Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SA Orthopaedic Journal
versão On-line ISSN 2309-8309
versão impressa ISSN 1681-150X
SA orthop. j. vol.11 no.4 Centurion Jan. 2012
CLINICAL ARTICLE
Massive bone loss around the knee - the orthopaedic oncological perspective
K Hosking
FCOrth, MBChB. (UCT)Orthopaedic Surgeon, Groote Schuur Hospital and Life Orthopaedic Hospital (SA). Department of Orthopaedic Surgery, University of Cape Town
ABSTRACT
Massive bone loss, as a consequence of tumour resection, has been dealt with historically by a variety of techniques. This article describes the techniques commonly used in the management of intercalary, intra-articular and extra-articular resections with uncontained bone defects.
Key words: bone tumour, knee, reconstruction, endoprosthetic replacement, allograft, vascularised fibula, massive bone defect.
Introduction
Prior to 1970 primary bone sarcomas had a less than 25% five-year survival and the primary surgical treatment was amputation. With the advent of chemotherapy and more advanced imaging techniques, it became evident that limb-sparing surgery was possible without affecting the overall oncological outcome and survival. Today patients with non-metastatic primary bone sarcoma have an up to 65% cure rate.
The most common indication for major structural allografts and endoprostheses is in orthopaedic oncological conditions. Seventy per cent of all primary bone sarcomas occur around the knee joint and are metaphyso-diaphyseal with frequent epiphyseal involvement. The management of these lesions is currently wide resection and adjuvant/neoadjuvant chemotherapy. Radiation is occasionally used post surgery.In 85% of extremity bone sarcomas, limb-sparing surgery is possible.
Patients with Anderson Orthopaedic Research Institute (AORI) type 3 defects requiring revision total knee arthroplasty (TKA) in the non-oncological population will occasionally require a so called 'tumour prosthesis' or bulk structural allograft when more conventional methods of reconstruction are not possible.
The focus of this paper is on the management of bone deficiency in patients with primary bone tumours. Massive bone loss around the knee has been managed historically with a massive tumour implant, an allograft or an allograft/prosthetic composite.
Options for reconstruction here are diverse and it is of utmost importance that the most effective technique, with the lowest complication rate, is used because a delay in resumption of chemotherapy (after more than 21 days post surgery) is associated with a poorer prognosis.1
The technique of reconstruction used is dependent on the age of the patient, the size and location of the tumour, the histological grade, surgeon preference/familiarity and availabilityof graft material and implants, namely, bulk allograft and custom endoprosthetics.
Classification
The majority of bone tumours will not involve a joint directly. In orthopaedic oncological conditions resulting in major bone loss, the resection and the remaining surgical defect have been classified by Enneking2 as follows (Figure 1):
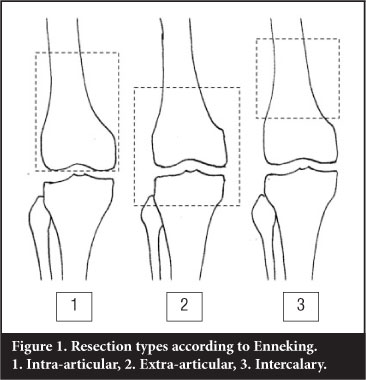
1. Intra-articular: one articular surface with the bone resected
2. Extra-articular: an extra-articular resection where the entire joint is removed en bloc
3. Intercalary: An intercalary defect involves mostly the diaphysis
Intra-articular resection
An intra-articular resection is where the joint is not involved,the tumour has not breached the joint and the resection is directly through the plane of the knee, i.e. the tibio-femoral articulation.
The majority of cases usually require an intra-articular resection of either the distal femur or proximal tibia. The affected segment of distal femur or proximal tibia is resected. This will invariably require division of the cruciate and collateral ligaments, capsule and periarticular muscle (Figure 2).

Extra-articular resection
An extra-articular resection involves the 'en bloc' resection of the joint with the resection lines being beyond the bounds of the joint.
The tumour has penetrated into the knee joint by way of direct perforation through the articular surface or via the collateral or cruciate ligaments or as a skip metastasis.The resection entails complete resection of the knee en bloc with the corresponding defect of both the tibia as well as femur. The posterior cruciate ligament inserts approximately 5 cm below the posterior joint line and the resection margin needs to be clear of this. These resections involve significant muscle loss and will often require an arthrodesis or proximal tibial and/or distal femoral prosthetic replacement and associated tendon/muscle transfers to cover the implant and power the joint (Figure 3).

