Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SA Orthopaedic Journal
versão On-line ISSN 2309-8309
versão impressa ISSN 1681-150X
SA orthop. j. vol.11 no.3 Centurion Jan. 2012
CLINICAL ARTICLE
Bone loss in shoulder replacement surgery: a review of current management
DTMcGuireI; B VrettosII; S RocheIII; J WaltersIV
IMBChB, FC(Orth)(SA), MMed(Orth)UCT. Department of Orthopaedic Surgery, University of Cape Town
IIMBChB(Zim), FRCS(Eng), FCS(SA)Orth, MMed(Orth)UCT. Department of Orthopaedic Surgery, University of Cape Town
IIIMBChB(UCT), LMCC, FCS(SA)Orth. Department of Orthopaedic Surgery, University of Cape Town
IVMBChB, FC(Orth)(SA) Department of Orthopaedic Surgery, University of Cape Town
ABSTRACT
Hemiarthroplasty was first introduced in 1951 by Boron and Sevin and the first series of total shoulder replacements (TSR) was reported in 1974 by Neer.1 The primary indications for the use of these implants have been non-reconstructable fractures and arthritis. The reverse TSR was first introduced in the 1970s, but had minimal clinical success due to poor design. It has since been redesigned and received approval from the US Food and Drug Administration for use in 2003 and now is commonly used for rotator cuff arthropathy, massive rotator cuff tears, arthritis and fractures.2
Bone loss may be encountered in primary and revision surgery. It is a difficult problem to manage and may even be a contraindication to surgery when severe. Bone loss may affect both the glenoid and humeral side of the joint. Multiple studies have shown component loosening and osseous deficiency to be much more common on the glenoid side than the humerus.3-7
Key words: Shoulder arthroplasty, bone deficiency, bone loss, bone graft, classification, management
The glenoid component
Bone loss due to arthritis
Arthritis is the predominant indication for primary TSR. These arthritic conditions cause different but predictable patterns of glenoid wear.6,8
Primary osteoarthritis is usually associated with wear of the posterior glenoid rim (Figure 1). Smaller defects of the posterior rim lead to a biconcave-shaped glenoid, in which the humeral head articulates with the posterior part of the glenoid.6 In more severe cases with larger defects the humeral head may sublux posteriorly.9

Inflammatory arthritis is usually associated with central glenoid erosion, which may be accompanied by the presence of cysts within the glenoid vault.10 With severe disease medialisation of the humeral head may preclude the implantation of a glenoid component.6
Recurrent instability or chronic dislocations can lead to an anterior or posterior glenoid rim defect and be associated with degenerative changes of the glenohumeral joint2 (Figure 2).
Posterior glenoid wear may also be iatrogenic after surgery for anterior instability where the anterior capsular structures are over-tightened and lead to posterior humeral head subluxation.
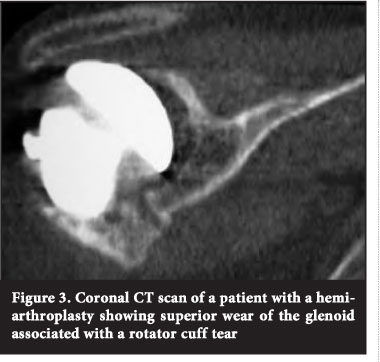
Advanced cuff tear arthropathy may cause flattening of the humeral head and superior glenoid erosion, as well as erosion of the under-surface of the acromion as a result of long-standing rotator cuff insufficiency and resultant proximal humeral migration6 (Figure 3).
These various forms of arthritis may have extensive cyst formation within the glenoid vault, sometimes almost entirely replacing the cancellous bone portion of the gle-noid.9
Classification
Glenoid morphology in osteoarthritis has been classified by Walch et al.11,12 This classification has helped with the understanding of glenoid bone deficiencies and the potential need for glenoid bone grafting. The glenoid could be divided into one of three types (Figure 4):
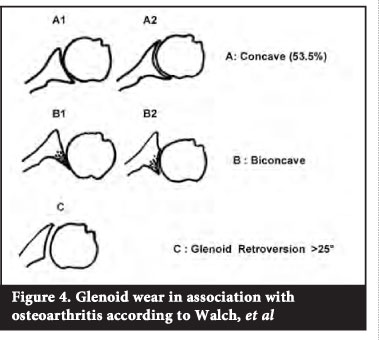
Type A (53.5%) -
characterised by an equal balance of forces acting on the glenoid and a centralised humeral head. There is central, concentric glenoid wear.
