Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SA Orthopaedic Journal
versión On-line ISSN 2309-8309
versión impresa ISSN 1681-150X
SA orthop. j. vol.12 no.4 Centurion dic. 2013
GENERAL
Biomarkers in rheumatoid arthritis - the old and new
Mahmood MTM Ally
MBBCh(Wits), FCP(SA) Rheumatology. Adjunct Professor Department of Internal Medicine/Rheumatology, University of Pretoria, Steve Biko Academic Hospital, South Africa
ABSTRACT
Rheumatoid arthritis (RA) is the prototype autoimmune disease but related mechanisms are involved in numerous other physiological and pathological conditions such as ageing, osteoporosis, chronic osteomyelitis and fracture healing. This article is a review of cellular and molecular components of the immune-inflammatory response in RA with a focus on candidate novel biomarkers that may take us one step closer to the modern quest for personalised medicine.
Key words: rheumatoid arthritis, mediators of inflammation, pathogenesis, biomarkers
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory arthritis of unknown aetiology. An autoimmune, auto-inflammatory pathogenic mechanism is central to disease initiation and perpetuation. Recent insights into the molecular pathways characterising these pathogenic processes have not only led to numerous innovative therapies but have also provided a better understanding of the immune-inflammatory response. RA is the prototype autoimmune disease but related mechanisms are involved in numerous other physiological and pathological conditions such as ageing, osteoporosis, chronic osteomyelitis and fracture healing. Despite clinical similarities, patients with RA are a very heterogeneous group with regard to disease progression and response to therapy; thus reliable, predictive biomarkers would be invaluable.
The immune response
The innate and adaptive systems are two components of the immune system acting in concert to defend against foreign infections and to promote healing following an injury. The innate response is the initial non-specific response to foreign antigens comprising cells of the macrophage monocyte lineage, neutrophils and complement: the predominant effector mechanism being phagocytosis.
Macrophages not only phagocytose foreign tissue but also process the antigen for presentation and activation of the adaptive immune system. The adaptive response is a delayed but specific response comprising T and B lymphocytes. It is initiated by antigen presentation and activation of naive T cells. Depending on the stimulus and host factors, T cells differentiate into a TH 1 (cell-mediated response), TH 2 (humoral/antibody-mediated response), TH 17 (pro-inflammatory response) and regulatory T cells (inhibitory response). A crucial host factor is the major histocompatibility complex (MHC) or human leukocyte antigen (HLA) which is the chromosomal area important for self-recognition and displaying of antigens to T cells. T-cell activation results in a response generating specificity and memory to a particular antigen. Cytokines are molecular messengers playing an integral role in the cross talk between various components of the immune response and host, allowing for a co-ordinated and regulated response.
Pathogenesis of RA
Despite intensive research, the inciting agent triggering RA remains elusive. Genetic and environmental factors play an important role in RA susceptibility and progression of disease. Genetic markers, termed the shared epitope, have long been associated with RA.
These represent a group of amino acids found on the HLA DRB1 region of antigen-presenting cells. This may result in altered response to certain antigenic stimuli. Infections with certain organisms causing periodontal disease and smoking have been implicated in the conversion of synovial arginine to citrulline and generation of RA-specific anti-cyclic citrullinated peptide antibodies (aCCP Ab).1
Genome wide association studies have also implicated a host of other, albeit less frequent, associations with RA including polymorphisms of molecules involved in:2
- T-cell co-stimulation or B-cell interaction (CD 28,CD 40,CTLA4)
- Cytokines that regulate activation of T cells (IL2,IL 21,TNF)
- Enzymatic conversion of arginine to citrulline (PAD14).
Once initiated this pathological loss of self-tolerance results in inflammation predominantly in synovial tissues, resulting in synovial hypertrophy and pannus formation. Inflammation and tissue destruction may result in increasing the antigenic load, consequently resulting in auto-inflammation and perpetuation of disease. Synovial pannus is characterised histologically by new vessel formation, cellular and molecular components of the immune response, synovial fibroblasts and destruction of adjacent cartilage and bone (Figure 1).