Historically these massive bone defects have been reconstructed by either an endoprosthesis or a bulk allograft and considerable debate around this topic still persists.
Intercalary resection
Intercalary resection involves resection of a diaphyseal section of the bone with the joint remaining intact.
Reconstructive options in intra/extra-articular resections
Bulk allografts/Allograft prosthetic composites
Bulk allograft has the attraction of increasing bone stock as well as offering the possibility of soft tissue attachment, particularly in the proximal humerus,femur and knee. The procedure is technically demanding and graft-related complications occur in up to 43% of major bulk allografts. Major complications include infection - up to 13% (and higher in some series); non-union in 17%-38%; and graft fracture and late dissolution in 16%-47% of cases.3-7
Adjuvant chemotherapy has been shown to delay union as well as increasing fracture risk5 and radiotherapy decreases the osteo-inductivity of an implanted allograft. This makes it a less desirable option in oncology patients.
Dissolution is due to an immune response with development of class 1 lymphocytotoxic antibodies in a haematoma around the graft. This causes a T-cell-mediated response with class 1 and 2 antigens being recognised.Subsequent osteoclastic resorption at varying depths of the graft occurs and there is a chronic immune response from the depot of antigen. This process occurs to a lesser extent with processed allografts, occurring least in freeze-dried allografts,but with a concomitant reduction of graft strength andhigher risk of fracture.
Matching of bone allograft for class 2 histo-compatibility antigens is associated with improved clinical outcomes.8,9
Surgical technique can play a role. Antibiotic-laden cement filling of the allograft canal as well as adequate soft tissue cover flaps can lower infection rates. Non-union incidence can be minimised by stable fixation.
Intramedullary nailing/prosthetic stem with a step-cut interlock between graft and host bone is superior to plating (Figure 4).

The most effective form of fixation is telescoping of the host bone into the allograft as this increases the surface area available for union by a factor of 3.142 times for every 1 cm of telescoping (Figure 5). Union occurs more slowly at cortico-cortical junction externally as opposed to at metaphyseal junctions by internal callus.4

Revision TKA with structural bulk allografting is associated with an up to 20% complication rate, usually related directly or indirectly to the allograft itself.10 For this reason endoprostheses are favoured by some authors.
Osteo-articular allografts
Historically in the 1970s there was a surge of interest in the use of bulk osteo-articular allografts. The benefit of osteo-articular grafting is joint preservation and soft tissue reattachment. However high complication rates were reported, particularly infection, fracture, non-union, graft dissolution and joint instability.11,12 Donor grafts need to be carefully matched to the recipient articular surface.The articular cartilage undergoes preservation in dimethyl sulfoxide and is stored at -80°C which results in very few of the chondrocytes remaining viable. This technique is seldom utilised today because of medium- and long-term failure. Hornicek et al reported that 25% of osteo-chondral allografts required surgical intervention due to degenerative joint disease at a mean of 6.6 years.11
Allograft arthrodesis
Many authors have reported poor long-term results and poor function13 leading to high conversion rates to rotating or fixed hinge implants. Arthrodesis implants are commercially available and are technically less demanding than allograft reconstruction.
Endoprosthetic replacement
Initial attempts to bridge large bone defects with endoprosthetics were first performed by Gaenslen prior to1930, using fabricated ivory. Vitallium was subsequently used by Moore and Bohlman in 1943. Kraft and Levinthal used acrylic to reconstruct a distal femur in 1954. Monticelli and Santori advocated the use of stainless steel implants and introduced the concept of modularity in 1975. In 1975, Scales in Stanmore (UK) reported an 80% success rate using titanium segmental implants in over 300 cases. These developments have led to the availability of the modular endoprosthetic replacement implant which is now widely used in orthopaedic oncological reconstructive procedures.
Reconstruction by insertion of an endoprosthetic replacement has become more universally recognised as the procedure of choice in this situation of massive bone loss around the knee. The high failure rates of loosening with fixed hinge cemented implants - 46% risk of revision at 10 years14 have been addressed with the rotating hinge prosthesis which has a reported failure rate of 3% at 10 years.14,15 In addition the concept of composite fixation has contributed to improved outcomes.