Type B (39.5%) -
asymmetrical posterior force distribution on the glenoid and a posterior subluxation of the humeral head with or without excessive posterior wear.
Type C (5%) -
arbitrarily defined as glenoid retroversion greater than 25 degrees. This is mostly seen in dysplasias.
Bone loss due to failed arthroplasty
Glenoid component loosening has been recognised as one of the more common aetiologies necessitating revision after TSR.7,13 Mechanical causes include a traumatic event, edge loading via a rocking horse mechanism in cuff-deficient patients, or fatigue failure.14 Polyethylene wear can lead to osteolysis and bone loss as a result of a macrophage response to particulate debris. Osteolysis is often a silent disease, and it can be quite common for patients not to have pain until the bone loss is very advanced. This response can lead to a massive loss of bone stock in both the proximal humerus and glenoid.15 Failure of a hemiarthroplasty due to severe glenoid erosion may also be a cause for revision surgery16 (Figure 3).
At the time of revision, the osseous deficiency of the glenoid may be too severe to support a glenoid component without reconstruction. Glenoid reconstruction, whether to enhance stability or to create a framework to secure a glenoid component, can be technically challenging and, if inadequate, may lead to early revision failure.16
Classification
Antuna et al have classified glenoid bone deficiencies after glenoid component removal into central, peripheral or combined, each with mild, moderate or severe degrees of bone loss.17 The deficiencies were classified as mild if they involved less than one-third of the glenoid surface or rim; moderate if they involved between one-third and two-thirds of the surface or rim; and severe if they involved more than two-thirds (Figure 5). Most deficiencies are central or combined.

Pre-operative planning
This includes an accurate history, an examination and radiological investigations.5 In the history it is important to note the aetiology as it may give a clue as to the amount and location of bone loss. Post-traumatic arthritis and avascular necrosis are unlikely to result in significant glenoid bone loss. As mentioned earlier, osteoarthritis, inflammatory arthritis and cuff arthropathy all exhibit their typical patterns of glenoid wear. The most severe patterns of bone loss, including cavitary and uncontained defects, are seen in revision cases.
A standard shoulder examination for motion, stability and muscle function should be performed. Stiffness suggests that glenoid exposure will be difficult, and postoperative motion difficult to restore. The rotator cuff should be tested if possible but pain and stiffness often make this difficult.5
If infection is suspected, blood workup should include inflammatory markers (ESR and CRP) and a white cell count. Pre-operative joint aspiration may be considered and intra-operative tissue samples should be obtained at the time of surgery if there is any suspicion. The use of bone graft is contraindicated where active infection is present.7
Radiological investigations
An accurate radiological assessment of the glenoid must be obtained before surgery. The exposure of the scapular neck is insufficient to appreciate the true orientation of the glenoid surface. In addition, the positioning of the patient on the operating table can influence the overall position of the scapula and thus contribute to a poor evaluation of possible glenoid erosion.6
The standard radiographic views should be obtained including a true anteroposterior (AP) view, a scapula lateral view and an axillary view, although these radiographic views alone are often not enough to fully evaluate the glenoid. The AP view is effective for evaluating superior migration, glenohumeral space narrowing and humeral head medialisation.5 The axillary view is effective for measuring posterior wear but is inadequate for measuring glenoid version and assessing glenoid deficiency because it is almost impossible to see the complete body of the scapula on this view.18
A CT scan is the radiological investigation of choice to evaluate the glenoid. The plane of the CT cut should be perpendicular to the glenoid surface for accurate measurement of the glenoid version.18 This can then be addressed at the time of surgery. A CT arthrogram may be superior to a standard CT scan by demonstrating a loose component with dye tracking behind the glenoid component, characterising the extent of the glenoid bone loss, and by evaluating the competency of the rotator cuff.7
For patients with suspected rotator cuff disease, MRI may be substituted for the CT scan, providing less bone detail but better information on the status of the soft tissue. Even when a failed prior implant is present, good information can be obtained with an MRI through special sequences.5
Surgical reconstruction for glenoid bone deficiencies
Current glenoid components are designed to rest on the subchondral bone of the glenoid with fixation points drilled or burred through the subchondral plate into the cancellous bone of the glenoid vault. In many cases of glenoid bone loss, the remaining subchondral bone is inadequate for support. Most solutions to this problem involve placement of structural grafts to restore the supporting surface so that the glenoid component can be reimplanted.19
The indications for bone-grafting in a patient with glenoid deficiency include:2
uneven wear that cannot be accommodated by small changes in glenoid or humeral component version
insufficient volume to support the glenoid component.
