Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SA Orthopaedic Journal
On-line version ISSN 2309-8309
Print version ISSN 1681-150X
SA orthop. j. vol.13 n.2 Centurion Apr./Aug. 2014
TUMOUR AND INFECTION
The management of chronic osteomyelitis: Part I - Diagnostic work-up and surgical principles
LC MaraisI; N FerreiraII; C AldousIII; TLB le RouxIV
IMBChB, FCS Orth(SA), MMed(Ortho); Tumour, Sepsis and Reconstruction Unit, Department of Orthopaedics, Grey's Hospital, University of KwaZulu-Natal
IIBSc, MBChB, FC Orth(SA), MMed(Orth); Tumour, Sepsis and Reconstruction Unit, Department of Orthopaedics, Grey's Hospital, University of KwaZulu-Natal
IIIBSc, BSc(Hons), MSc, PhD; Medical Research Scientist, School of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal
IVMBChB, FCS Orth(SA), MMed(Ortho); Professor and Head of Department, Department of Orthopaedics, I Military Hospital, University of Pretoria
ABSTRACT
To date, no evidence-based guidelines for the treatment of chronic osteomyelitis exist. Owing to certain similarities, treatment philosophies applicable to musculoskeletal tumour surgery may be applied in the management of chronic osteomyelitis. This novel approach not only reinforces certain important treatment principles, but may also allow for improved patient selection as surgical margins may be customised according to relevant host factors. When distilled to its most elementary level, management is based on a choice between either a palliative or curative approach. Unfortunately there are currently no objective criteria to guide selection of the most appropriate treatment pathway.
The pre-operative diagnostic work-up should be tailored according to the relevant objective, albeit confirming the clinical suspicion of the presence of infection, host stratification, anatomical disease classification, pre-operative planning or post-operative follow-up. MRI and PET-CT are emerging as the imaging modalities of choice. interleukin-6, in combination with CRP, has been shown to have excellent sensitivity in the diagnosis of implant-associated infection. Molecular methods are growing rapidly as the method of choice in pathogen detection.
Chronic osteomyelitis, as is the case with musculoskeletal tumours, can only be eradicated through complete resection of all infected bone. Chemotherapy, in the form of antibiotics, only plays an adjuvant role. Dead space management is essential following debridement, and the appropriate strategy should be selected according to the anatomical nature of the disease. Provision of adequate bony stability is crucial as it promotes revascularisation and maximisation of the host's immune response. Although there is currently a variety of fixation options available, external fixation is generally preferred.
Key words: osteomyelitis, chronic, management, review
Introduction
When contemplating open fractures Hippocrates stated that 'One should especially avoid such cases if one has reasonable excuse, for the risks are great and rewards are few'.1 This statement still rings true today for chronic osteomyelitis. Prior to the implementation of contemporary classifications systems poor results were universally reported in the management of chronic osteomyelitis. The Mayo Clinic, for example, reported a failure rate of 20% and this figure deteriorated to failure in over 60% of patients in the presence of mixed aerobic and anaerobic infections.2
Chronic osteomyelitis can only be eradicated through complete resection of all infected bone
The poor outcome of treatment in chronic bone infections has inspired many changes in our management strategy over the past few decades. The 1970s can be seen as the era of secondary healing. During this period sequential debridement, healing by secondary intention and long-term antibiotic treatment were the order of the day. Reconstruction options were often limited to open sky techniques (Papineau grafting) or bypass grafting. As a result of these limitations the extent of surgical debridement was restricted and residual fibrotic and ischaemic tissue was often left behind, impairing the host's ability to launch an effective defence against bacterial persistence. In the 1980s wound revitalisation, involving thorough wound debridement with excision of all ischaemic tissue, became the mainstay of treatment. In conjunction with systemic and local antibiotic therapy, wound revitalisation allowed early closure of wounds following the debridement.3 The era of revascularisation followed as a result of this new-found ability. In the 1990s free tissue transfer involving microvas-cular anastomosis became an integral part of the post-infective reconstruction process. The advances in soft tissue management culminated in the creation of a wound bed that was able to withstand the metabolic demands of more complex limb reconstruction procedures. In the past two decades, the potential for skeletal reconstruction has reached new heights. Salvage protocols for peri-prosthetic infection, incorporating staged endo-prosthetic replacement, have grown in popularity. The propagation of the science of distraction osteogenesis and Ilizarov techniques outside of Russia has allowed surgeons the opportunity to reconstruct much larger bone defects than before. Most recently the induced membrane technique, popularised by Masquelet, has emerged as a useful adjunct in the management of large bone defects following debridement.