A porous collar allowing for composite extracortical fixation16 was introduced by Chao in 1983 and subsequently most tumour endoprosthetic manufacturers have incorporated this feature in their implants (Figure 6). The concept is similar to extracortical bridging callus in bulk allografts. Endoprosthetic replacement insertion is technically easier than fashioning an allograft and has the additional benefit of permitting immediate post operative weight bearing and movement and has a less complicated post-operative course, thereby facilitating adjuvant oncological management.

Stem fixation
Three basic forms of implant stem fixation have been used in customised implant reconstruction:
Mechanical fixation through interference fit
Fixation of the stem through interference press fit does not provide intimate bonding due to the straight geometry of the often encountered mid-cortical diaphysis. A fibrous layer is formed which may consolidate to bone if micromotion is prevented. Fixation may be enhanced by porous and HA coating.
Fixation using bone cement
Cement due to its low modulus of elasticity allows gradual transference of stress to bone. To date this still displays the best long-term survivorship.
Biological fixation of implant to bone
Porous coating of an implant enhances osseo-integration. This can paradoxically also then act as a stress riser in cases where diaphyseal fixation alone is employed, leading to bone resorption and ultimate stem fracture.
Modularity has made for easier surgery with no change in implant survival. The reported survival rates of cemented stem prosthetics with an hydroxyapatite porous collar are higher than with cementless fixation.14,15 However, a great deal of controversy still exists as to which modality is superior. A central registry similar to the Swedish/Norwegian one does not exist for custom endoprosthetics.
Infection
Infection rates of up to 11% have been reported.17 This is more common in the paediatric extensile, proximal tibial replacements, pelvic and previously irradiated group of patients. Should infection occur, two-stage revision is successful in 72% cases, with a 32% probability of amputation if the second stage procedure is unsuccessful. The infection rate with endoprostheses is significantly lower in the non-oncological group of patients.
More recently there has been an upsurge in interest in the application of a silver coating to large endoprosthetic implants with both Unwin in Stanmore and Gosheger in Munster publishing promising initial results.18
Overall survival rates for large endoprosthetic replacements around the knee have been reported as 60%-90% at 5years and 40%-80% at 10 years, with the worst overall group being the proximal tibial replacements.
Special considerations
The growing child
Most patients with bone sarcoma are skeletally immature and resection can result in significant leg length inequality once skeletal maturity is attained. Initially, an invasive extendible implant using a custom segment sequential replacement was used. Subsequently a minimally invasive extendible implant with a key-and-gear concept was introduced. This was also associated with high rates of infection and more recently a non-invasive extensile implant using a system of electromagnetic motors and gears has been introduced (Wright Medical, Mutars, Stanmore). The complications have been lowered by more frequent controlled non-invasive lengthening procedures; however, cost is still a major factor and the procedure is still not without significant problems.19
Soft tissue cover
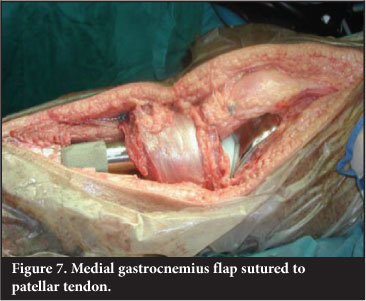
It is imperative that adequate soft tissue cover over an implant or graft is obtained. Use of a medial gastrocnemius flap to cover the defect associated with a proximal tibial endoprosthetic replacement has reduced infection rate from 25% to 6%.20 The medial half of gastrocnemius is transposed anteriorly and the patellar tendon is sutured directly to the flap. This reconstructs the extensor mechanism and allows for good muscle cover (Figure 7). An extensor lag is usually evident.Patients are educated to compensate by 'back-setting' the knee in the stance phase of gait - usually with a rotating hinge-type knee prosthesis.
Soft tissue loss in distal femoral replacement is less frequently encountered. Usually two of the quadriceps group of muscles remain in Enneking type 2b resections. Occasionally it is necessary to perform a hamstring to rectus tendon transfer or a latissimus dorsi free flap.