Bone-grafting can restore glenoid volume and version. Lesser degrees of bone loss can be compensated for by changing the humeral component version, lowering the high side of the glenoid with reaming or using an augmented glenoid component.2
Alternatively, the surgeon may decide not to resurface the glenoid but to perform a humeral hemiarthroplasty.
Although controversy exists with regard to the indications for use of a glenoid component, most data indicate that prosthetic glenoid resurfacing results in more predictable pain relief than hemiarthroplasty alone.19,20
Durability of the glenoid component remains an issue, especially with posterior glenoid deficiency.19,20 Therefore, the goals of treatment for glenoid bone loss should be more frequent glenoid resurfacing (preferably in a single stage), durable component fixation, diminished wear, and greater intra-operative component flexibility with regard to the types of bone loss encountered.19
Bone grafting for cavitary defects
Most cavitary defects can generally be managed with morcellised bone impaction grafting from the humeral head.6 In revision cases the humeral head is not available for morcellised bone graft. In these cases, if autograft is desired, the iliac crest may be used. Larger defects may need the addition of allograft cancellous bone chips or by simply using allograft alone. Very large contained defects may be addressed with structural autogenous graft.7
Some have advocated covering the grafted glenoid with a biologic resurfacing (Achilles tendon allograft, fascia lata, capsule).4 The soft tissue can be sutured to the perimeter of the glenoid rim using suture anchors much like an arthroscopic Bankart repair. This provides a means of containing the impacted graft material while also providing a smooth articulation for the humeral head.16 The results of this technique are conflicting in the available literature. Our limited use of this technique has not been encouraging in the past and we have abandoned it.
Bone grafting for eccentric glenoid wear
In primary TSR uneven or eccentric glenoid wear is present in most patients. If this wear is mild, and there is enough glenoid bone stock, it can usually be corrected by asymmetric reaming. As described earlier, the quantification of the wear and the feasibility of the technique can be assessed using a pre-operative CT scan.6
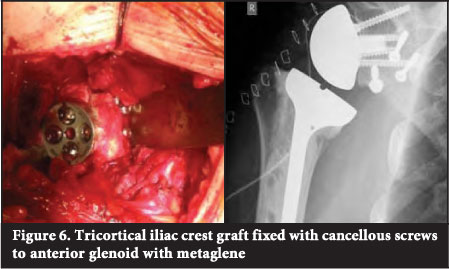
With more severe glenoid wear and bone loss, especially in cases with a deficient rim, a structural bone graft is required. If autograft is desired, tricortical iliac crest autograft can be used21 (Figure 6).
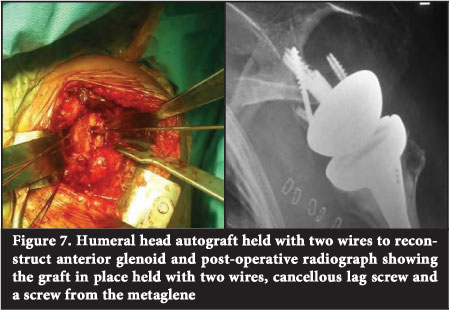
A femoral head provides a convenient source of allograft in that a sufficient volume of dense cancellous bone may be extracted and contoured while being typically available in most institutions7 (Figure 7).