In Part I of this two-part series we will discuss the management strategies currently available for the management of chronic osteomyelitis. Certain novel concepts, key to the decision-making process, will also be introduced. The different diagnostic modalities, which may be employed in the conformation of the presence of infection or during the pre-operative workup of the patient, will also be explored. Finally we will discuss the surgical management strategies that may be implemented during the first stage of treatment, with specific reference to debridement techniques, pathogen detection, dead space management and skeletal stabilisation. In Part II of this series on the management of chronic osteomyelitis, which will be published in the next issue of this journal, we will review antibiotic therapy, as well as soft tissue and skeletal reconstruction following debridement.
Management strategies
To date, no evidence-based guidelines exist in terms of the treatment of chronic osteomyelitis.4 When distilled to its most elementary level, management is based on a choice between either a palliative or curative approach. Management strategies, aimed at eradication of infection and limb reconstruction, incorporate a wide array of surgical procedures and techniques in terms of debridement, dead space management, soft tissue cover and skeletal reconstruction. While curative management strategies usually involve multiple surgical procedures, palliative treatment strategies, on the other hand, are much less invasive and typically involve the use of chronic suppressive antibiotic therapy. Thus the most important decision a surgeon faces is whether to embark on either a curative or a palliative treatment strategy.
This decision regarding cure or palliation requires consideration of several factors, foremost of which is the host's physiological status. As described by Cierny, a C-host should be palliated, whereas A- and B-hosts may be considered for a curative treatment protocol. The main risk involved in certain curative treatment strategies, is the fact that treatment failure may result in unplanned amputation of the limb. If, for example, wide resection and limb reconstruction through bone transport is embarked upon in a patient who is unable to cope with the physical or physiological demands of the process, failure of the reconstruction process may result in amputation. To justify the morbidity and risk of limb salvage, the expected outcome must offer distinct advantages over an amputation or palliation alone. If treatment aimed at cure is contraindicated or excessive, as a result of the risk it entails, the patient should be classified as a C-host and offered palliation (incision and drainage, oral antibiotics, ambulatory aides, and pain medication). Amputation may be indicated when limb salvage and palliation are neither safe nor feasible.5 The main problem we currently face, however, is the absence of objective criteria according to which a C-host should be defined.
Owing to various similarities, principle among which is the high recurrence rate following incomplete excision, certain treatment philosophies applicable to musculoskeletal tumour surgery may also be applied when formulating a treatment plan for chronic osteomyelitis. Excision margins, for example, may be thought of in oncological terms with a simple sequestrectomy representing an intralesional excision, direct or indirect unroofing a marginal excision, and finally a complete resection can be seen as a wide excision. Similarly antibiotic therapy can be thought of as chemotherapy which may be instituted in a neo-adjuvant, adjuvant and palliative setting. This novel approach to chronic osteomyelitis not only reinforces certain important treatment principles, but may also allow for improved patient selection as surgical margins may be customised according to relevant host factors.
Pre-operative considerations
Clinical evaluation
Patient evaluation should include a meticulous history taking and careful examination.
Information should be gathered regarding the main complaint, associated problems, medical history, previous surgical history and prior therapeutic interventions. Examination should include a systemic evaluation as well as a thorough assessment of the local pathology, skeletal stability, the condition of the soft tissues, vascularity and neurological status.
Imaging
Imaging modalities should be tailored to the relevant objective, albeit confirming the clinical suspicion of the presence of infection, anatomical disease classification, pre-operative planning or post-operative follow-up.