Soft tissue attachment
Resection of a large tumour and insertion of a prosthesis presents difficulty with respect to attachment of soft tissue. The major benefit of a prosthetic/allograft composite is that the reattachment of the soft tissue is facilitat-ed.21 Methods to allow for soft tissue attachment around a prosthetic implant are:
1. Madreporique surface (Howmedica),
2. Trevira tube - porous mesh sutured around the implant onto which the soft tissue can be attached (Mutars), and more recently,
3. Trabecular metal has shown promise in facilitating soft tissue ingrowth.22
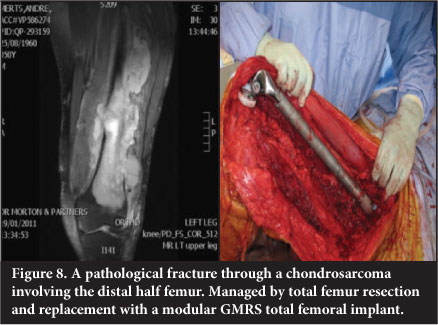
Total femur replacement
Total femur replacement is used in massive reconstruction as an alternative to through-hip disarticulation in extensive tumours and/or where inadvertent contamination has occurred. It has applications in revision surgery where patients have an ipsilateral failed hip and knee prosthesis with massive bone loss (Figure 8).
Reconstructive options in joint-sparing procedures/intercalary defects
Intercalary defects are significantly easier to manage and have a superior functional outcome.
Bone transport
Bone transport is seldom used due to the high maintenance demands of the procedure combined with adjuvant oncological treatment. It is useful in lower grade sarcoma, e.g. grade 1 osteosarcoma, adamantinoma where chemo-and or radiotherapy is not required.
Intercalary allograft
Intercalary grafts are subject to the same problems associated with bulk allografts. The results of intercalary allografts are however superior to allograft/prosthetic composites and in some series have a reported ten-year survival of 84%.23
Intercalary autograft
Usually the defect post tumour resection is too large to be bridged by an autograft and a vascularised free fibula is preferable.
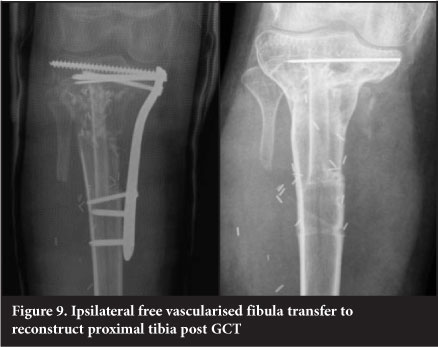
Vascularised free fibular transfer
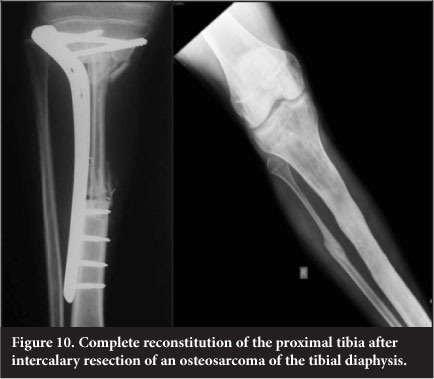
Vascularised free fibular transfer was first described by Taylor et al in 1977.24 The fibula is noted to incorporate and hypertrophy, however and has been associated with high rates of fracture. Various modes of fixation have been advocated. The use of a 'slot in' technique whereby the fibula is firmly compressed into the defect has been utilised at our unit since 2003 and has become the mainstay of treatment for intercalary defects. This is combined with titanium plate internal fixation (Figure 9). This has a success rate of 96%, with fracture occurring in 7.6% of patients. The presence of a fracture is usually associated with exuberant subsequent callus formation which increases the strength of the graft. The fibula is fixed directly to the cortex into a slot as opposed to being centralised in the canal and is always slotted in under pressure to stimulate the graft. The use of more flexible titanium plates is also felt to assist with graft stimulation. Complete remodelling of the shaft is an expected outcome (Figure 10).