Neer and Morrison in 1988 were able to monitor 19 of 20 shoulders having a large segmental graft for eccentric glenoid wear.22 The patients were followed up for a minimum of 2 years (average 4.4 years). The clinical outcome was excellent in 16 and satisfactory in one. None of the glenoid components clinically loosened or moved, and none required further surgical treatment. The authors commented that this technique provided sufficient osseous support to allow secure fixation of a glenoid component in the face of eccentric glenoid wear.
However, the results of other reviews using this technique were not so favourable. Steinmann and Cofield in 2000 reported 28 patients having glenoid bone grafting for segmental glenoid wear.23 Twenty-five patients had slight pain and three had moderate pain. Applying Neer's result rating, 13 shoulders had excellent results, ten had satisfactory results, and five had unsatisfactory results due to glenoid loosening in two, reoperation for instability in two, and persistent pain in one. Thus, in this series, four of 28 glenoids had not remained securely fixed, and the risk of recurrence of instability despite correction of glenoid component alignment was introduced.
A review article by Gerber in 2001 reported poor results with this technique due to high rates of glenoid loosening. The high failure rate led them to reconsider hemiarthroplasty for such cases.6 It should be noted that even when performing hemiarthroplasty, the glenoid version and inclination should still be corrected with a structural bone graft or asymmetric reaming because the functional results of hemiarthroplasty for osteoarthritis with asymmetrical glenoid wear are inferior to the functional results of hemiarthroplasty for concentric wear.6,24 We have found in our own unit that hemiarthroplasty with bone graft from the humeral head tends to resorb, and now prefer tricortical iliac crest graft.
Another study reporting on segmental bone grafting of the glenoid included a more difficult patient group.2 All patients had pre-operative instability. There were 12 posterior and five anterior glenoid bone defects. The humeral head was used for grafting in 15 shoulders, and the iliac crest was used in two. There were five revisions due to component issues in three, instability in two, and recurrent rotator cuff tearing in one. The authors commented that cortico-cancellous grafting of the glenoid can restore glenoid version and volume and did so effectively in 14 of the 17 shoulders in their study. Associated issues are often present, however - most notably glenohumeral instability, which is typically present in the face of uneven glenoid wear and may persist or recur. We have found that humeral head grafts and coracoid grafts have also not consistently helped to prevent recurrent instability in those patients with longstanding chronic dislocations with large bony glenoid deficits (Figure 8).,

Glenoid bone grafting for failed arthroplasty
Revision cases pose a more complex problem than primary TSR. Glenoid component loosening is the most common cause of failure of a TSR.6,25 Patients who require glenoid bone grafting at the time of primary TSR have a tenfold higher rate of glenoid component failure than those who have adequate glenoid version and volume.2
Because of the small anatomic size of the glenoid, bony deficiencies frequently compromise component fixation and pose considerable reconstructive challenges. In many instances, the bony deficiency on the glenoid side could be complex and massive, which precludes placement of a glenoid component.16 When glenoid components fail, most often due to loosening with some degree of bone resorption, it may be possible to place a new glenoid component with or without some degree of bone grafting, which may be done in one or two stages.
The addition of bone graft should be considered in any revision where glenoid defects occur.7 If a structural bone graft is used it should be of sufficient size to address the defect as well as normalise the version, inclination and offset (Figure 9).