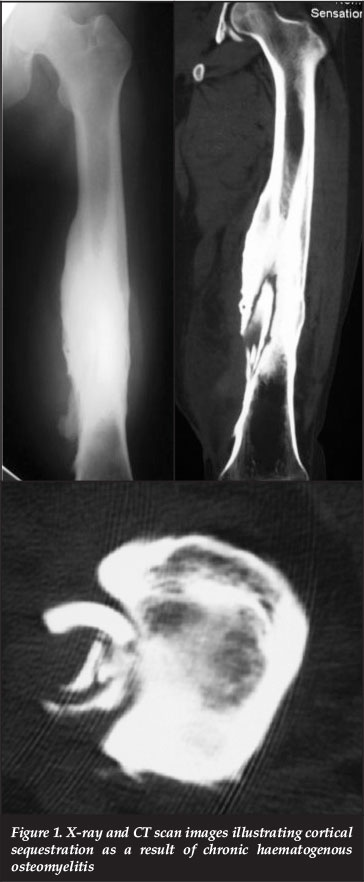
Ultrasonic waves do not cross cortical bone but ultrasound is still useful in the assessment of the presence of periosteal reaction or purulent collections. Ultrasound may also be utilised as a guide during deep aspiration of fluid collections for culture and sensitivity. X-rays and CT scanning are useful in localising sequestra or cloacae and also aid in the assessment of skeletal integrity and stability (Figure 1).
MRI has evolved as the modality of choice, especially in light of the modern oncologically oriented approach. It provides the most accurate information on extent of disease in bone and soft tissue and is therefore especially useful when planning a marginal or wide resection6(Figure 2).

Positron emission tomography (PET) scanning has also gained popularity and has surpassed MRI as the most sensitive and specific imaging modality to diagnose the presence of infection.7 It has also been shown that 18F-FDG PET/CT is a highly sensitive and specific method in the evaluation of chronic post-traumatic infection. PET/CT allows precise anatomical localisation and characterisation, demonstrating the extent of involvement with a high degree of accuracy.8
Laboratory investigations
As is the case with imaging modalities, laboratory investigations may be used in several contexts. In all patients a comprehensive haematological and biochemical profile, including a full blood count, renal and liver function tests, as well as an electrolyte and nutritional profile is required in order to stratify the host's physiological status. In addition, supplementary tests may be required to ascertain the degree of systemic compromise as a result of certain specific disorders. Examples include HbA1C assessment in the case of diabetes mellitus, creatinine clearance in patients with chronic renal failure, and CD4 counts and viral loads in HIV-infected individuals.
The second capacity in which laboratory studies can be utilised is as a diagnostic tool in the confirmation of the presence of sepsis. In this respect Lautenbach has shown that iron studies are particularly useful with an increased ferritin:iron ratio (in excess of 7), a decrease in iron saturation, as well as a decrease in mean cell volume and mean cell haemoglobin, all pointing to the presence of underlying infection.9 Routine infection markers, including the leukocyte count (WBC), erythrocyte sedimentation rate (ESR), and C-reactive protein level (CRP) may be used in both the diagnosis of the presence of infection as well as the follow-up of the patient. It should however be kept in mind that the WBC and ESR may be normal in extensive non-inflamed, and localised lesions (grade 6 and 7 infections according to the Lautenbach classification system). Pro-calcitonin (PCT) is currently routinely used in the diagnosis of the presence of severe infections in critically ill patients.10 Pro-calcitonin however has a limited role in the diagnosis of the presence of osteoarticular infection, with a sensitivity of only 16.6% in osteomyelitis and 33% in peri-prosthetic infections.11,12 In addition PCT does not appear to be superior to CRP in the post-operative follow-up of patients.13 In contrast to pro-calcitonin, the combination of abnormal CRP and inteleukin-6 has been shown to be 100% sensitive in the diagnosis of deep infection in the presence of an implant.10
On the other hand tumour necrosis factor and interleukin-8 have been shown to be elevated in acute, but not in chronic post-traumatic osteomyelitis.14 These pro-inflammatory cytokines have unfortunately not been studied in the setting of chronic haematogenous osteomyelitis.
