Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SA Orthopaedic Journal
On-line version ISSN 2309-8309
Print version ISSN 1681-150X
SA orthop. j. vol.13 n.1 Centurion Jan./Mar. 2014
INFECTION
The classification of chronic osteomyelitis
LC MaraisI; N FerreiraII; C AldousIII; TLB le RouxIV
IMBChB, FCS Orth (SA), MMed (Orth). Tumour, Sepsis and Reconstruction Unit, Department of Orthopaedics, Grey's Hospital, University of KwaZulu-Natal
IIBSc, MBChB, FC Orth (SA), MMed (Orth). Tumour, Sepsis and Reconstruction Unit, Department of Orthopaedics, Grey's Hospital, University of KwaZulu-Natal
IIIBSc, BSc (Hons), MSc, PhD. Medical Research Scientist, School of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal
IVMBChB, FCS Orth (SA), MMed (Orth). Professor and Head of Department, Department of Orthopaedics, I Military Hospital, University of Pretoria
ABSTRACT
As a result of the heterogeneous nature of chronic osteomyelitis and the complexity of management strategy formulation, more than ten classification systems have been published over the past 40 years. Historical systems, used in the classification of chronic osteomyelitis, remain useful in terms of the description of the nature and origin of the disease. They fail, however, to provide the user with sufficient information in order to select the appropriate treatment strategy. As a result, more comprehensive classifications have subsequently been proposed. Accurate host stratification, in particular, is considered to be essential. The physiological status of the host serves as the primary indicator of the patient's ability to effect healing of bone and soft tissues, as well as their ability to launch an effective immune response in conjunction with antibiotic therapy. Despite the development of more comprehensive classification systems, many shortcomings remain within the domain of disease classification and host stratification.
Key words: osteomyelitis, chronic, classification.
Introduction
Chronic osteomyelitis, as a clinical entity, encompasses a wide array of clinical scenarios, including chronic haematogenous osteomyelitis, post-traumatic osteomyelitis, periprosthetic infections and contiguous osteomyelitis. Owing to the heterogeneous nature of disease, the wide variety of patients affected and the multitude of factors that need to be considered during the formulation of a treatment strategy, more than ten classification systems of chronic osteomyelitis have been published over the past 40 years. None of these classifications is universally accepted. Some of the systems simply classify the nature of the disease while others attempt to guide the treating surgeon on certain aspects of the management of chronic osteomyelitis or enable comparison of the outcome of different treatment strategies.1
Formulating the appropriate management strategy, albeit palliative or curative, is a complex task. The decision-making process requires consideration of multiple factors including the impairment resulting from the disease, the patient's functional requirements, local and systemic risk factors, the anatomic nature of the disease and the realistic goals of therapy. When considering the risk-benefit ratio of any proposed management strategy, the host's physiological status remains the main determinant of the risk involved with a specific intervention. This is illustrated by previous studies which have identified the physiological status of the host as the most important predictor of treatment failure.2 The significant impact of inadequate or incorrect host stratification and risk assessment is epitomised by the fact that failure of a curative (limb reconstruction) strategy often results in the inevitable amputation of the involved limb.
This article aims to review the available classification systems for chronic osteomyelitis and highlight some of their shortcomings. Furthermore we will evaluate how the existing classification systems relate to new and evolving principles and techniques utilised in the management of chronic osteomyelitis.
Historical perspectives
Traditionally, osteomyelitis has been classified according to the system described by Waldvogel in 1970.3 This was a descriptive classification system incorporating the source of the infection (haematogenous or contiguous), the presence of generalised vascular disease and the duration of the infection (acute, sub-acute and chronic). Haematogenous chronic osteomyelitis of long bones typically presents as recurrence at a previous site of acute haematogenous osteomyelitis, while haematogenous periprosthetic infections involve seeding from a distant infective focus. Contiguous osteomyelitis may be the result of either direct inoculation (as is the case in post-traumatic and postoperative infections) or, alternatively, continuous spread from an adjacent septic focus (pressure sore or vascular ulcer, for example). As the frequency of surgical intervention increased, so did our need to classify contiguous osteomyelitis. Kelly subsequently published an aetiological classification which distinguished haematogenous from post-surgical and post-traumatic causes (with or without the presence of non-union).4
Ger's classification, published in 1977, recognised that the condition of the soft tissues plays an important role in the surgical decision-making process. According to this system the condition of the soft tissue is classified as a simple sinus, chronic superficial ulcer, multiple sinuses or multiple skin-lined sinuses.5 In 1984 Weiland et al. introduced an anatomical classification system based on the nature of skeletal involvement in order to guide the utilisation of free tissue transfers during the reconstruction process. Type I lesions were defined as soft tissue infection with exposed bone. Type II lesions were characterised as circumferential endosteal and cortical infection, while type III lesions involved endosteal and cortical infection in the presence of a segmental bone defect.