A large allograft such as a cadaveric femoral head can be appropriately contoured to fit the defect and secured with inter-fragmentary screw fixation. After it is secured, in situ contouring can re-establish the appropriate glenoid version. A high speed burr is helpful in contouring the graft. A useful technique credited to Iannotti can be used to help shape a structural graft to fit a defect. This involves using a methylmethacrylate impression mould to help guide the creation of the graft. Hand-mixed cement, when in its doughy phase, is pressed into the defect while the excess cement is cleared away. Before final curing, it is removed so that it may act as a template for the graft to be fashioned. A screw can also be placed by hand in the cement before curing to act as a convenient handle to help with extraction of the mould. This is especially useful for moulds within a contained vault defect.7
With the glenoid reconstituted, the decision can be made to implant a new glenoid component or leave the grafted glenoid to articulate directly with the humeral prosthesis (i.e. revised to a hemiarthroplasty).7 This decision is a multifactorial one, which has not been well defined in the literature. Factors such as size of the graft, patient activity level and surgeon experience all influence the final decision. In some cases where a large volume of glenoid bone graft has been required, a prosthetic glenoid may rest entirely on graft material. Because some graft resorption will always occur, even with the use of structural autograft, the risk of component loosening is high in this setting. This may jeopardise the glenoid component and should influence the surgeon to consider implanting the prosthesis in a staged manner after the graft has incorporated and stabilised.7
In cases where a TSR is being converted to a hemiarthroplasty, the addition of bone graft offers the potential to provide a stable platform on which the humeral head may articulate and offers the best opportunity to replenish glenoid bone stock should prosthetic glenoid resurfacing be contemplated at a future date.7,25
In 2006 a review was undertaken by three centres.21 Nineteen patients underwent reimplantation of cemented glenoid components, 12 glenoplasty with or without bone grafting, five reverse TSR, and one resection arthroplasty for infection. At a minimum follow-up of 12 months the Constant-Murley score improved in all patients. Cemented glenoid reimplantation led to considerably improved function. Conversion to a reverse implant in patients with cuff tears led to improved pain scores.
Neyton et al reviewed nine patients undergoing revision to a reverse prosthesis in 2009.26 These patients underwent, in one or two stages, glenoid bone grafting and implantation of a reverse TSR. The indications for this procedure included revision shoulder arthroplasty and cuff tear arthropathy. At a minimum follow-up of 24 months most patients were satisfied with their results chiefly because of pain relief.
A review by Antuna et al reported 48 shoulders that underwent revision surgery.17 Of the 48 glenoid components that required revision, 18 underwent removal of the component and bone grafting for bone deficiencies with no glenoid reimplantation, and 30 underwent implantation of a new glenoid component. At a minimum follow-up of 2 years there was considerable pain relief (86%) and improvement in range of motion in the group of patients who underwent revision of the glenoid component. The group of patients without a glenoid component was less satisfied than the group with glenoid reimplantation and pain relief was achieved in only 66%.
Phipatanakul and Norris reviewed 24 patients who underwent conversion of a TSR to a humeral head replacement with glenoid bone grafting for glenoid loosening due to osteolysis.14 Of the 24 patients, 18 had satisfactory pain relief at a mean follow-up of 33 months. Four had good pain relief with conversion back to total shoulder replacement at a mean of 11 months after the index procedure. Two patients continued to report significant pain and were not satisfied with the procedure. They concluded that bone grafting of glenoid defects in revision arthroplasty provides satisfactory improvement in terms of pain relief and, by improving bone stock, allows for placement of a glenoid component at a later date if there is persistent pain.
Elhassan et al retrospectively reviewed 21 patients who underwent glenoid bone grafting using corticocancellous bone grafting or impaction grafting using cancellous bone graft.16 Three patients underwent revision TSR, five patients hemiarthroplasty, ten patients hemiarthroplasty with biologic resurfacing of the glenoid, and three patients revision to reverse TSR for large rotator cuff tears. After a minimum of 25 months' follow-up all patients had improvement in their range of motion and the Constant-Murley score. Most improvement occurred in patients with glenoid reimplantation. They concluded that revision TSR with glenoid bone grafting can produce good short-term outcomes and glenoid component reinsertion should be attempted whenever possible.
There is little literature analysing the results of a two-stage procedure where the glenoid component is removed and bone grafted and then at a later setting a new glenoid is implanted. In the setting where patients have undergone glenoid component removal and bone grafting but have persistent pain and limitation of movement this procedure may be considered.27 A review of seven patients was performed by Cheung et al in 2007.28 Five of the patients had reduced pain and expressed satisfaction with the result but two patients developed a low grade Propionibacterium acnes infection and underwent re-revision. They concluded that reimplantation of a glenoid component offers an improved clinical outcome in most patients, particularly reduction in pain and improvement in satisfaction.