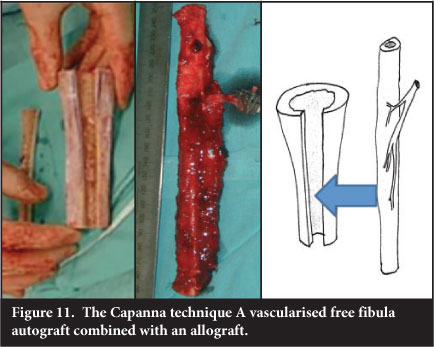
Allograft/vascularised free fibular composite
Combining a bulk allograft with central vascularised free fibula, known as the Capanna technique, was introduced due to the high incidence of fracture in the free fibular grafts. The fibula is inserted into the centre of the allograft via a slot and the allograft then fixed to the host bone with the fibula centralised in the host canal (Figure 11). The fibula has been evaluated by serial CT and has been shown to undergo either incorporation into the allograft, fracture with exuberant callus formation and incorporation or no detectable response.25
The combination with allograft is felt to assist with stability and lower the fracture risk, which has been reported as being as high as 13%.26
Endoprosthetic replacement
Endoprosthetic replacement of the diaphysis is usually indicated in metastatic disease with a poor long-term prognosis where an immediate weight-bearing reconstruction is needed, as opposed to intramedullary nail fixation.This procedure has been used in a small series to reconstruct intercalary defects in primary tumours but is not the method of choice and is associated with high complication rates.27 Tibial diaphyseal endoprosthetic replacement has been reported in recent publications as having a 22% infection rate.
More recently transphyseal resection in children with bone sarcoma has been advocated.28 With careful planning and technique the bony margins of resection are significantly closer with no appreciable increase in local recurrence rates in carefully selected patients. Reconstruction is performed using a custom-made joint-sparing endoprosthetic replacement (Figure 12). Computer-assisted surgery/navigation has improved the accuracy of the cut and therefore the fit of the custom endoprosthesis, which in the young child is usually a non-invasive growing implant.

Resection and re-implantation after autoclaving or irradiation
This technique whereby the resected specimen is denuded of soft tissue and subsequently irradiated to 50G or autoclaved is a useful reconstruction technique, particularly where the anatomy makes for difficult reconstruction. The local recurrence rates have not been shown to be elevated.29,30
Computer-assisted surgery/navigation has improved the accuracy of the cut and therefore the fit of the custom endoprosthesis
Summary
The surgical management of massive bone and soft tissue loss around the knee is challenging. The techniques and developments in the field of limb-sparing surgery that have evolved over the past few decades in the treatment of orthopaedic oncological disease have greatly improved limb salvage rates. This group of patients is immunocompromised and it is essential to perform the procedure with the lowest complication rate to facilitate resumption of chemotherapy/oncological management. Advances in prosthetic engineering as well as the understanding of the biology of allografts should lead to more consistent and predictable outcomes.
No benefits of any form have been received from a commercial party related directly or indirectly to the subject of this article. The content of the article is the sole work of the authors.
References
1. Imran H. Effect of time to resumption of chemotherapy after definitive surgery on prognosis for non-metastatic osteosarcoma. J Bone Joint Surg (Am.)2009;91:604-12. [ Links ]
2. Wolf RE, Enneking WF. The staging and surgery of musculoskeletal neoplasms. OrthopClin N Am 1996;27(3):473-81. [ Links ]
3. Brien EW, Terek RM, Healey JH, Lane JM. Allograft reconstruction after proximal tibial resection for bone tumors. An analysis of function and outcome comparing allograft and prosthetic reconstructions. ClinOrthopRelat Res 1994 Jun;(303):116-27. [ Links ]
4. Gebhardt MC, Flugstad DI, Springfield DS, Mankin HJ. The use of bone allografts for limb salvage in high-grade extremity osteosar-coma. Clin Orthop Relat Res 1991 Sept (270):181-96. [ Links ]
5. Donati D, Di Liddo M, Zavatta M, et al. Massive bone allograft reconstruction in high-grade osteosarcoma. Clin Orthop Relat Res 2000 Aug;(377):186-94. [ Links ]
6. Lord CF, Gebhardt MC, Tomford WW, Mankin HJ. Infection in bone allografts. J Bone Joint Surg (Am) 1988;70-A:369-76. [ Links ]