Although the abovementioned classification systems are useful in terms of describing the nature and origin of the disease, they fail to provide the treating physician with guidance regarding the management of the patient. May and Jupiter addressed these shortcomings in 1989 through the publication of their classification system, which focused on the status of the tibia and ipsilateral fibula as a guide during the selection of the appropriate reconstruction procedure (Table I).6

Gordon et al. simplified the approach to post-infective reconstruction by condensing the classification of tibial defects into three groups, namely, no significant bone loss, <3 cm of bone loss and >3 cm bone loss.7 This classification system was, however, specifically designed to prognosticate patients following free muscle transfers. Romano et al. subsequently proposed a more extensive classification system for bone defects, which included defects frequently seen following periprosthetic infections. According to this system, type 1 lesions were defined as cavitatory defects within a stable bone segment, type 2 lesions represented epiphyseal lesions with joint involvement and type 3 lesions involved a segmental bone defect. Type 3 bone defects were sub-classified as either less than 1cm, between 1 and 3 cm, or more than 3 cm.8
Prior to 2006 there was no published classification for infections following osteosynthesis. Romano et al. responded to this omission with the publication of the ICS (Infection, Callus, Stability) classification. According to this system, type I infection occurs in the presence of stable internal fixation and progression of union on serial X-rays. In terms of the management of type I infections, they suggested conservative measures until union was achieved. Type II infections were defined as infections in the presence of stable osteosynthesis without the progression of callus. The authors suggested managing this type of infection with control of the infection (as for type I), acceleration of bone healing through physical stimulation (low-intensity pulsed ultrasound, for example), biological factors (bone morphogenetic protein, platelet-rich plasma, etc.) and limited surgical procedures (e.g. dynamisation of intra-medullary nail fixation). For type III infections, involving unstable fixation and the absence of callus formation, revision surgery was recommended.
The abovementioned classification systems are all useful, especially in terms of the description of the nature and origin of the disease. With exception of the ICS classification system they fail, however, to provide the user with sufficient information to formulate a treatment strategy. The need had thus arisen to develop a more comprehensive classification system which incorporated several criteria and was able to guide the treating orthopaedic surgeon towards the correct management strategy.
Comprehensive classification systems
Cierny and Mader revolutionised our approach to osteomyelitis in 1984 through the publication of a classification system which emphasised a more holistic approach to the patient, recognising the importance of immune competency and the physiological ability of the host to effect healing.9 This system involved classification according to the host's physiological status and the anatomic nature of the disease (Table II).