Anecdotally from meetings, discussion and our experience,29 failed conventional arthroplasty is more readily converted to reverse TSR. The reason for this is probably multifactorial. These failures are often associated with a dysfunctional rotator cuff due to tears, stiffness and fatty change of the muscle. Septic cases often have rotator cuff deficiencies and bone loss. The exposure of the glenoid is better when doing a reverse and therefore easier to bone graft. Good function is probably more reliably returned to the shoulder with the biomechanical advantage of the reverse TSR. The altered biomechanics of the reverse TSR probably allow better forces to act across the bone-grafted glenoid rather than across a glenoid of a conventional TSR, and therefore fewer failures are being seen.
The humeral component
Loosening humeral component is rarely a cause for revision in shoulder replacement surgery. Similarly bone loss is also an infrequent cause for revision when compared to the glenoid component. A 2010 study by Cil et al reviewed the survivorship of the humeral component in shoulder replacement surgery.30 They reviewed 1 584 cases of hemi- and total arthroplasty over a 20-year period. They found an 83% survival of the humeral component at 20 years when the end point was defined as humeral component revision or removal. Certain risk factors were identified as an increased risk for humeral component failure, i.e. young age, male gender, uncemented shoulder replacement, and replacement due to post-traumatic arthritis. Their overall conclusion however, was that glenoid-related problems, glenoid arthritis in hemiarthroplasties and the glenoid component in total shoulder replacements are the weak links in shoulder replacement surgery, resulting in humeral component revision or removal.
Verborgt et al evaluated the long-term results of uncemented humeral components at an average of 9.2 years.31 No complications or revisions occurred related to the uncemented humeral component. Of the components, 59% showed radiolucency, 32% had endosteal erosion and 14% had tilted. Of the humeral components, 19% were judged to be at risk for loosening. The prevalence of an at-risk humeral component was not associated with increasing pain or deteriorating function.
There is a paucity of literature addressing the subject of humeral bone loss, mainly due to the fact that this problem is not commonly encountered. In cases of massive bone loss of the humerus due to multiple revisions, fracture and infection, a tumour endoprosthesis is an option. A case report by Wang et al describes a patient with severe rheumatoid arthritis who had multiple revision total shoulder and elbow arthroplasties, which were complicated by infection and fracture.32 The patient had a dysfunctional flail limb with progressive humeral and bone loss, which was treated with a total humeral prosthesis and a vascular mesh allograft. At 14-month follow-up the patient reported continued pain relief and good elbow function.
Very often with these types of cases there is full rotator cuff loss. We have been using a reverse prosthesis combined with tendon transfers (latissimus dorsi), with the addition of suture holes to the custom prosthesis to try to restore some external rotation for these patients. Most of these are failed fracture fixations or a failed arthroplasty used to treat a fracture. The tumours are dealt with by the tumour surgeons.
Emerging techniques
Arthroscopy can be a useful adjunct to both diagnosis and treatment in cases of suspected glenoid loosening. Namdari et al have had success with arthroscopically assisted removal of the loose glenoid and bone grafting of defects with cancellous allograft bone chips.27 Their indications for arthroscopically assisted glenoid removal and bone grafting are limited to aseptic loosening of the glenoid component with resultant contained glenoid defects. They state that this technique allows for a theoretically less morbid approach to conversion of a painful TSR to a hemiarthroplasty with restoration of glenoid bone stock, but is inappropriate in cases with severe capsular contraction, need for significant bony and soft-tissue reconstruction and balancing, or metal-backed glenoid components or in any cases in which the surgeon expects significant technical difficulty in accessing and visualising the glenohumeral joint with an arthroscope. We have limited experience in using arthroscopy for diagnostic and occasional therapeutic use in arthroplasty patients. We have not used it to remove glenoids yet, but certainly confirmed loose glenoids when there has been doubt.
Although glenoid bone deficiency may preclude the placement of a traditional component, some investigators have looked towards the design of alternative glenoid components. A recent biomechanical study by Kirane et al in 2012 reviewed a posterior-step prosthesis that has been designed to compensate for biconcave glenoid defects (specifically type B2).33 Mechanical performance was evaluated based on periglenoid bone strains under consistent loading conditions in shoulder specimens. No significant difference was observed in the performance of the posterior-step prosthesis in the presence of a biconcave defect as compared with that of a conventional prosthesis in the absence of a defect. They concluded that this prosthesis may be a viable treatment option for posterior glenoid defects but further testing is required.