Positron emission tomography (PET) scanning has also gained popularity and has surpassed MRI as the most sensitive and specific imaging modality to diagnose the presence of infection
Host stratification and optimisation
Following the confirmation of the presence of infection and determination of the severity of the disease, attention should shift towards accurate anatomical and physiological classification. Numerous classification systems have been described. The Cierny and Mader system remains the most popular classification system in use today. The most important decision is to embark on either a curative or palliative treatment strategy. Once a curative management option is selected emphasis should be placed on host optimisation, and modifiable risk factors should be addressed. Reversal of these risk factors will improve the outcomes in B-hosts to more closely resemble the results seen in A-hosts.15 Cessation of smoking, tight glycaemic control and dietary supplementation, for example, take precedence over any surgical intervention.
Pathogen identification
Cierny has previously recommended that attempts be made to identify the pathogen prior to the first formal surgical debridement through biopsy of deep granulation tissue.16 This view is not uniformly held and not routinely implemented. In cases without significant local or systemic septic complications, pathogen detection may be delayed to after the primary debridement procedure. In certain scenarios pre-operative ('neo-adjuvant') antibiotics may be mandatory, for example in patients with significant local (cellulitis in the region of the incision) or systemic compromise (systemic sepsis or septic shock). In such cases pre-operative identification of the causative organism is essential and samples for microscopy, culture and sensitivity (MCS) should be obtained either through open biopsy or deep aspiration under ultrasound guidance, prior to definitive surgery.
Contrary to popular belief swab culture from a sinus may offer some diagnostic benefit. Firstly, the identification of methicillin-resistant S. aureus (MRSA) or vancomycin-resistant enterococcus necessitates the implementation of stringent infection control measures during hospitalisation. Secondly, isolation of S. aureus from a superficial culture has a high degree of correlation with deep cultures.17
In cases without significant local or systemic septic complications, pathogen detection may be delayed to after the primary debridement procedure.
Surgical management
Debridement techniques
As is the case with musculoskeletal tumours, eradication of chronic osteomyelitis can only be achieved through adequate resection. Chemotherapy only plays an adjuvant role. Unless a palliative treatment pathway has been chosen, all necrotic or ischaemic tissues should be excised.16 All foreign bodies and surgical implants need to be removed, with the exception of early infection following osteosynthesis where union is expected to occur. Soft tissues, and especially scar tissue, should be resected to a supple, well-perfused margin.18 In terms of the bony debridement several techniques are currently available including simple sequestrectomy, intra-medullary reaming (indirect unroofing), tangential excision (direct unroofing), segmental resection and amputation. Despite the fact that several techniques have been described in order to determine the viability of bone, 'point-of-care testing' (POCT) remains the most trustworthy tool.19 This technique involves intra-operative assessment of bone colour, bone sound, bone texture, as well as the quality of the cancellous bone and surrounding soft tissues in order to distinguish vital bone from vital-affected bone or devitalised bone. Devitalised bone should be excised to the point where punctate bleeding, also known as the 'paprika sign', is noted.20
Schmidt et al. illustrated that osteitis can only truly be eradicated through complete resection of all infected bone, and that remaining infected or devitalised bone segments may act as a source for persistent infection or result in late reactivation. On the other hand, the authors pointed out that affected bone may recover when it is surrounded by vital, healthy soft tissue.21 When contemplating the extent of the debridement the anatomic nature of the disease, the physiological condition of the host and the proposed skeletal reconstruction technique should be considered. Compromised hosts, for example, are theoretically best treated with a wide resection of all infected tissues and subsequent limb recon-struction.22 But herein resides one of the main problems a surgeon faces when dealing with a compromised host. Wide resection and limb reconstruction is advised to achieve cure, but the reconstruction procedures required, typically involving bone transport or extensive bone grafts, are fraught with danger in the poor host and failure frequently results in the amputation of the limb.
Pathogen detection
Routine microscopy, culture and sensitivity (MCS) of tissue, bone and exudates taken under aseptic condition in the absence of antibiotic therapy in the preceding ten days, still serves as the primary diagnostic modality in order to confirm the presence of infection.23 Multiple samples should be acquired early in the procedure from fluid collections, soft tissue, bone and foreign materials or sequestra.
Samples should undergo aerobic and anaerobic incubation for prolonged periods, at least seven days, in order to increase detection of fastidious organisms.
