaZulu-Natal
IIBSc, MBChB, FC Orth(SA), MMed(Orth); Tumour, Sepsis and Reconstruction Unit, Department of Orthopaedics, Grey's Hospital, University of KwaZulu-Natal
IIIBSc, BSc(Hons), MSc, PhD; Medical Research Scientist, School of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal
IVMBChB, FCS Orth(SA), MMed(Ortho); Professor and Head of Department, Department of Orthopaedics, I Military Hospital, University of Pretoria
ABSTRACT
Over the past few decades considerable progress has been made in terms of our ability to reconstruct post-infective soft tissue and bone defects. Soft tissue reconstruction is not always required and it is frequently possible to achieve a tension-free closure of well-perfused tissue following debridement. It is now generally accepted that primary closure of the wound, be it by direct suturing or tissue transfer, may be performed at the same sitting as the debridement. In cases were debridement has resulted in tissue loss, muscle or musculocutaneous flaps appear to be superior to random-pattern flaps in achieving resolution of infection. The management of bone defects is dependent on several factors including the host's physiological status, the size of the defect, duration of the defect, quality of the surrounding soft tissue, the presence of deformity, joint contracture / instability or limb length discrepancy, as well as the experience of the surgeon.
Surgery remains the mainstay of treatment when a curative treatment strategy is selected. As is the case with chemotherapy for bone tumours, antibiotic therapy fulfils an adjuvant role in curative management strategies. The choice of antibiotic, in this setting, remains a very difficult one and there are many problems with the interpretation of 'cure rate' data. The controversy surrounding the optimal duration and route of antibiotic therapy has not been resolved. The second role of antibiotics in the management of chronic osteomyelitis is disease suppression as part of a palliative treatment strategy. Further studies are required to clarify which patients may successfully be treated with antibiotics alone.
Keywords: osteomyelitis, chronic, management, review.
Part I of this article - Diagnostic work-up and surgical principles - was published in the previous issue of the South African Orthopaedic Journal, Winter 2014, vol 13, no 2
Introduction
The complex and heterogeneous nature of chronic osteomyelitis necessitates a multi-disciplinary approach, involving experts in the field of orthopaedic tumour, infection and limb reconstruction surgery, plastic surgery, microbiology, nursing, physiotherapy and psychology. Numerous surgical techniques and adjuvant therapies have been developed during the past three decades in order to deal with the wide spectrum of pathology that falls under the heading of chronic osteomyelitis. Despite these developments, the outcome of current treatment protocols remains unsatisfactory, with failure of therapy reported in up to 20% of cases.1
The preceding article in this series aimed to elucidate current concepts in the diagnostic work-up and surgical management of chronic osteomyelitis. In this paper post-infective soft tissue and skeletal reconstruction, as well as the principles of antibiotic therapy, will be addressed. There are several controversial issues related to these subjects. The optimal choice for soft tissue cover following debridement, for example, remains controversial. Although several techniques have been described to deal with bone defects, a comprehensive contemporary strategy has not yet been described. In terms of antibiotic therapy, clear evidence-based guidelines are also lacking, especially in terms of the selection of the appropriate antibiotic agents, the optimal duration of treatment and the ideal route of administration.
Post-debridement reconstruction
Soft tissue reconstruction
In many cases it is possible to achieve a tension-free closure of well-perfused tissue following debridement. Unfortunately the excision of ischaemic tissue and sinuses frequently result in a soft tissue defect. It is now generally accepted that primary closure of the wound, be it by direct suturing or tissue transfer, may be performed at the same sitting as the debridement.2,3 Cierny, however, emphasises the importance of systemic and local antibiotics, as well as a double setup in case of a single stage procedure. This involves re-scrubbing of all staff members, repeat preparation and draping of the patient, as well as the use of new instruments for the reconstructive part of the procedure.4 Delayed primary closures may still be required in certain cases where, for example, a second look at the viability of remaining tissue is required, soft tissues are not amenable to closure due to swelling or induration, or where a second team is required to perform a complex free flap.