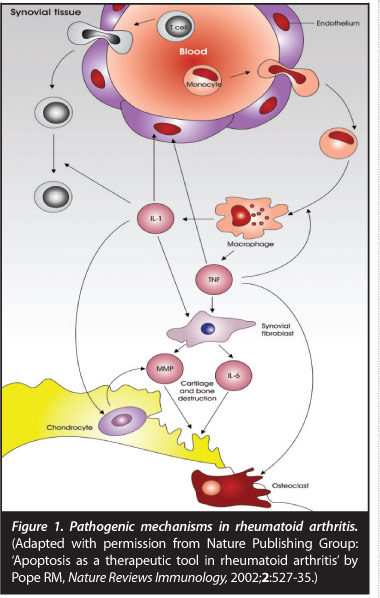
Joint destruction is mediated by pro-inflammatory cytokines tumour necrosis factor (TNF), interleukin 1 (IL 1) and inter-leukin 6 (IL 6) stimulating synovial fibroblasts, chondrocytes and osteoclasts.3 Matrix metalloproteinases cause enzymatic degradation of cartilage resulting in production of a cartilage breakdown product: cartilage oligomeric protein (COMP). Bony changes in RA include focal erosions, peri-articular osteopaenia and generalised osteoporosis. Various components of the immune-inflammatory response mediate bony resorption with specific cytokines such as receptor activator of nuclear factor icB ligand (RANKL) and osteo-protegerin (OPG) playing a key role. RANKL binds to its receptor RANK stimulating osteoclastogenesis and bony resorption. OPG acts as a decoy receptor binding to RANKL resulting in an inhibitory effect on osteoclast activation.4 Bony resorption also requires proteases such as cathepsin K and metalloproteinases to break down non-mineral matrix. More recently with MRI imaging, an area of pre-erosive disease unrelated to synovial pannus has been identified as bone marrow oedema which histologically contains macrophages, T cells, B cells and plasma cells suggestive of a primary bone marrow site of inflammation.5
Biomarkers in RA
With the recent advances in the management of patients with RA, the need for early diagnosis and rapid disease control is paramount to limit morbidity and premature mortality from RA. Clinical, imaging and laboratory biomarkers are being used to diagnose and monitor disease progression, usually as composite scores of a range of markers. Current biomarkers in use, although valuable, fail to diagnose or monitor disease activity effectively. Candidate novel markers that could contribute to improving RA management are those integrally involved in the immuno-pathogenesis of RA.
Clinical measures and acute phase response measures
Clinical measures such as joint counts for swelling or tenderness and global assessments of disease activity by the physician and patient using a visual analog scale, are commonly used to assess disease activity and response to therapy. The erythrocyte sedimentation rate and C-reactive protein (CRP) are included in some of the disease activity scores. Unfortunately even in patients with low disease activity scores, patients may have ongoing radiographic damage and significant disease progression.
Imaging markers
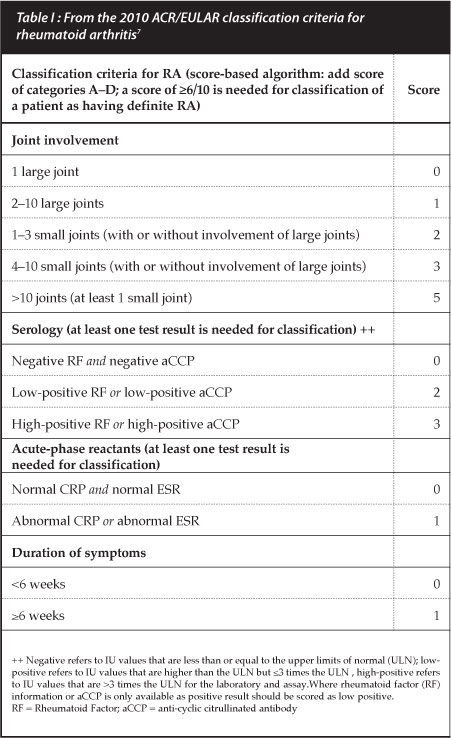
Plain X-rays of hands and feet are used as surrogate markers of disease progression with radiographic scoring taking into account the degree of bony destruction and joint space narrowing representing cartilage loss. Two popular scoring measures are the Larsen and Sharp van der Heijde methods but these are used mostly in clinical trials. The simplified erosion and narrowing score (SENS), proposed by van der Heijde may be more practical for clinical use.6 However, radiographic changes are not commonly present in early disease and are hence no longer listed in the new RA classification criteria (Table I).7
Newer imaging modalities such as ultrasound and MRI hold promise in monitoring disease progression and response to therapy early in disease management. Doppler ultrasound activity and MRI marrow oedema have been associated with erosive radiographic progression.
Genotyping
Meyer et al in a cohort of predominantly black South African RA patients, showed an association of the shared epitope with RA in 88% of patients similar to other studies in Caucasians, but the association did not add more predictive value than the presence of RA-specific anti-cyclic citrullinated peptide antibody (aCCP Ab), thus limiting the clinical utility of shared epitope genotyping.8 The presence of the shared epitope may also have prognostic value as several studies have shown an association with more aggressive disease.
Auto-antibodies
Rheumatoid factor (RF) is one of the well-established laboratory markers associated with RA but sensitivity and specificity, especially in early disease, may be as low as 50%. The addition of the aCCP Ab increases specificity to greater than 90%.1 Antibody positivity also has prognostic value with aCCP Ab being associated with erosive disease and radiographic progression. Titres of these anti-bodies are now considered important as greater than a three-fold elevation increases the likelihood of diagnosing RA, using the new criteria (Table I).7 A decrease in aCCP Ab titres with biologic DMARDs has correlated with clinical response and may be a marker of response to therapy. A cost-effective recommendation for clinical use of aCCP Ab is to request the test only if RA is suspected and RF is negative.9 The clinical utility of using these auto-antibodies to monitor disease progression has not yet been established.