Owing to the fact that only a minor fraction of biofilm-based micro-organisms is planktonic in nature (and thus available for culture) and small colony variants may enter a latent metabolic state, traditional culture techniques are frequently unreliable in the identification of the causative pathogen embedded in the biofilm covering implants or necrotic bone. Culture yield from implants or sequestra can be enhanced through sonication, a process utilising ultrasound to shear organisms from the biofilm on the substrate.24 This technique may be especially valuable in low-grade periprosthetic infections.
Molecular methods have, however, grown rapidly as the method of choice in pathogen detection. These techniques are based on characterisation of the causative organism's genome. Polymerase chain reaction (PCR) pyrosequencing is currently the most popular technique. It can be performed on any specimen and is able to reliably identify the micro-organism involved, irrespective of its phenotype (culturability), prior antibiotic therapy or metabolic state.18
Dead space management
Several alternatives are available to deal with the dead space resulting from the excision of necrotic and devitalised tissue. Contemporary techniques include gentamycin-impregnated polymethylmethacrylate (PMMA) beads, Lautenbach irrigation systems, physician-directed antibiotic-impregnated PMMA spacers or intra-medullary nails, as well as antibiotic-loaded calcium sulphate pellets. All of these methods incorporate local adjuvant antibiotics, aimed at eradicating persistent bacterial contamination. The choice of dead space management is generally determined by the patho-anatomical nature of the disease and the volume of the dead space. Continuous irrigation, as popularised by Lautenbach, remains a versatile dead space management technique and is commonly utilised in Cierny and Mader type I post-operative infections.25,26 Alternatively, antibiotic-impregnated PMMA intramedullary nails may be used in type I infections, especially in the setting of post-operative sepsis.27 Dead space following debridement of type II lesions are typically dealt with through local or free soft tissue transfer procedures. Gentamycin-impreg-nated PMMA beads remain useful in type III lesions despite the fact that they require removal at a subsequent procedure. This disadvantage has prompted the use of several alternative, absorbable products including antibiotic-impregnated lyophilised collagen sponge, calcium sulphate pellets and bioactive glass.28,29 Concerns have, however, been raised regarding the occurrence of aseptic wound dehiscence with the use of calcium sulphate pellets.30 Antibiotic-impregnated PMMA spacers, commonly utilised in the setting of peri-prosthetic infection, have gained much popularity in the management of other Cierny and Mader anatomical type IV infections following the encouraging results with the induced-membrane technique reported by Masquelet31(Figure 3).

The optimal composition of physician-directed antibiotic-impregnated PMMA spacers has been investigated. Using a combination of antibiotic agents improves antibiotic release and inhibition of bacterial growth.32 Gentamicin/vancomycin-loaded spacers were most effective against S.epidermidis and MRSA, while gentamicin/teicoplanin-combination spacers showed the best results against E. faecalis and S. aureus. Proportional weights of up to approximately 5 weight/weight % (2 g vancomycin per 40 g cement powder) have a negligible influence on the mechanical strength of the cement.33 When mechanical strength is not a consideration, the antibiotic content may be increased to 10%, although concentrations as high as 20% have been used.34,35 As a result of the formation of a richly vascularised membrane around the PMMA spacer, this form of dead space management has become known as the induced membrane or Masquelet technique.31 This technique offers several mechanical and biological advantages. The first is the fact that the induced membrane, which can be likened to an artificial periosteum, secretes several growth factors including VEGF and BMP-2.36 Furthermore extracts from the membrane have been shown to stimulate bone marrow cell proliferation and differentiation to osteoblastic lineage. These factors combine to result in reduced resorption of cancellous bone graft inside the membrane.31 Secondly, as illustrated in an animal model, the induced membrane prevents adjacent soft tissue from protruding into the defect, adheres to the resected bone edges and does not collapse following removal of the spacer, thus delineating a cavity corresponding to the volume of the retrieved cement spacer.37
This so-called 'spacer effect' has proven very useful in the reconstruction of bone defects, where the resulting cylinder forms a stable receptacle for bone graft and also serve as a framework through which a bone segment may be transported.