In the past post-debridement soft tissue defects were often left to heal by secondary intention or dealt with through the use of open-sky techniques like Papineau bone grafting. These methods have subsequently fallen out of favour, and authors like Ger have promoted the principle of muscle flap coverage in order to achieve improved cure rates.5,6 This approach was justified through animal studies which showed that muscle or musculocutaneous flaps were superior to random-pattern flaps (i.e., local flaps) in achieving resolution of infection.7 In an experimental model, Feng and colleagues were able to explain this phenomenon by showing increased blood flow and more consistent leukocyte mobilisation in musculocutaneous flaps when compared to random flaps. In addition, the oxygen tension in soft tissue defects covered by muscle flaps was shown to be higher than those covered through random-pattern flaps. The advantages of muscle flaps have also been illustrated in the clinical setting, although a recent review still questioned the clinical validity of the theoretical advances of muscle flaps in the setting of infection.8,9
With the advances in microsurgical techniques in the recent past, free tissue transfer has become more accessible. The success achieved with free flaps in the management of open fractures has prompted utilisation of these techniques in the management of chronic osteomyelitis. The excellent results, in terms of bony union and eradication of infection, with free muscle transfer in chronic osteomyelitis, have also been attributed to the dramatic increase in the local blood supply.10 In addition, performing a debridement and free flap in a single sitting has been shown to be reliable in achieving cure.3 Recently, perforator free flaps have gained much popularity in the management of open fracture and have been suggested to be superior in the management of tibial osteomyelitis.11 Although free anterolateral thigh fasciocutaneous flaps have been shown to be effective in the management of open tibia fractures, it is technically challenging and free muscle- or musculocutaneous flaps are still considered the method of choice in coverage of lower leg defects.12
Several other salvage techniques have emerged in the recent past. Negative pressure dressing has been employed successfully in the management of many soft tissue defects. It has, however, a limited role in the management of chronic osteomyelitis as it results in the formation of dense and poorly vascularised scar tissue. The application of vacuum dressings to draining sinuses in particular is discouraged as it significantly complicates subsequent surgery.4 Vacuum dressing may occasionally be considered in severely compromised hosts where tissue transfer is deemed impossible. More recently negative pressure wound therapy combined with the instillation of solution in the local area (VAC instil therapy) has been proposed as a viable alternative in the management of osteomyelitis-associated soft tissue defects.13 This form of therapy is attractive as it offers the theoretical advantages of both the Lautenbach technique and negative pressure wound therapy. As a last resort, in certain cases where the local soft tissue condition does not permit flap coverage, open skeletal transport (in accordance with Ilizarov principles) may be considered.
Skeletal reconstruction
Cierny and Mader type I, II and III lesions are, per definition, stable and generally do not require reconstruction of the defect left by the debridement. Type IV lesions, on the other hand, are characterised by instability and routinely require stabilisation and reconstruction of osseous defects resulting from the debridement. Existing classification systems for post-osteomyelitis bone defects, including those suggested by May and Gordon, have failed to keep up with the modern trends in limb reconstruction surgery and have therefore lost some of their value.14,15
Acute shortening, with primary docking of the bone ends, of up to 4 cm has been advocated for post-traumatic bone loss.16 Unfortunately the soft tissue scarring associated with chronic osteomyelitis rarely permits acute shortening beyond 2 cm. Not only does acute shortening in the presence of significant scar tissue present technical difficulties with wound closure, it also carries a particular risk of vascular compromise as a result of kinking of blood vessels which are immobilised by rigid soft tissues. Acute shortening of 1-2 cm can, however, be used as part of a combined strategy, which may include the induced membrane technique along with bone grafting or bone transport.
Unfortunately the soft tissue scarring associated with chronic osteomyelitis rarely permits acute shortening beyond 2 cm
The advent of induced membrane techniques has increased the potential for the use of cancellous bone graft in much larger defects
The size of a segmental bone defect which should be considered critical, and thus not suitable for autologous cancellous bone grafting, remains controversial. Traditionally approximately 4 cm has been recommended as the cut-off point.17, 18 The first problem with cancellous bone grafting is its dependence on the surrounding soft tissues for nourishment. Large grafts may undergo central necrosis in the absence of an excellent soft tissue envelope (bone bed).19 Secondly the regenerated segment is often weak and prone to fracture as a result of partial graft resorption.20 As a result, Tiemann et al. recommended 2 cm as the maximum size of a segmental diaphyseal tibial defect that can be managed with autologous cancellous grafting.19
The advent of induced membrane techniques has, however, increased the potential for the use of cancellous bone graft in much larger defects. Masquelet reported the successful use of this technique in 35 cases, with defects ranging from 4-25 cm.21 Others have been able to reproduce these results.
