Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SA Orthopaedic Journal
versión On-line ISSN 2309-8309
versión impresa ISSN 1681-150X
SA orthop. j. vol.11 no.3 Centurion ene. 2012
CLINICAL ARTICLE
Revision total hip arthroplasty: addressing acetabular bone loss
C ReidI; GP GroblerII; BJ DowerIII; MB NortjeIV; J WaltersV
IMBChB, FC(Orth)(SA) Clinical Fellow: Hip and knee arthroplasty, Groote Schuur Hospital. Department of Orthopaedic Surgery, University of Cape Town
IIMBChB, FRCS(Edin), FC(Orth)(SA), MMed Orthopaedic Surgeon, Groote Schuur Hospital and Vincent Pallotti Hospital, Cape Town. Department of Orthopaedic Surgery, University of Cape Town
IIIMBChB, FC(Orth)(SA) Orthopaedic Surgeon, Groote Schuur Hospital and Vincent Pallotti Hospital, Cape Town. Department of Orthopaedic Surgery, University of Cape Town
IVMBChB, FC(Orth)(SA), MMed Orthopaedic Surgeon, Groote Schuur Hospital and Claremont Hospital, Cape Town. Department of Orthopaedic Surgery, University of Cape Town
VMBChB, FC(Orth)(SA) Orthopaedic Surgeon. Department of Orthopaedic Surgery, University of Cape Town
ABSTRACT
Managing deficient acetabular bone in primary and revision total hip arthroplasty requires thought and planning. This paper focuses on the management of bone loss in revision arthroplasty and presents an overview of the literature, the careful pre-operative assessment required prior to surgery and the surgical options available to achieve an optimal outcome.
Key words: Acetabular bone loss, acetabular deficiency, revision hip arthroplasty, acetabular management
Introduction
In most cases of revision of the acetabular component in total hip arthroplasty (THA) there will be some degree of bone loss. Contained defects with an intact rim are not usually problematic. The majority of these cases can be managed with an uncemented hemispherical cup.1,2 Impressive outcomes have also been reported for acetabular revision with impaction bone grafting and cemented cups.3,4 Uncontained defects, or defects associated with pelvic discontinuity, pose a more challenging problem. High morbidity and failure rates are associated with reconstruction of these deficiencies.5,6
Several authors have classified acetabular bone loss.7-12 These classifications aim to guide management or to compare outcomes but often have poor inter- and intra-observer correla-tion.i3-i5 Part of the problem is that accurate assessment of bone loss can be difficult and intra-operative findings may not correlate with radiological assessment.
Numerous techniques have been described to address acetabular deficiencies. The purpose of this paper is to review the current literature and to provide guidelines for assessing and managing bone loss in acetabular revision surgery.
History and clinical assessment
A thorough history and clinical assessment of the patient with possible acetabular bone loss following previous THA is important before any special investigations are requested. Questions that may be relevant pertain to possible current or previous infection, progressive limb length discrepancy and the number and extent of previous operations. It is important to enquire about the indication for the primary THA as the acetabulum might have been deficient before the initial surgery (e.g. acetabular dysplasia or trauma).
Patients often present to a different institution or practice from where they had their index surgery. Clinical notes or correspondence with the index surgeon must be obtained if possible, along with details of the implants used.
Pain is the most common presenting symptom and it is important to enquire about the nature of the pain. 'Startup' pain is often experienced with loosening of prostheses. A loose acetabular component often causes groin or buttock pain. Persistent pain from the time of surgery suggests infection, fracture, impingement or failure of initial stability of uncemented prostheses. Late onset pain is associated with aseptic loosening, low grade infection, osteolysis or instability.16
Clinical examination starts with assessment of gait. Acetabular component migration due to bone loss will affect the biomechanics of the hip. Proximal or medial displacement of the hip centre of rotation shortens the abductor lever arm resulting in a Trendelenburg gait. Marked shortening (>2 cm) will result in a short-limb gait.
Investigations
Before formulating a management plan for the THA patient with bone loss, special investigations are aimed at:
determining whether implants are loose or well fixed
quantifying bone loss
confirming or excluding infection
Plain radiographs
Subtle changes on a single radiograph can be difficult to interpret and bone loss is usually underestimated.17 Serial radiographs are the most important radiological investigation for assessing acetabular bone loss in a patient with previous THA.