The importance of the consideration of the physiological host status of patients with osteomyelitis was validated through Cierny and Mader's study involving 189 patients. The host classification facilitated the decision-making process in terms of offering the patient the alternatives of amputation or limb salvage surgery. Forty-six patients required amputation in order to achieve cure, while arrest of disease was achieved in 93.6% of patients in the limb salvage group.10
In our opinion the anatomical sub-section of the Cierny and Mader classification remains applicable today, although the definition of the subtypes has been refined over the years. Type I lesions imply infection limited to the medulla, while type II lesions refer to infection limited to the cortex. Type III and IV infections involve both medullary and cortical bone, with type IV being differentiated by the presence of instability prior to or following the debridement. Although initially included as an anatomic type IV infection, peri-prosthetic infection has subsequently also been allocated its own classification system.11
The Cierny and Mader classification however failed to provide specific, objective criteria according to which the C-host, whom they deemed unsuitable for surgery, should be defined. McPherson et al. attempted to address the shortcomings of the Cierny and Mader host classification system by modifying it to include specific objective criteria (Table III).12

The McPherson system divides patients into three classes, A, B or C, based on the number of comorbid conditions that a patient has in common with a list of 14 immune-compromising factors. Patients with no compromising factors are in class A, while patients in class B have fewer than three compromising factors. Patients in class C have three or more compromising factors and/or one of the following conditions: an absolute neutrophil count less than 1 000; a CD4 count less than 100; intravenous drug abuse; chronic active infection of another site; or dysplasia or a neoplasm of the immune system. This classification system was, however, developed specifically for use in terms of planning for second stage revision arthroplasty in patients with infection following total hip replacement. The criteria suggested by them are conservative in terms of their numerical values and may not be appropriate when applied to chronic osteomyelitis in the South African clinical setting. Several criteria have been omitted, with specific reference to physical impairment, the state of the soft tissue, arterial and venous sufficiency, age, diabetic control (HbA1c), albumin and haemoglobin values, which may play a critical role in the decision-making process in the case of chronic osteomyelitis. The McPherson modification of the Cierny and Mader host classification system has, nevertheless, also been used in other clinical settings. Bowen and Widmaier looked at the incidence of infection following open fractures in three cohorts of patients, who were classified according to the McPherson modification.13 They found that type B hosts were 2.86 times, and type C hosts 5.72 times more likely than type A hosts to develop infection following open fractures.
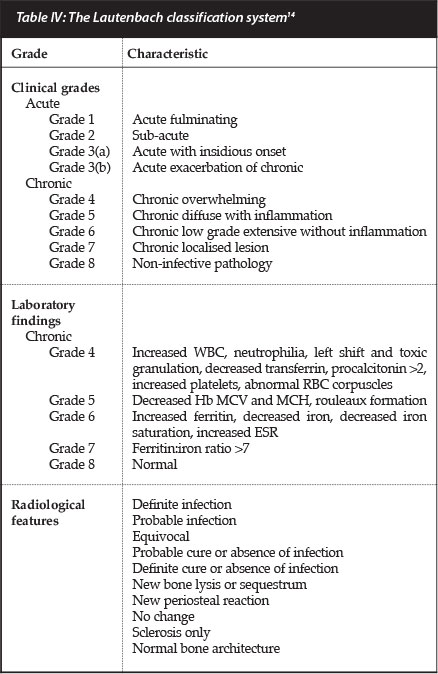
Lautenbach developed a staging system that integrates clinical, laboratory and radiological features in an incremental manner.14 This classification is based on the severity of the disease and describes certain characteristic laboratory abnormalities which may be utilised to confirm the presence underlying infection in equivocal cases.
The classification system consists of eight escalating grades of severity (three grades of acute and five grades of chronic osteomyelitis), which are each defined by characteristic clinical and laboratory features (Table IV). As the grades of chronic osteomyelitis increase in intensity we see progressive abnormalities of the laboratory findings, especially in terms of iron studies, which may then be utilised in the diagnosis and stratification of disease severity.
Recently Romanò et al. again highlighted the shortcomings of the Cierny and Mader host stratification system as a subjective evaluation of the host's physiological ability to deal with infection.15 Their Seven-Item Comprehensive Classification System (SICCS) of bone and joint infections for adults is based on the clinical presentation, aetiopatho-genesis, anatomo-pathological characteristics (incorporating the Cierny and Mader anatomical sub-section for long bones), the McPherson modification of host classification (further subdivided according to age as less than 2 years, less than 14 years and more than 14 years of age), causative microorganism, the bone defect (in accordance with Romano's earlier classification system), as well the state of the soft tissues (Table V).