Stafford reported a 90% union rate of defects ranging from 1-25 cm (average 5.8 cm) with the use of reamer-irrigation-aspiration graft.22 Although the induced membrane technique offers several theoretical and practical advantages, caution should be applied in the use of cancellous bone graft in tibial defects exceeding 4 cm, especially in the absence of periosteal new bone formation (Figure 1).19
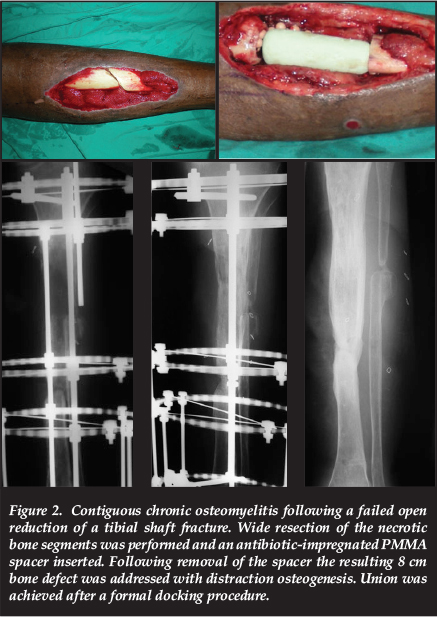
Distraction osteogenesis, in accordance with the Ilizarov method, remains the gold standard in the management of post-debridement bone defects of 4 cm or more.16,23 This may take the form of acute shortening with subsequent lengthening or, more commonly, bone transport into the defect. Distraction osteogenesis offers several advantages in the management of chronic osteomyelitis, including the increase of regional blood flow for a period of up to 17 weeks following the corticotomy.24 Large defects can be dealt with through simultaneous multifocal transport, sequential transport or cable-transport techniques. The upper limit of the size of defects which may be dealt with through distraction osteogenesis is, however, highly dependent on the surgeon's experience with the technique (Figure 2).
Circular fixation and bone transport is associated with its own subset of complications and a second procedure involving cancellous grafting of the docking site is generally recommended
Circular fixation and bone transport is associated with its own subset of complications and a second procedure involving cancellous grafting of the docking site (formal docking) is generally recommended.25 Following a comparative study, El-Gammal and colleagues suggested that defects smaller than 12 cm should be reconstructed with Ilizarov bone transport while free vascularised fibula grafts performed better in defects larger than 12 cm.26 Although vascularised fibula grafts, fibula-pro-tibia (fibula centralisation) or fibula bypass grafting remain options for defects in excess of 12 cm these procedures involve donor site morbidity and is often complicated by non-union or fracture of the graft during the period of hypertrophy (Figure 3).27
A combination of techniques is commonly used. Ultimately the management of bone defects is dependent on several factors including the host's physiological status, the size of the defect, duration of the defect (i.e. acute or chronic), quality of the surrounding soft tissue, the presence of deformity, joint contracture/instability or limb length discrepancy, as well as the experience of the surgeon.
Antibiotic therapy
It is important to note that surgery remains the mainstay of treatment when a curative treatment strategy is selected. As is the case with chemotherapy for bone tumours, antibiotics fulfil an adjuvant role in curative management strategies. Curative surgery should ideally involve a wide resection with clear margins. This goal is however frequently unachievable as it may result in unreconstructable loss of bone that is vital to the survival and function of the limb. Marginal resection may, on the other hand, leave behind colonised bone or soft tissue that may serve as a nidus for recurrent infection.28 Even in wide resections the remaining bone and soft tissue bed should also be considered contaminated. Antibiotics are, therefore, used in wide and marginal resections (curative surgical strategies) in an attempt to sterilise the remaining bone and soft tissues. In the curative setting empirical adjuvant antibiotics are typically started immediately following the debridement, and the regimen is modified once the culture and sensitivity results become available.