Skeletal stabilisation
It is has been shown that skeletal stability results in a statistically significant reduction in the incidence of infection following open fractures.38 This principle also applies to skeletal reconstruction following debridement of infected bone. In an animal model it was found that the union of an infected fracture is directly related to the degree of bony stability.39 The theory is that stability promotes revascularisation, resulting in enhanced perfusion and maximisation of the host's immune response.40
A variety of fixation options is currently available, although external fixation is generally preferred. Intramedullary PMMA nails do provide some stability, but cannot achieve the level of stability provided by external fixation. Curtis and colleagues found, in an experimental model, that infected osteotomies stabilised with external fixation had fewer and less severe infections than those stabilised with either a reamed or unreamed intra-medullary nail.41 Circular external fixators have gained much popularity in the field of post-infective reconstruction as a result of their modularity, minimally invasive nature and ability to effect bone transport and deformity correction.
Stability promotes revascularisation, resulting in enhanced perfusion and maximisation of the host's immune response
The attributes of fine wire fixators, in particular, are commonly used in the setting of septic non-unions and post-infective skeletal reconstruction. When dealing with a bone transport docking site, for example, the aim is to create the optimal biological milieu through the use of osteo-inductive materials in combination with the ideal mechanical environment. External fixation cannot achieve the level of stability required for primary bone healing, and union is therefore generally achieved through enchondral ossification. As predicted by the inter-fragmentary strain theory this can only be achieved under conditions resulting in inter-fragmentary strain of 2 to 10%.42 This mechanical environment can reliably be created through the use of fine wire circular fixators. Tensioned fine wires exhibit increased axial stiffness with higher loads.43 This non-linear, load-dependent axial stiffness is similar to the viscoelastic properties of tendons and ligaments. As result of these biomechanical attributes fine wire circular external fixators can be described as the only true form of true biological fixation.44
Conclusion
Many questions regarding the management of chronic osteomyelitis remain unanswered. The wide variety of treatment options currently available, combined with the advances in our surgical reconstruction abilities, makes disease classification and accurate host stratification now more important than ever.
A dilemma commonly encountered is whether to embark on a palliative or curative treatment pathway. Although existing classification systems do offer some guidelines, the lack of objective selection criteria makes the decision a subjective one. The critical significance of correct patient selection is epitomised by the fact that failure of a curative (limb reconstruction) strategy invariably results in amputation of the involved limb.
Surgical debridement offers definite advantages in terms of achieving eradication of infection. However, not all cases require surgical intervention in order to achieve quiescence and certain patients may be successfully treated with antibiotics alone. In the second part of this series on the management of chronic osteomyelitis, the principles of antibiotic therapy, as well as the current concepts in postdebridement reconstruction, will be explored.
References
1. Weiland AJ, Moore JR, Daniel RK. The efficacy of free tissue transfer in the treatment of osteomyelitis. J Bone Joint Surg Am 1984;66-A:181-93. [ Links ]
2. Hall BB, Fitzgerald RH, Rosenblatt JE. Anaerobic osteomyelitis. J Bone Joint Surg Am 1984;65-A:30-35. [ Links ]