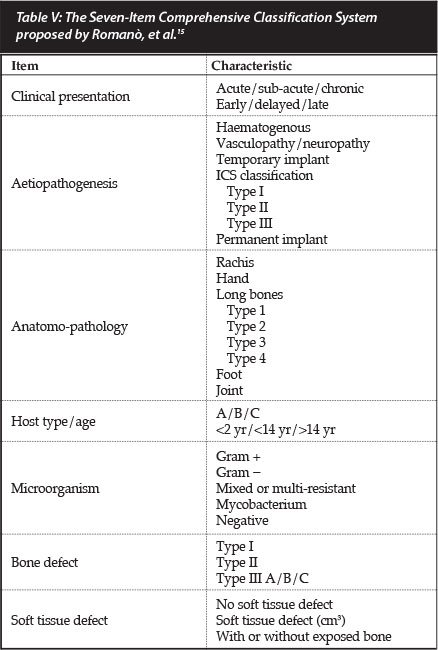
The SICCS is descriptive in nature, incorporating existing classification systems. In contrast with the Cierny and Mader classification system it was not designed to guide management, but is rather intended for didactic and scientific purposes in order to compare results from different clinical trials.
Importance of accurate host stratification
The clinical manifestations of osteomyelitis are the result of the complex interplay between the host's immune defence system and the causative organisms' attempts to establish a biofilm-based colony on a sequestrum, surgical implant or foreign body. The host's physiological status in particular, has been identified as a crucial factor, determining the course and clinical manifestations of the disease. The host status also serves as the primary indicator of the patient's ability to effect healing of bone and soft tissues, as well as their ability to launch an effective immune response in conjunction with antibiotic therapy. Without a competent immune response from the host, any attempt at surgical eradication of the infection may be futile.
The physiological host status does not only determine the suitability of a treatment strategy for the patient, be it curative or palliative, it also guides the surgeon in terms of the appropriate surgical margin during debridement. Traditional teaching regarding the surgical management of chronic osteomyelitis advocates the excision of all necrotic and ischaemic bone and soft tissue, to a clean, well-perfused surgical margin.16 The importance of the extent of debridement has been investigated in both normal and compromised hosts. Compromised patients (B-hosts) treated with marginal resection (clearance margin of <5 mm) had a higher rate of recurrence than normal patients (A-hosts), whereas a marginal resection may be acceptable in normal hosts.17 Thus, compromised hosts are theoretically best treated with a wide resection and subsequent limb reconstruction. These reconstruction procedures, involving bone transport or extensive bone grafts, are however fraught with danger, and failure invariably results in the amputation of the limb.
The decision-making process is further complicated by that fact that many patients should not receive surgery because the risk of surgery may outweigh the benefit thereof. For example, patients may have little pain and minimal disability, with only intermittent drainage from a sinus. Embarking on major limb reconstruction surgery may be inappropriate in such a case, due to the risk of ablation. Thus, further consideration should also be given to the patient's current functional status and the realistically achievable goals of treatment.
In South Africa the high prevalence of immune compromise, malnutrition and other risk factors present unique challenges during host stratification. Classifications previously devised in developed countries have been found to be either inadequate or inappropriate in a resource-poor clinical setting.
In stark contrast with the South African public sector, where approximately one-third of patients are classified as C-hosts, developed countries deal with a much lower percentage. In a review of 2 207 patients seen over approximately 30 years, Cierny reported an incidence of only 4% type C-hosts in his American practice.18 Clinical experience in South Africa has therefore revealed the need for accurate and objective host stratification to enable the selection of a safe, appropriate and patient-specific treatment plan. Ultimately the patient's physiological status should be considered as a critical factor during the formulation of the appropriate treatment strategy for an individual.
Shortcomings of existing classification systems
The first major shortcoming of existing classification systems relates to host stratification. The stratification strategies currently available have failed to determine specific objective criteria whereby which patients who are unsuitable for a curative management strategy (a type C-host) can be identified. According to Cierny type C-hosts should not be offered definitive care, but rather palliated or simply treated expectantly.18 The type C-host, as defined by Cierny and Mader, is a patient in whom the risk or morbidity of treatment outweigh the benefits thereof or, in other words, the treatment or results of treatment of chronic osteomyelitis are more compromising to the patient than the disability caused by the disease itself. This definition encompasses a large group of patients, including patients with minimal disability as a result of the disease as well as patients who are not suitable candidates for complex bone and/or soft tissue reconstruction. The limitation of this definition is the fact that it is subjective (with a poor inter-observer reliability), case dependent and susceptible to widely varying interpretation depending on the surgeon's experience.