The first role of antibiotics in the management of chronic osteomyelitis, is adjuvant therapy as part of a curative treatment strategy. The choice of antibiotic, in this setting, remains a very difficult one and there are many problems with the interpretation of 'cure rate' data. Firstly there are no standardised definitions for cure or failure of treatment and no universally accepted host stratification system. In addition, many of the historical studies evaluated the efficacy of antibiotics in the absence of surgical debridement or surgical implants. Studies often include a heterogeneous group of patients in terms of their physiological status, the aetiological source of the infection and the anatomical/ pathological nature of the disease. Finally, in vivo effect does not always mirror the high degree of efficacy predicted by in vitro investigations. Empirical antibiotics should be selected on the basis of the aetiology of the infection as well as local pathogen profiles. (β-lactams and vancomycin are the most commonly used antimicrobials in the medical management of osteomyelitis.29
In terms of contiguous infections, The Bone Infection Unit in the United Kingdom recommends empirical parenteral vancomycin and meropenem.2 Although this protocol covers a broad range of pathogens there are some potential concerns. Vancomycin offers excellent activity against MRSA and ampicillin-resistant enterococci. Unfortunately it has several drawbacks including poor bone penetration, increased minimal inhibitory concentrations among many S. aureus strains and has been shown to have increased recurrence rate when compared with cefazolin or ceftriaxone.30, 31 β-lactam antibiotics (penicillins, cephalosporins and carbapenems) exhibit poor bone penetration, with levels reaching only approximately 5-20% of serum concentrations. Fortunately serum levels of parenteral β-lactams are so high that the resulting bone levels most likely exceed the necessary minimum inhibitory concentration (MIC).32 Cefepime appears to be a reasonable alternative to meropenem, offering good activity against Gram-negative organisms, and it has been shown to have excellent bone penetration, with bone concentration reaching 97-100% of serum levels.33
In terms of the route of administration, oral antibiotic agents which exhibit high bioavailibility are an acceptable alternative to parenteral therapy.32 Several randomised clinical trials have found similar cure rates in patients treated with oral and parenteral antibiotic therapy.34,35 In addition parenteral antibiotics are associated with an increased incidence of moderate or severe side-effects.30 Preferred oral agents, based on clinical and pharmacokinetic data, include fluoroquinolones and trimethoprim-sulfamethoxazole (cotrimoxazole).32 Studies involving fluoroquinolones have found high cure rates, although failure of treatment may occur in Pseudomonas or S.aureus infections, especially when used as monotherapy.36 In addition, it is a matter of concern that fluoroquinolones have been associated with impaired bone healing and these agents may need to be avoided in cases of septic nonunion or in the setting of post-infective reconstruction.37
Cotrimoxazole exhibits concentration-dependent killing, therefore higher than usual doses (7-8 mg/kg/day trimethoprim) are recommended in the treatment of chronic osteomyelitis.38 De Barros et al. reported an impressive 98% cure rate with 6 months of cotrimoxazole therapy following surgical debridement, although it may be argued that the extended duration of therapy may have resulted in disease quiescence through suppression.39 Rifampicin achieves bone levels equivalent to serum concentrations and when used in conjunction with other agents there appears to be a clear benefit in terms of cure rates.40,41 It should however never be used as monotherapy due to the risk of the development of resistance. Sanchez et al. reported a 100% cure rate in staphylococcal infections with surgical debridement in conjunction with double the standard dose of cotrimoxazole combined with rifampicin for a mean of five weeks.42 Similarly, cotrimoxazole combined with rifampicin achieved similar cure rates to both linezolid with rifampicin, as well as eight weeks of intravenous cloxacillin monotherapy, in the treatment of chronic osteomyelitis and infections associated with surgical implants.43,44 It is important to note that oral dosing of β-lactam antibiotics results in serum levels of less than 10% of parenteral administration. This pharmacokinetic characteristic raises concern regarding the ability of β-lactams to reach adequate MIC in bone, despite the fact that their penetration is better in infected than in uninfected bone.45 Clindamycin exhibits good bone penetration and many methicillin-resistant S. aureus strains are susceptible to the agent. Despite these characteristics there are no recent studies investigating the use of clindamycin in the management of osteomyelitis.