The following X-ray protocol is suggested (Figure 1):
Asymptomatic THA with minor bone loss on X-ray (small lucencies, minimal osteolysis): Repeat X-ray at 3/12. If still asymptomatic and no progression of X-ray changes then repeat AP pelvis and lateral view of hip at 12 months.
Asymptomatic THA with major bone loss on X-ray (extensive osteolysis) but components not obviously loose: Manage on merit. A patient with progressive bone loss may benefit from early intervention, even without any symptoms.
Symptomatic THA with minor bone loss on X-ray (excluding patients with obvious or likely infection): Repeat AP pelvis and lateral view of hip in 3 months.
Loose component (symptomatic or asymptomatic): Book for revision if patient medically fit.
Computerised tomography
CT scan provides more information about bone quantity and quality than plain X-ray.17 It is however an expensive investigation that exposes the patient to additional radiation. It is not a routine pre-operative investigation for all revision cases but can add valuable information in cases with severe bone loss.
Ultrasound
Ultrasound provides little information about bone loss but can be of use when a pseudo-tumour is suspected.18
Magnetic resonance imaging
Metal artefact reduction sequence magnetic resonance imaging (MARS MRI) now allows for much improved MRI imaging of THA with greatly reduced artefact from metal implants.19,20 The typical signal characteristics of osteolysis on MRI are: areas of low T1 signal and intermediate to slightly increased T2 signal (similar to skeletal muscle), with a well-defined additional line of low signal surrounding areas of marrow replacement.19 In quantifying bone loss it is inferior to CT scanning and the main indication is for soft tissue assessment. In patients with metal-on-metal (MoM) bearing THA, MRI may be a useful pre-operative investigation for suspected adverse reactions to metal debris (including pseudo-tumours and metallosis).
Nuclear studies
Technetium-99 methylene diphosphonate (Tc) bone scan is less accurate in assessing loosening of components than plain X-rays but may increase the accuracy of the diagnosis when combined with plain X-ray.21 For assessment of possible infection, Tc scanning provides high sensitivity but does not differentiate bone infection from fracture, new bone formation, heterotopic ossification or arthritis.2122 Because of its sensitivity it remains a useful examination to exclude infection (good negative predictive value).
It has been shown however to remain positive for up to 2 years following THA in asymptomatic patients and is of questionable value during this period.21
Gallium-67 citrate (Ga) is used as an adjunct to increase specificity but accuracy in diagnosis of peri-prosthetic infection remains low with the sequential Tc-Ga technique.23
Indium-111 (In) labelled leukocyte scanning provides improved specificity and will increase accuracy of diagnosis if combined with Tc scan.24 Leukocytes accumulate in areas of infection but not in areas of increased bone turnover due to other causes. This is however a costly and time-consuming procedure and positive predictive value remains low. False positives may occur because of the presence of leukocytes in bone marrow, and specificity might be increased by correlating images with bone marrow images of the ilium. Scher et al25 do not recommend the routine use of In scanning to determine the presence of infection in the loose or painful total joint arthroplasty. Glithero et al26 report false negatives (poor sensitivity) in chronic peri-prosthetic infection. Leucocyte scans might improve in the future with improved labelling.
Positron emission tomography (PET) with fluorodeoxyglucose (FDG) measures biological activity of particularly macrophages and neutrophils, with an increased glucose uptake in areas of infection. This is a very promising modality that is less time-consuming and potentially more accurate than other nuclear studies27,28
Classification
Several classifications exist for acetabular bone loss in THA.712 The two most commonly cited classifications are those by Paprosky7 and DAntonio8 (AAOS classification).
Paprosky classification
Paprosky's classification7 is based on assessing the remaining host bone available to provide support for the acetabular component (Table I and Figure 2). The remaining superior dome, medial wall, anterior and posterior columns are assessed. Defects are classified as types I-III. A type I defect has an intact rim (walls and dome) and no cup migration. The teardrop is present (medial wall uninvolved) and there is no ischial bone lysis (posterior wall present). The remaining bone is completely supportive. In type II defects the remaining host bone is partially supportive. The rim is distorted but the columns are intact and supportive. This group is sub-classified according to the location of the defect. Type IIA defects are oval enlargements of the acetabulum with superior bone lysis but an intact superior rim. There is superior migration of the cup (<2 cm).