The second limitation of existing chronic osteomyelitis classification systems lies in the patho-anatomical characterisation of lesions. There is currently no universally accepted classification system for either bone or soft tissue defects. The problem is further confounded by the fact that the magnitude of a bone defect that should be considered as critical and thus not manageable with cancellous bone graft, remains contro-versial.19 Older classifications systems have failed to keep up with contemporary reconstruction techniques. The classifications proposed by May and Jupiter, for example, fail to mention the induced-membrane technique popularised by Masquelet.20 Furthermore, the classification of bone defects varies widely in terms of cut-off points and each system reflects the unique preferences and abilities of the authors. While some surgeons, for example, feel comfortable transporting bone for a defect in excess of 6 cm, others would prefer the use of a vascularised fibula graft.
A problematic decision commonly faced when utilising the Cierny and Mader classification's anatomical subsection, is whether a specific lesion should be graded as a type III or type IV lesion. This decision is complicated by the fact that the distinction between the two grades is defined as instability following debridement.
The classification of a lesion as either type III or IV is, therefore, completely subjective and arbitrary, depending on the surgeon's choice of resection margin. If an infected section of bone is critical for axial stability, the surgeon has two choices: either resection of the bone with subsequent destabilisation of the limb (which will require complex reconstruction procedures), or leaving the infected bone behind and attempting to suppress the infection. The former type of wide resection with 'clear' margins (resecting any avascular material) remains the ideal, but it is frequently unachievable as it may involve resecting bone or soft tissue that is vital to the survival and function of the limb. On the other hand marginal resections may leave behind soft tissue or bone which contains bacteria and may serve as a nidus for recurrence of infection. The major limitation of the Cierny and Mader system is that it unfortunately does not provide any guidelines regarding the selection of the appropriate surgical margin.
The most prominent inadequacy of existing classification systems rests in the fact that they fail to guide the user in selecting the appropriate treatment strategy from the myriad of contemporary treatment options available. Although the Seven-Item Comprehensive Classification System, proposed by Romano et al., is useful when describing the nature of the infection, it is complex and does not offer any guidelines for the selection of the applicable treatment strategy. In fact, the authors conclude that the classification system should find application in the comparison of outcomes, rather than being used as a guide to management. This problem is not unique to the SICCS and is a feature common to the other classification systems. The treatment guidelines offered by Cierny and Mader have failed to keep up with modern trends in the surgical management of chronic osteomyelitis.10 Although the basic premise remains sound, some of the modalities suggested in the original publication has fallen out of favour. The use of open-sky (Papineau) bone grafting, for example, has been all but abandoned. This point is further illustrated by the fact that Cierny abandoned the original guidelines in a more recent publication, opting for a more generic approach to management.21
The final limitation of existing classification systems lies in the structure of the decision-making process. While there are three host types described there are only two major treatment options, namely cure or palliation.22 In order to appear logical and aid in the therapeutic decision-making process each host group should ideally be matched with its own unique management strategy. This will require revision of existing systems and the establishment of a new unified classification which incorporates all the relevant selection criteria, as well as all contemporary interventional strategies and techniques.
Conclusion
As stated by Cierny, the selection of patient-matched treatment options (for example low risk treatment in high risk patients) closes the gap in successful outcomes between health-compromised patients (B- or C-hosts) and patients without compromise (A-hosts).23 Ultimately the patient's physiological status is considered to be the single most important factor that needs to be considered when stratifying patients and during the formulation of the appropriate treatment strategy for any individual.
Despite the development of comprehensive classification systems, many shortcomings remain within the domain of disease classification and host stratification. The failure of existing classification systems to keep pace with contemporary management philosophies and modern reconstructive techniques has resulted in the need for the development of a new classification system which allows integration of host factors with the oncological-oriented approach which is currently being popularised in the surgical management of chronic osteomyelitis.