In terms of the route of administration, oral antibiotic agents which exhibit high bioavailibility are an acceptable alternative to parenteral therapy
Linezolid, the first antimicrobial in the new oxazolidinone class, was initially received with much enthusiasm as a result of its high bioavailability following oral administration and excellent activity against staphylococci, streptococci and vancomycin-resistant enterococci. Unfortunately clinical studies have shown cure rates of only 60% and prolonged used has been associated with pancytopaenia, peripheral neuropathy and optic neuritis.46,47 The use of linezolid is therefore typically limited to patients with osteomyelitis resulting from vancomycin-resistant enterococci or patients who are intolerant of vancomycin.46 Daptomycin, a new lipopeptide antimicrobial agent, exhibits good activity against Gram-positive bacteria including methicillin-resistant S. aureus and glycopeptide-resistant enterococci. It has been studied extensively in the management of osteomyelitis and has been shown to be a good salvage option in cases which failed to respond to standard therapy.48
The optimal duration of antimicrobial therapy following surgical debridement remains unknown. The traditional duration of treatment is four to six weeks. This is based on experience with the management of acute osteomyelitis in children, where extended periods of antibiotics are required, as well as the results of animal studies which illustrated that six weeks of antibiotics was effective in sterilising diseased bone.49 This traditional recommendation is also derived from the assumption that revascularisation of bone following debridement takes about four weeks.50 Several studies have failed to demonstrate increased efficacy of extended duration antibiotic therapy.51 Furthermore, the absence of standardised treatment algorithms makes interpretation of the data very difficult. Many studies did not include surgical debridement or removal of surgical implants, and thus this form of treatment should rather be viewed as palliative intervention. Because of the historical absence of standardised definitions and treatment strategies, older antibiotic treatment protocols are inconsistent with our current way of thinking. The duration of antibiotic treatment should rather be based on the treatment strategy selected, the realistic aim of treatment and the extent of the surgical margin. In theory, curative management strategies involving wide resection would only require a short period of antibiotics in order to sterilise the remaining soft tissue. In practice truly wide margins are, however, very difficult to achieve. Traditional thinking dictates a minimum of six weeks treatment in curative treatment protocols involving marginal debridement. Unfortunately there is insufficient evidence to make definitive recommendations and further studies are required in this respect.32 In palliative treatment strategies or in cases treated with intra-lesional debridement extended periods of antibiotics appear to remain appropriate.
The second role of antibiotics in the management of chronic osteomyelitis is disease suppression as part of a palliative treatment strategy. This form of treatment appears to be justified by the successful use of suppressive antibiotics in peri-prosthetic infections of hip or knee replacements.52,53 Success rates of between 60 and 75% have also been reported in cases of infection associated with osteosynthesis through the use of long-term antibiotics without surgical removal of the implants.38,54
The efficacy of suppressive treatment in chronic osteomyelitis without an implant has, however, not been determined. In addition many of the older studies looking at long-term antibiotic therapy included patients with and without surgical implants as well as surgically and non-surgically managed patients. This lack of uniformity made comparison of results impossible and, again, illustrates the urgent need for the establishment of standardised nomenclature and treatment strategies in the management of chronic osteomyelitis.
Chronic suppressive antibiotic therapy forms the cornerstone of palliative management in C-hosts. This form of treatment typically involves antibiotics that are prescribed for a period six months. If quiescence or sufficient suppression is achieved the antibiotics can be stopped. If the infection recurs after discontinuation of the therapy, a lifelong suppressive regimen should be considered.50 Various antibiotic regimens have been investigated. Due to the inferior results reported with single agents, and the efficacy shown with the addition of a second agent in the setting of implant-related infections, most chronic suppressive regimens generally involve the combination of two agents.55,56 Antibiotics used in suppressive regimens include cotrimoxazole, rifampicin, ciprofloxacin, cloxacillin, fusidic acid and clindamycin.38,45,57,58 Although directed therapy according to culture and sensitivity results is the ideal, this is frequently not practical and possibly not necessary in order to achieve clinical quiescence. The available literature suggests that cotrimoxazole and rifampicin can be considered as first line chronic suppressive antibiotic therapy.33 If these agents fail to achieve clinical suppression during the first six months, second line therapy may be instituted in the form of clindamycin or cloxacillin in combination with rifampicin, ciprofloxacin or fusidic acid.
Conclusion
Over the past few decades considerable progress has been made in terms of our ability to reconstruct post-infective soft tissue and bone defects. Muscle or musculocutaneous flaps appear to be superior to random-pattern flaps (i.e. local flaps) in achieving resolution of infection and it is now generally accepted that primary closure of the wound may be performed at the same sitting as the debridement. Several factors need to be considered when dealing with post-infective bone defects, and the size of the defect serves as a useful guideline when selecting the appropriate treatment strategy. The soft tissue scarring associated with chronic osteomyelitis rarely permits acute shortening beyond 2 cm. Good results have been reported with cancellous grafting into an induced membrane and the Masquelet technique may be utilised in cases with bone loss of more than 2 cm. For bone defects larger than 4 cm distraction osteogenesis may be appropriate, while free vascularised fibula grafts may have to be considered for defects in excess of 12 cm.