3. Cierny G, DiPasquale D. Adult osteomyelitis protocol. Osteomyelitis.com; Avaliable from: http://www.osteomyelitis.com/pdf/treatment_protocol.pdf.Last accessed 05 March 2013. [ Links ]
4. Walter G, Kemmerer M, Kappler C, Hoffmann R. Treatment Algorithms for Chronic Osteomyelitis. Dtsch Arztebl Int 2012;109(14):257-64. [ Links ]
5. Cierny G, DiPasquale D. Treatment of chronic infection. J Am Acad Orthop Surg 2006;14:S105-S110. [ Links ]
6. Gross T, Kaim AH, Regazzoni P, Widmer AF. Current concepts in posttraumatic osteomyelitis: A diagnostic challenge with new imaging options. J Trauma 2002;52:1210-19. [ Links ]
7. Termaat MF, Raijmakers PGHM, Scholten HJ, et al. The accuracy of diagnostic imaging for the assessment of chronic osteomeylitis: A sytematic review and meta-analysis. J Bone Joint Surg Am 2005;87-A:2464-71. [ Links ]
8. Hartmann A, Eid K, Dora C, et al. Diagnostic value of 18F-FDG PET/CT in trauma patients with suspected chronic osteomyelitis. Eur J Nucl Med Molec Imag 2007;34(5):704-14. [ Links ]
9. Lautenbach EEG. Calibrating the Systemic Effects of Infection with Laboratory Investigations. European Bone and Joint Infection Society Congress, S11.2, September 2009,Vienna, Austria. J Bone Joint Surg Br 2011; 93-B (Supp III):S333. [ Links ]
10. Simon L, Gauvin F, Amre DK, et al. Serum pro-calcitonin and C-reactive protein levels as markers of bacterial infections: a systematic review and meta-analysis. Clin Infect Dis 2004;39:1867-68. [ Links ]
11. Faesch S, Cojoru B, hennequin C, et al. Can procalcitonin measurement help the diagnosis of osteomyelitis and septic arthritis? A prospective trial. Italian J Pediatrics 2009;35:33. doi:10.1186/1824-7288-35-33 [ Links ]
12. Bottner F, Wegner A, Winkelmann W, et al. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br 2007;89-B(1):94-99. [ Links ]
13. Uckay I, Garzoni C, Ferry T, et al. Postoperative serum procalcitonin and C-reactive protein levels in patients with orthopaedic infections. Swiss Med Weekly 2010;140:w13124 [ Links ]
14. Evans CAW, Jellis J, Hughes SPF, et al. Tumour necrosis factor-a, interleukin-6, and interleukin-8 secretion and the acute-phase response in patients with bacterial and tuberculous osteomyelitis. J Inf Dis 1998;177:1582-87. [ Links ]
15. Cierny G, Zorn K. Segmental tibial defects: Comparing conventional and Ilizarov methodologies. Clin Orthop Relat Res 1994;301:118-23. [ Links ]
16. Cierny G, Mader JT, Penninck JJ. A clinical staging system for adult osteomyelitis. Contemporary Orthopaedics 1985;10:17-37. [ Links ]
17. Mackowiak PA, Jones SR, Smith JW. Diagnostic value of sinus tract cultures in chronic osteomyelitis. JAMA 1978;239:2772-75. [ Links ]
18. Cierny G. Surgical treatment of osteomyelitis. Plast Reconstr Surg 2011;127(1) Suppl:190S-204S. [ Links ]
19. Tiemann AH, Schmidt HGK, Braunschweig R, Hofmann GO. Strategies for the analysis of osteitic bone defects at the diaphysis of long bones. Strat Traum Limb Recon 2009;4:13-18. [ Links ]
20. Mader JT, Calhoun JH, Lazzarini L. Adult long bone osteomyelitis. In: Calhoun JH, Mader JT, eds. Musculoskeletal Infections. 1st Edition. New York, NY. Marcel Dekker, Inc.2003:149-82. [ Links ]
21. Buhler M, Engelhardt M, Schmidt HGK. Septische postoperative komplikationen. Springer, Wien. ISBN 3-211-838112:174-86. [ Links ]
22. Simpson AH, Deakin M, Latham JM. Chronic osteomyelitis. The effect of the extent of surgical resection on infection-free survival. J Bone Joint Surg Br 2001;83:403-407. [ Links ]
23. McNally M, Nagarajah K. Osteomyelitis. Orthop Trauma 2010;24(6):416-29. [ Links ]
24. Kobayasjo M, Bauer TW, Tuohy MJ, et al. Brief ultrasonication improves detection of biofilm-formative bacteria around metal implants. Clin Orthop Relat Res 2007;457:210-13. [ Links ]
25. Lautenbach E. Chronic osteomyelitis: irrigation and suction after surgery. Bone Joint Surg Br 1975;57-B(2):245-62. [ Links ]