Another emerging technique is the use of patient-specific instrumentation. Mulder et aí29 presented the results of a pilot study of the use of a jig designed from a CT scan-derived CAD 3-dimensional model of the scapula. This jig allowed correct placement of a metaglene glenoid base plate for a reverse shoulder replacement. The jig was tested on two cadavers and five patients. The post-operative CT scans showed the average centre peg deviation from the intended inclination was 5.7 degrees and the version 3.3 degrees from the intended position. They concluded that this shows good positioning, but added that the numbers were too small and a follow-up clinical trial is required.
The pre-built mould (Figure 10) is placed on the patient's glenoid at the time of surgery and drill holes are then drilled through the predetermined holes in the mould to ensure accurate placement of screws in the glenoid (Figure 11).


It has been well shown that correct placement of the glenoid is important to prevent notching and failure of the reverse TSR. We are currently designing a mould for use in standard TSR as well.
Summary
First, it should be clear to the surgeon and patient that these are complex and challenging cases and as such both parties should have realistic expectations. No criteria are yet defined in the literature as to the amount of bone loss required before bone grafting should be performed in both primary and revision TSR, but rather each case should be considered on an individual basis. Again in revision TSR there are no criteria available to guide the surgeon as to whether a glenoid can be reimplanted or not, either as a one-stage or two-stage procedure. However the studies reviewed consistently show better outcome scores (pain relief and satisfaction) when a glenoid is reimplanted as opposed to revising a total arthroplasty to a hemiarthroplasty. The reverse TSR in our hands seem to be more amenable to bone graft and reimplantation of a glenoid in revision cases.
No benefits of any form have been received from a commercial party related directly or indirectly to the subject of this article. The content of the article is the sole work of the authors.
References
1. Neer CS 2nd. Replacement arthroplasty for gleohumeral arthritis. J Bone Joint Surg Am 1974; 56:1-13. [ Links ]
2. Nam D, Kepler CK, Neviaser AS, Jones KJ, Wright TM, Craig EV, Warren RF. Reverse total shoulder arthroplasty: current concepts, results, and component wear analysis. J Bone Joint Surg Am 2010; 92(suppl 2):23-35. [ Links ]
3. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am 2001; 83(6):877-83. [ Links ]
4. Namdari S, Goel DP, Warner JP. Managing glenoid bone loss in revision total shoulder arthroplasty: a review. Univ Pennsylvania Ortho J 2010; 20:44-49. [ Links ]
5. Klepps S, Hazrati Y, Flatow E, May LaPW. Management of glenoid bone deficiency during shoulder replacement. Tech Shoulder Elbow Surg 2003; 4(1):4-17. [ Links ]
6. Gerber AMD, Warner JP MD. Management of glenoid bone loss in shoulder arthroplasty. Tech Shoulder Elbow Surg 2001; 2(4):255-66. [ Links ]
7. Scalise JJ. Management of glenoid bone loss in revision total shoulder arthroplasty. Tech Shoulder Elbow Surg 2008;9(2):85-90. [ Links ]
8. Wiater JM JM. Shoulder arthroplasty: prosthetic options and indications. J Am Acad Orthop Surg 2009;17(7):415-25. [ Links ]
9. Cofield RH. Bone grafting for glenoid bone deficiencies in shoulder arthritis: A review. J Shoulder Elbow Surg 2007;16(5):S273-S281. [ Links ]
10. Strauss EJ, Roche C, Flurin PH, Wright T, Zuckerman JD. The glenoid in shoulder arthroplasty. J Shoulder Elbow Surg 2009;18:819-33. [ Links ]
11. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty 1999;14(6):756-60. [ Links ]
12. Walch G, Boulahia A, Boileau P, Kempf JF. Primary glenohumeral osteoarthritis: clinical and radiographic classification. Acta Orthop Blegica 1998;64(suppl 2):46-52. [ Links ]