In terms of antibiotic therapy clear evidence-based guidelines are lacking, especially in terms of the selection of the appropriate antibiotic agents, the optimal duration of treatment and the ideal route of administration. Oral antibiotic agents that exhibit high bioavailability appear to be an acceptable alternative to parenteral therapy.
Over the past few decades considerable progress has been made in terms of our ability to reconstruct post-infective soft tissue and bone defects
Preferred oral agents, based on clinical and pharmacokinetic data, include fluoroquinolones, rifampicin and trimethoprim-sulfamethoxazole. In theory, curative management strategies involving wide resection would only require a short period of antibiotics in order to sterilise the remaining soft tissue. Traditional thinking dictates a minimum of six weeks of treatment in curative treatment protocols involving marginal debridement. In palliative treatment strategies and in cases treated with intra-lesional debridement, extended periods of antibiotics remain appropriate.
The content of this article is the sole work of the authors. The primary author has received a research grant from the South African Orthopaedic Association for research relating to chronic osteomyelitis.
References
1. Haas DW, McAndrew MP. Bacterial osteomyelitis in adults: evolving considerations in diagnosis and treatment. Am J Med 1996;101:550-61. [ Links ]
2. McNally M, Nagarajah K. Osteomyelitis. Orthop Trauma 2010;24(6):416-29. [ Links ]
3. Singh J, Marwah S, Mustafa J, et al. Outcome of single stage treatment of chronic osteomyelitis. J Bone Joint Surg Br 2012;94-B(Suppl XXXVII):495. [ Links ]
4. Cierny G. Surgical treatment of osteomyelitis. Plast Reconstr Surg 2011;127(1) Suppl:190S-204S. [ Links ]
5. Ger R, Efron G. New operative approach in the treatment of chronic osteomyelitis of the tibial diaphysis: a preliminary report. Clin Orthop Rel Res 1970;70:165-69. [ Links ]
6. Ger R. Muscle transposition for treatment and prevention of chronic post-traumatic osteomyelitis. J Bone Joint Surg Am 1977;59-A:784-91. [ Links ]
7. Chang N, Mathes SJ. Comparison of the effect of bacterial inoculation in musculo-cutaneous and random pattern flaps. Plast Reconstr Surg 1983;70:1-10. [ Links ]
8. Anthony JP, Mathes SJ, Alpert BS. The muscle flap in the treatment of chronic lower extremity osteomyelitis: Results in patients over 5 years after treatment. Plast Reconstr Surg 1991;88(2):311-18. [ Links ]
9. Tintle SM, Levin LS. The reconstructive microsurgery ladder in orthopaedics. Injury, Int. J. Care Injured 2013;44:376-85. [ Links ]
10. Lee JM, Song KH, Park JH. Muscle free flap transplantation in chronic osteomyelitis of the lower extremities. J Korean Soc Microsurg 2009;18(2):49-54. [ Links ]
11. Park G, Kim H. Treatment of chronic osteomyelitis using the medial sural perforator flap. J Plast Reconstr Aesth Surg 2010;63(1):153-59. [ Links ]
12. Demitras Y, Kelahmetoglu O, Cifci M, et al. Comparison of free anterolateral thigh flaps and free muscle-musculocutaneous flaps in soft tissue reconstruction of lower extremity. Microsurgery 2010;30(1):24-31. [ Links ]
13. Rashid MA, Vincent M, Dennison MG, et al. Management of chronic osteomyelitis - role of combined topical negative pressure dressings and local instillation of antibiotic solution. J Bone Joint Surg Br 2010;92-B(Suppl III):402. [ Links ]
14. May JW, Jupiter JB, Weiland AJ, Byrd HS. Clinical classification of post-traumatic tibial osteomyelitis. J Bone Joint Surg Am 1989;71-A(9):1422-28. [ Links ]
15. Gordon L, Chiu EJ. Treatment of infected non-unions and segmental defects of the tibia with staged microvascular muscle transplantation and bone-grafting. J Bone Joint Surg Am 1988;70-A:377-86. [ Links ]