26. Hashmi MA, Norman P, Saleh M. The management of chronic osteomyelitis using the Lautenbach method. J Bone Joint Surg Br 2004;86-B:269-75. [ Links ]
27. Madangopal S, Seligson D, Roberts CS. The antibiotic cement for infection after tibial nailing. Orthopedics 2004;27(7):709-12. [ Links ]
28. McKee MD, Wild LM, Schemitsch EH, et al. The use of anantibiotic-impregnated, osteoconductive, bioabsorbable bone substitute in the treatment of infected long bone defects: Early results of a prospective trial. J Orthop Trauma 2002;16:622-27. [ Links ]
29. Lidfors NC, Hyvonen P, Nyssonnen H, et al. Bioactive glass S53P4 as bone graft substitute in the treatment ofosteomyelitis. Bone 2010;47:210-18. [ Links ]
30. Robinson D, Alk D, Sandbank J, Farber R, Halperin N. Inflammatory reactions associated with a calcium sulfate boneTransplantation Society 1999;4(3-4):91-97. [ Links ]
31. Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin N Am 2010;41:27-37. [ Links ]
32. Anagnostakos K, Kelm J, Regitz T, et al. In vitro evaluation of antibiotic release from and bacteria growth inhibition by antibiotic-loaded acrylic bone cement spacers. J Biomed Mater Res B Appl Biomater 2005;72(2):373-78. [ Links ]
33. Lautenschlager EP, Jacobs JJ, Marshall GW, et al. Mechanical properties of bone cements containing large doses of antibiotic powders. J Biomedical Mater Res 1976;10(6):929-38. [ Links ]
34. Hsieh P-H, Shih C-H, Chang Y-H, et al. Treatment of deep infection of the hip associated with massive bone loss. Two-stage revision with an antibiotic-loaded interim cement prosthesis followed by reconstruction with allograft. J Bone Joint Surg Br 2005;87(6):770-75. [ Links ]
35. Anagnostakos K, Furst O, Kelm J. Antibiotic-impregnated PMMA hip spacers. Acta Orthopaedica 2006;77(4):628-37. [ Links ]
36. Viateau V, Bensidhoum M, Guillemin G, et al. Use of the induced membrane technique for bone tissue engineering purposes: Animal studies. Orthop Clin N Am 2010;41:49-56. [ Links ]
37. Viateau V, Guillemin G, Yang YC, et al. A technique for creating critical-size defects in the metatarsus of sheep for use in investigation of healing of longbone defects. Am J Vet Res 2004;65:1653-57. [ Links ]
38. Worlock P, Slack R, Harvey L, Mawhinney R. The prevention of infection in open fractures: an experimental study of the effect of fracture stability. Injury 1994;25(1):31-38. [ Links ]
39. Rittman WW, Perren SM. Cortical bone healing after internal fixation and infection. Springer-Verlag. 1974. [ Links ]
40. Rodner CM, Browner BD, Pestani E. Chronic osteomyelits. In: Skeletal Trauma. Saunders. 2003:483-506. [ Links ]
41. Curtis MJ, Brown PR, Dick JD, et al. Contaminated fractures of the tibia: A comparison of treatment modalities in an animal model. J Orthop Res 1995;13:286. [ Links ]
42. Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: Choosing a new balance between stability and biology. J Bone Joint Surg Br 2002;84-B:1093-110. [ Links ]
43. Caja VJ, Kim W, Larsson S, Chao EYS. Comparison of the mechanical performance of three type of external fixators: linear, circular and hybrid. Clin Biomech 1995;10:401-406. [ Links ]
44. Ferriera N, Mare PH, Marais LC. Circular external fixator application for midshaft tibial fractures: Surgical technique. SA Orthop J 2012;11(4):39-42. [ Links ]
 Correspondence:
Correspondence:
Dr LC Marais
Department of Orthopaedic Surgery Grey's Hospital School of Clinical Medicine
University of KwaZulu-Natal
Private Bag X9001 Pietermaritzburg 3201
Tel: +27 033 897 3299
Fax: +27 33 897 3409
Email: Leonard.Marais@kznhealth.gov.za
The content of this article is the sole work of the authors. The primary author has received a research grant from the South African Orthopaedic Association for research relating to chronic osteomyelitis.
This article is also available online on the SAOA website (www.saoa.org.za) and the SciELO website (www.scielo.org.za). Follow the directions on the Contents page of this journal to access it.