13. Petersen SA HR. Revision of failed total shoulder arthroplasty. Orthop Clin North Am 1998;29:519-33. [ Links ]
14. Phipatanakul WP NT. Treatment of glenoid loosening and bone loss due to osteolysis with glenoid bone grafting. J Shoulder Elbow Surg 2006;15:84-87. [ Links ]
15. Klimkiewicz JJ, Iannotti JP, Rubash HE, Shanbhag AS. Aseptic loosening of the humeral component in total shoulder arthroplasty. J Shoulder Elbow Surg 1998;7:422-26. [ Links ]
16. Elhassan B, Ozbaydar M, Higgins LD, Warner JP. Glenoid reconstruction in revision shoulder arthroplasty. Clin Orthop Relat Res 2008;466:599-607. [ Links ]
17. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg 2001; 10:217-24. [ Links ]
18. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am 1992;74:1032-37. [ Links ]
19. Williams GR I,JP. Options for glenoid bone loss: composites of prosthetics and biologics. J Shoulder Elbow Surg 2007; 16(5 Suppl):S267-72. [ Links ]
20. Edwards TB, Kadakia NR, Boulahia A, Kempf JF, Boileau P, Nemoz C. A comparison of hemiarthroplasty and total shoulder arthroplasty in the treatment of primary glenohumeral osteoarthritis: results of a multicenter study. J Shoulder Elbow Surg2003;12:207-13. [ Links ]
21. Neyton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid corticocancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg 2006; 15:173-79. [ Links ]
22. Neer CS 2nd, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am 1988;70(8):1154-62. [ Links ]
23. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg 2000;9(5):361-67. [ Links ]
24. Levine WN, Djurasovic M, Glasson JM, Pollock RG, Flatow EL, Bigliani LU. Hemiarthroplastyfor glenohumeral osteoarthritis: results correlated to degree of glenoid wear. J Shoulder Elbow Surg 1997;6:449-54. [ Links ]
25. Hawkins RJ, Greis PE, Bonutti PM. Treatment of symptomatic glenoid loosening following unconstrained shoulder arthroplasty. Orthopedics 1999;22(229-34). [ Links ]
26. Neyton L, Boileau P, Nové-Josserand L, Edwards TB, Walch G. Glenoid bone grafting with a reverse design prosthesis. J Shoulder Elbow Surg 2007; 16(3):S71-S78. [ Links ]
27. Namdari S, Goel DP, RomanowskiJ, Glaser D, WarnerJP. Principles of glenoid component design and strategies for managing glenoid bone loss in revision shoulder arthroplasty in the absence of infection and rotator cuff tear. J Shoulder Elbow Surg2011;20(6):1016-24. [ Links ]
28. Cheung EV, Sperling JW, Cofield RH. Reimplantation of a glenoid component following component removal and allogenic bone-grafting. J Bone Joint Surg Am 2007;89(8):1777-83. [ Links ]
29. Mulder M, Booyens M, Honiball R, Du Toit W, Smit A, Roche S. Accurate screw placement for the glenoid component in reverse shoulder arthroplasty. Report of new technique and results of preliminary cases. Paper presented at SAOA congress; 2011 September 7; Sun City, South Africa. [ Links ]
30. Cil A, Veillette CJH, Sanchez-Sotelo J, Sperling JW, Schleck CD, Cofield RH. Survivorship of the humeral component in shoulder arthroplasty. J Shoulder Elbow Surg 2010;19(1):143- 50. [ Links ]
31. Verborgt O, El-Abiad R, Gazielly DF. Long-term results of uncemented humeral components in shoulder arthroplasty. J Shoulder Elbow Surg 2007;16(3):S13-S18. [ Links ]
32. Wang ML, Ballard BL, Kulidjian AA, Abrams RA. Upper extremity reconstruction with a humeral tumor endoprosthesis: A novel salvage procedure after multiple revisions of total shoulder and elbow replacements. J Shoulder Elbow Surg 2011;20(1):1-8. [ Links ]
33. Kirane YM, Lewis GS, Sharkey NA, Armstrong AD. Mechanical characteristics of a novel posterior-step prosthesis for biconcave glenoid defects. J Shoulder Elbow Surg 2012;21:105-15. [ Links ]
 Reprint requests:
Reprint requests:
Dr S Roche
E-mail: sroche@iafrica.com