16. Lerner A, Fodor L, Soudry M, et al. Acute shortening: modular treatment modality for severe combined bone and soft tissue loss of the extremities. J Trauma 2004;57:603-608. [ Links ]
17. Tiemann AH, Hofmann GO. Principles of the therapy of bone infection in adult extremities. Strat Traum Limb Recon 2009;4:57-64. [ Links ]
18. Lasanianos NG, Kanakaris NK, Giannoudis PV. Current management of long bone large segmental defects. Orthop Trauma 2009;24(2):149-63. [ Links ]
19. Tiemann AH, Schmidt HGK, Braunschweig R, Hofmann GO. Strategies for the analysis of osteitic bone defects at the diaphysis of long bones. Strat Traum Limb Recon 2009;4:13-18. [ Links ]
20. Masquelet AC. Muscle reconstruction in reconstructive surgery: soft tissue repair and long bone reconstruction. Langenbecks Arch Surg 2003;388:344-46. [ Links ]
21. Masquelet AC, Fitoussi F, Begue T, Muller GP. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann Chir Plast Esthet 2000;45(3):346-53 [ Links ]
22. Stafford PR, Norris BL. Reamer-irrigator-aspirator bone graft and bi Masquelet technique for segmental bone defect nonunions: a review of 25 cases. Injury, Int J Care Injured 2010;41(Suppl 2):S72-S77. [ Links ]
23. Rodner CM, Browner BD, Pestani E. Chronic osteomyelits. In: Skeletal Trauma. Saunders. 2003:483-506. [ Links ]
24. Aronson J. Temporal increases in blood flow during distraction osteogenesis. Clin. Orthop 1994;301:124-31. [ Links ]
25. Giotakis N, Narayan B, Nayagam S. Distraction osteogenesis and nonunion of the docking site: Is there an ideal treatment option? Injury, Int J Care Injured 2007:38 (Suppl1): S100-S107. [ Links ]
26. El-Gammal TA, Shiha AE, El-Deen MA, et al. Management of traumatic tibial defects using free vascularized fibula or Ilizarov bone transport: A comparative study. Microsurg 2008;28(5):339-46. [ Links ]
27. Arai K, Toh S, Tsubo K, et al. Complications of vascularized fibula graft for reconstruction of long bones. Plast Recon Surg 2002;109(7):2310-06. [ Links ]
28. Roa N, Ziran BH, Lipsky BA. Treating osteomyelitis: antibiotics and surgery. Plast Reconstr Surg 2011;127 (Suppl):177S-187S. [ Links ]
29. Tice AD, Hoaglund PA, Shoultz DA. Outcomes of osteomyelitis among patients treated with outpatient parenteral antimicrobial therapy. Am J Med 2003;114:723-28. [ Links ]
30. Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy: DSA guidelines. Clin Infect Dis 2004;38:1651-72. [ Links ]
31. Tice AD, Hoaglund PA, Shoultz DA. Outcomes of osteomyelitis among patients treated with outpatient parenteral antimicrobial therapy. Am J Med 2003;114:723-28. [ Links ]
32. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis 2012;54(3):393-407. [ Links ]
33. Breilh D, Boselli E, Bel JC, et al. Diffusion of cefepime into cancellous and cortical bone tissue. J Chemother 2003;15:134-38. [ Links ]
34. Gentry LO, Rodriguez GG. Oral ciprofloxacin compared with parenteral antibiotics in the treatment of osteomyelitis. Antimicrob Agents Chemother 1990;34:40-43. [ Links ]
35. Euba G, Murillo O, Fernandez-Sabe N, et al. Long-term follow-up trial of oral rifampin-cotrimoxazole combination versus intravenous cloxacillin in treatment of chronic staphylococcal osteomyelitis. Antimicrob Agents Chemother 2009;53:2672-76. [ Links ]
36. Greenberg RN, Kennedy DJ, Reilly PM, et al. Treatment of bone, joint, and soft-tissue infections with oral ciprofloxacin. Antimicrob Agents Chemother 1987;31:151-55. [ Links ]
37. Huddleston PM, Steckelberg JM, Hanssen AD, et al. Ciprofloxacin inhibition of experimental fracture healing. J Bone Joint Surg Am 2000;82-A:161-73. [ Links ]
38. Stein A, Bataille JF, Drancourt M, et al. Ambulatory treatment of multidrug-resistant Staphylococcus-infected orthopedic implants with high-dose oral co-trimoxazole (trimethoprim-sulfamethoxazole). Antimicrob Agents Chemother 1998;42:3086-91. [ Links ]