7. Brigman BE, Hornicek FJ, Bebhardt MC, Mankin HJ. Allografts about the knee in young patients with high grade sarcoma. Clin Orthop 2004;421:232-39. [ Links ]
8. Muscolo DL, Ayerza MA, Calabrese ME, et al. Human leukocyte antigen matching, radiographic score, and histologic findings in massive frozen bone allografts. Clin Orthop Relat Res 1996 May;(326):115-26. [ Links ]
9. Friedlaender GE, Strong DM, Tomford WW, Mankin HJ. Long-term follow-up of patients with osteochondral allografts. A correlation between immunologic responses and clinical outcome. Orthop Clin North Am 1999;30:583. [ Links ]
10. Bauman RD. Limitations of structural allograft in revision total knee arthroplasty. Clin Orthop Relat Res. 2009 March;467(3):818-24. [ Links ]
11. Hornicek FJ Jr, Mnaymneh W, Lackman RD, et al. Limb salvage with osteoarticular allografts after resection of proximal tibia bone tumors. Clin Orthop Relat Res 1998 Jul;(352):179-86. [ Links ]
12. Mnaymneh W, Malinin TI, Lackman RD, Hornicek FJ, Ghandur-Mnaymneh L. Massive distal femoral osteoarticular allografts after resection of bone tumors. Clin Orthop 1994;303:103-15. [ Links ]
13. Donati D, Giacomini S, Gozzi E, et al. Allograft arthrodesis treatment of bone tumors: a two-center study. Clin Orthop Relat Res 2002 Jul;(400):217-24. [ Links ]
14. Myers G, Abudu S, Carter SR, Tillman RM, Grimer RJ. The long term results of endoprosthetic replacement of the proximal tibia for bone tumours. J Bone Joint Surg (Br) December 2007;89-b(12):1632-37. [ Links ]
15. Myers G, Abudu S, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg (Br.) 2007 May;89(5):706. [ Links ]
16. Chao EY. A composite fixation principle for modular segmental defect replacement (SDR) prostheses. The Orthopedic Clinics of North America 1989;20(3):439-53. [ Links ]
17. Jeys l. Periprosthetic infection in patients treated for an orthopaedic oncological condition. J of Bone and Joint Surgery (Am.) 2005;87:842-49. [ Links ]
18. Unwin P. Surface treatment of mega-prostheses by 'stitching-in' silver. J Bone Joint Surg Br 2012vol. 94-B no.SUPP XXV 251. [ Links ]
19. Picardo NE. The medium-term results of the Stanmore non-invasive extendible endoprosthesis in the treatment of paediatric bone tumours. J Bone Joint Surg (Br) March 2012;94-b(3):425-30. [ Links ]
20. Grimer RJ. Endoprosthetic replacement of the proximal tibia. J Bone Joint Surg(Br) 1999;81-B:488-94. [ Links ]
21. Jofe MH, Gebhardt W, Tomford WW, Mankin HJ. Reconstruction for defects of the proximal part of the femur using allograft arthroplasty. J Bone Joint Surg (Am) 1988;70-A:507-16. [ Links ]
22. Reach, JS. Direct tendon attachment and healing to porous tantalum: an experimental animal study. J Bone and Joint Surg (Am) 2007;89:1000-1009. [ Links ]
23. Bullens P. Survival of massive allografts in segmental oncological bone defect reconstructions. Int Orthop. 2009 June;33(3):757-60. [ Links ]
24. Taylor GI. Microvascular free bone transfer: A clinical technique. Orthop Clin North Am 1977;8:425. [ Links ]
25. Manfrini M. Imaging of vascularized fibula autograft placed inside a massive allograft in reconstruction of lower limb bone tumours. American Journal of Roentgenology. April 2004;182(4):963-70. [ Links ]
26. Bakri, K. Combined massive allograft and intramedullary vascularized fibula transfer: The Capanna technique for lower-limb reconstruction. SeminPlast Surg. 2008 August;22(3):234-41. [ Links ]
27. Sewell MD. Intercalary diaphyseal endoprosthetic reconstruction for malignant tibial bone tumours. J Bone Joint Surg (Br) August 2011;93-b(8):1111-17. [ Links ]
28. Abed R, Grimer R. Surgical modalities in the treatment of bone sarcoma in children. Cancer Treatment Reviews 2010. [ Links ]
29. Manabe J, Ahmed AR, Kawaguchi N, Matsumoto S, Kuroda H. Pasteurized autologous bone graft in surgery for bone and soft tissue sarcoma. Clin Orthop 2004;419:258-66. [ Links ]
30. Uyttendaele D, De Schryver A, Claessens H, Roels H, Berkvens P, Mondelaers W. Limb conservation in primary bone tumours by resection, extracorporeal irradiation and reimplantation. J Bone Joint Surg (Br) 1988;70:348-53. [ Links ]
 Reprint requests:
Reprint requests:
Email: keith.hosking@coru.co.za