References
1. Mader JT, Shirtliff M, Calhoun JH. Staging and staging application in osteomyelitis. Clin Inf Dis 1997;25:1303-309. [ Links ]
2. Haas DW, McAndrew MP. Bacterial osteomyelitis in adults: evolving considerations in diagnosis and treatment. Am J Med 1996;101:550-61. [ Links ]
3. Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: A Review of Clinical Features, Therapeutic Considerations and Unusual Aspects. N Engl J Med 1970;282:198-206. [ Links ]
4. Kelly PJ. Infected nonunion of the femur and tibia. Orthop Clin North Am 1984;15:481-90. [ Links ]
5. Ger R. Muscle transposition for treatment and prevention of chronic traumatic osteomyelitis of the tibia. J Bone Joint Surg Am 1977;59-A:784-91. [ Links ]
6. May JW, Jupiter JB, Weiland AJ, et al. Clinical classification of post-traumatic tibial osteomyelitis. J Bone Joint Surg Am 1989;71-A(9):1422-28. [ Links ]
7. Gordon L, Chiu EJ. Treatment of infected non-unions and segmental defects of the tibia with staged microvascular muscle transplantation and bone-grafting. J Bone Joint Surg Am 1988;70-A:377-86. [ Links ]
8. Romano CL, Meani E. Il difetto osseo nelle infezioni: proposta di classificazione e opzioni di trattamento. Arch Ortop Reumatol 2006;117:14-15. [ Links ]
9. Cierny G, Mader JT. Adult chronic osteomyelitis. Orthopedics 1984;7:1557-64. [ Links ]
10. Cierny G, Mader JT, Penninck JJ. A clinical staging system for adult osteomyelitis. Contemporary Orthopaedics 1985;10:17-37. [ Links ]
11. Cierny G, DiPasquale D. Periprosthetic total joint infections. Staging, treatment, and outcomes. Clin Orthop Relat Res 2002;403:23-28. [ Links ]
12. McPherson EJ, Woodson C, Holtom P, et al. Periprosthetic total hip infection. Outcomes using a staging system. Clin Orthop Relat Res 2002;403:8-15. [ Links ]
13. Bowen TR, Widmaier JC. Host classification predicts infection after open fractures. Clin Ortho Rel Res 2005;433:205-11. [ Links ]
14. Lautenbach EEG. Calibrating the systemic effects of infection with laboratory investigations. European Bone and Joint Infection Society Congress, S11.2, September 2009,Vienna, Austria. J Bone Joint Surg Br 2011; 93-B:Supp III S333. [ Links ]
15. Romano CL, Romano D, Logoluso N, Drago L. Bone and joint infections in adults: a comprehensive classification proposal. Eur Orthop Traumatol 2011;1:207-17. [ Links ]
16. Rao N, Ziran, BH, Lipsky BA. Treating osteomyelitis: Antibiotics and surgery. Plast Reconst Surg 2011;127(1):177S-187S. [ Links ]
17. Simpson AH, Deakin M, Latham JM. Chronic osteomyelitis. The effect of the extent of surgical resection on infection-free survival. J Bone Joint Surg Br 2001;83:403-407. [ Links ]
18. Cierny G. Surgical treatment of osteomyelitis. Plast Reconstr Surg 2011;127(1)Suppl:190S-204S. [ Links ]
19. Tiemann AH, Hofmann GO. Principles of the therapy of bone infection in adult extremities. Strat Traum Limb Recon. 2009;4:57-64. [ Links ]
20. Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin N Am 2010;41:27-37. [ Links ]
21. Cierny G, DiPasquale D. Treatment of chronic infection. J Am Acad Orthop Surg 2006;14:S105-S110. [ Links ]
22. Walter G, Kemmerer M, Kappler C, Hoffmann R. Treatment algorithms for chronic osteomyelitis. Dtsch Arztebl Int 2012;109(14):257-64 [ Links ]
23. Cierny G. Patient selection in osteomyelitis. Osteomyelitis.com 2009; Available from: http://www.osteomyelitis.com/public/blog/wp-content/uploads/2009/11/Treatment-Modification3.JPG [ Links ]
 Correspondence:
Correspondence:
Dr LC Marais
Department of Orthopaedic Surgery Grey's Hospital School of Clinical Medicine University of KwaZulu-Natal
Private bag X9001
Pietermaritzburg 3201
Tel: +27 33 897 3299
Fax: +27 33 897 3409
Email: Leonard.Marais@kznhealth.gov.za
The content of this article is the sole work of the author. The primary author, LC Marais, has received a research grant from the South African Orthopaedic Association for research relating to chronic osteomyelitis.