39. De Barros JW, Calapodopulos CJ, Oliveira DJ, et al. The treatment of chronic osteomyelitis. Rev Soc Bras Med Trop 1992;25:235-39. [ Links ]
40. Norden CW, Fierer J, Bryant RE. Chronic staphylococcal osteomyelitis: treatment with regimens containing rifampin. Rev Infect Dis 1983;5(Suppl 3):S495-501. [ Links ]
41. El Helou OC, Berbari EF, Lahr BD, et al. Efficacy and safety of rifampicin containing regimen for Staphylococcal prosthetic joint infections treated with debridement and retention. Eur J Clin Microbiol Infect Dis 2010;29:961-67. [ Links ]
42. Sanchez C, Matamala A, Salavert M, et al. Cotrimoxazole plus rifampicin in the treatment of staphylococcal osteoarticular infection. Enferm Infecc Microbiol Clin 1997;15:10-13. [ Links ]
43. Nguyen S, Pasquet A, Legout L, et al. Efficacy and tolerance of rifampicin-linezolid compared with rifampicin-cotrimoxazole combinations in prolonged oral therapy for bone and joint infections. Clin Microbiol Infect 2009;15:1163-69. [ Links ]
44. Euba G, Murillo O, Fernandez-Sabe N, et al. Long-term follow-up trial of oral rifampin-cotrimoxazole combination versus intravenous cloxacillin in treatment of chronic staphylococcal osteomyelitis. Antimicrob Agents Chemother 2009;53:2672-76. [ Links ]
45. Landersdorfer CB, Bulitta JB, Kinzig M, et al. Penetration of antibacterials into bone. Clin Pharmacokinetics 2009;48(2):89-124. [ Links ]
46. Berbari EF, Steckelberg JM, Osmon DR. Osteomyelitis. In Mandell, Douglas and Bennet's Principles and Practice of Infectious Diseases. Churchill Livingstone. 2009:1457-67. [ Links ]
47. Birmingham MC, Rayner CR, Meagher AK, et al. Linezolid for the treatment of multidrug-resistant, grampositive infections: experience from a compassionate-use program. Clin Infect Dis 2003;36:159-68. [ Links ]
48. Finney MS, Crank CW, Segreti J. Use of daptomycin to treat drug resistant gram-positive bone and joint infections. Curr Med Res Opin 2005;21:1923-26. [ Links ]
49. Norden CW, Dickens DR. Experimental osteomyelitis III. Treatment with cephaloridine. J Infect Dis 1973;127:525-28. [ Links ]
50. Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Joint Surg Am 2004;86-A:2305-18. [ Links ]
51. Haidar R, Der Boghossian A, Atiyeh B. Duration of post-surgical antibiotics in chronic osteomyelitis: empiric or evidence-based? Int J Infect Dis 2010;14:e752-58. [ Links ]
52. Goulet JA, Pellicci PM, Brause BD, Salvati EM. Prolonged suppression of infection in total hip arthroplasty. J Arthroplasty 1988;3:109-16. [ Links ]
53. Segreti J, Nelson JA, Trenholme GM. Prolonged suppressive antibiotic therapy for infected orthopedic prostheses. Clin Infect Dis 1998;27:711-13. [ Links ]
54. Javaloyas de Morlius M, Monreal Portella M. Oral antibiotic therapy in the adult bacterial osteomyelitis: results after two years of follow-up. Med Clin (Barc) 1999;113:488-89. [ Links ]
55. Zimmerli W, Widmer AF, Blatter M, et al. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 1998; 279:1537-41. [ Links ]
56. Saengnipanthkul S, Pongvivat T, Mahaisavariya B, LaupattarakasemW. Co-trimoxazole in the treatment of chronic osteomyelitis. J Med Assoc Thai 1988;71:186-91. [ Links ]
57. Pontifex AH, McNaught DR. The treatment of chronic osteomyelitis with clindamycin. Can Med Assoc J 1973;109:105-107. [ Links ]
58. Atkins B, Gottlieb T. Fusidic acid in bone and joint infections. Int J Antimicrob Agents 1999;12 (Suppl 2):S79-93. [ Links ]
 Correspondence:
Correspondence:
Dr LC Marais
Department of Orthopaedic Surgery
Grey's Hospital
School of Clinical Medicine
University of KwaZulu-Natal
Private Bag X9001
Pietermaritzburg 3201
Email: Leonard.Marais@kznhealth.gov.za
Tel: +27 033 897 3299
Fax: +27 33 897 3409
This article is also available online on the SAOA website (www.saoa.org.za) and the SciELO website (www.scielo.org.za). Follow the directions on the Contents page of this journal to access it.