Novel laboratory markers
Cytokines are important molecular components of the immune-inflammatory response and integrally involved in the pathogenesis of RA. Their importance is underscored by the success of numerous newer therapeutic agents targeting specific cytokines or their biologic pathways. Baseline RANKL levels and serum RANKL/OPG ratio may identify a subset of patients more likely to respond to TNF treatment, and patients with very high serum TNF values were found to be more refractory to treatment.10,11 Biomarkers reflecting enzymatic processes associated with breakdown of bone and cartilage have been shown to be associated with radiographic progression. Independent predictors of radiographic outcome include matrix metalloproteinase -3(MMP-3) and COMP.12 MMP- 3 has also been found to correlate with erosive disease despite a normal CRP. In a cohort of 128 early RA patients, MMP-3 levels correlated with measures of disease activity but did not add any more value than the CRP.13 Serum levels of cathepsin K have been shown to correlate with radiological damage in patients with RA.12 However, serum correlations of these novel biomarkers with disease activity or as predictors of response to treatment has been inconsistent and of limited clinical use as independent markers. Recent developments in the use of some of these novel biomarkers as composite scores together with other measures of disease activity have been made commercially available but their clinical utility needs to be validated.
Conclusion
As the aetiopathogenic mechanisms underlying RA unfold, more candidate biomarkers and potential therapeutic targets are being explored. Recent advances in RA management have brought with them challenges to select specific therapies for specific situations, and no single measure is likely to emerge as the gold standard. Composite scores using clinical, imaging and laboratory measures are likely to come closest to meeting the ideals of personalised medicine.
References
1. Puszczewicz M, Iwaszkiewicz C. Role of anti-citrullinated protein antibodies in diagnosis and prognosis of rheumatoid arthritis. Arch Med Sci 2011;7(2):1989-94. [ Links ]
2. McInnes BI, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205-19. [ Links ]
3. Cantley MD, Smith MD, Haynes DR. Pathogenic bone loss in rheumatoid arthritis: mechanisms and therapeutic approaches. Int J Clin Rheumatol 2009;4(5):561-82. [ Links ]
4. Tana S. Regulation of bone destruction in rheumatoid arthritis through RANKL-RANK pathways. World J Orthop 2013 January 18;4(1):1-6. [ Links ]
5. McQueen FM. Bone marrow edema and osteitis in rheumatoid arthritis: the imaging perspective. Arthritis Res Ther 2012;14:224. [ Links ]
6. Van der Heijde D, Dankert T, Nieman F, et al. Reliability and sensitivity to change of the simple erosion narrowing score compared with the Sharp-van der Heijde method for scoring radiographs in rheumatoid arthritis. Rheumatology 1999;38:941-47. [ Links ]
7. Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria. An American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Arthritis Rheum 2010;62:2569-81. [ Links ]
8. Meyer PW, Hodkinson B, Ally M, et al. HLA-DRB1 shared epitope genotyping using the revised classification and its association with circulating autoantibodies, acute phase reactants, cytokines and clinical indices of disease activity in a cohort of South African rheumatoid arthritis patients. Arthritis Res Ther 2011;13(5):R160. [ Links ]
9. Hodkinson B, Meyer P, Musenge E, et al. The diagnostic utility of the anti-CCP antibody test is no better than rheumatoid factor in South Africans with early rheumatoid arthritis. Clin Rheumatol. 2010 Jun;29(6):615-18. [ Links ]
10. González-Alvaro I, Ortiz MA, Tomero EG, et al. Baseline serum RANKL levels may serve to predict remission in rheumatoid arthritis patients treated with TNF antagonists. Ann Rheum Dis 2007;66:1675-78. [ Links ]
11. Halilova IK, Brown EE, Morgan SL, et al. Markers of Treatment Response to Methotrexate in Rheumatoid Arthritis: Where Do We Stand? International Journal of Rheumatology volume 2012, Article ID 978396, 7 pages. [ Links ]
12. Karsdal AM, Woodworth T, Henriksen K, et al. Biochemical markers of ongoing joint damage in rheumatoid arthritis-current and future applications, limitations and opportunities. Arthritis Res Ther 2011;13:215. [ Links ]
13. Ally M, Hodkinson B, Meyer P, et al. Serum matrix metallo-proteinase-3 in comparison with acute phase proteins as a marker of disease activity and radiographic damage in early rheumatoid arthritis. Mediators Inflamm 2013;2013:1836563. [ Links ]
 Correspondence:
Correspondence:
Prof M Ally
PO Box 13044 Laudium 0037
Tel: 012 354 1277 Fax: 086 663 9874
Email: tar@up.ac.za