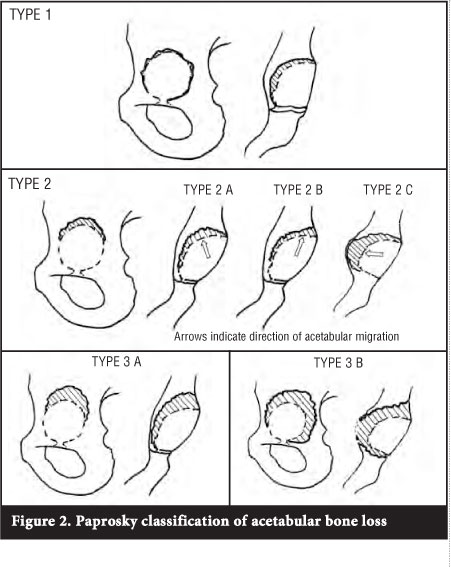
In type IIB defects the dome is more distorted and the superior rim absent. The component migrates superolaterally. When the medial wall is absent, the teardrop is obliterated and there is medial migration of the component. This defect is classified as a type IIC. In type III defects the remaining host bone is non-supportive. This occurs when there is destruction of the acetabular rim and either or both columns are non-supportive. There is superior migration of the component by more than 2 cm. In a type IIIA defect there is a rim deficiency from 10 o'clock to 2 o'clock. There is moderate, but not complete, destruction of the teardrop, and moderate lysis of the ischium. Migration is usually superolaterally because there is some medial wall still left intact. Type IIIB defects have rim deficiencies from 9 o'clock to 5 o'clock, there is complete obliteration of the teardrop and severe lysis of the ischium. Component migration is usually superomedially.
Paprosky developed the classification evaluating 147 patients. Acetabular defects were graded pre-operatively on a plain AP radiographs. Intra-operatively 11% of grade II defects were upgraded to type III and 5% of type III defects were downgraded to type II. The intra- and inter-observer reliability of the Paprosky classification of plain radiographs have been found to be moderate to poor by other authors.13-15
AAOS classification
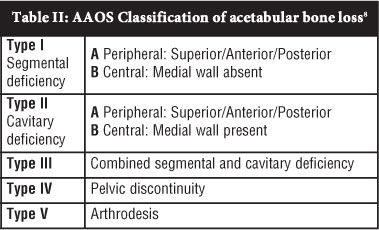
The American Academy of Orthopaedic Surgeons (AAOS) classification8 distinguishes between segmental and cavitary defects (Table II). Type I are segmental defects that are peripheral (IA), involving superior, anterior or posterior rim, or central (IB) with absent medial wall. Cavitary defects or volumetric expansions are classified as type II and sub-classified once again into peripheral (IIA) and central (IIB). Combined segmental and cavitary defects are classified as type III, pelvic discontinuity type IV and arthrodesis type V. This is a descriptive classification that does not provide the surgeon with a guide for reconstruction options. Poor reliability has also been demonstrated with this classification system.13-15
Saleh classification
Like the Paprosky classification, the Saleh classification9 aims to estimate the remaining supportive host bone stock following removal of the implant (Table III). The authors identify specific deficiencies that provide challenges at revision surgery without suggesting reconstruction options. Type I defects have no significant bone loss. Type II are contained defects with an intact rim. Uncontained defects with less than 50% segmental loss of the acetabulum are classified as type III, and those with more than 50% segmental loss are classified as type IV. Pelvic discontinuity is classified as type V. The Saleh classification has been shown to have higher inter-observer reliability than other acetabular bone loss classifications.15
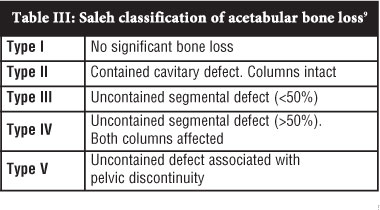
The complexity of the problem of acetabular bone loss in revision surgery and the limitations of radiographic assessment make it impossible to devise a perfect classification system that is simple, reproducible, suggests management options and predicts outcome. Johnson et aP compared 6 classifications and found that the Saleh classification most reliably describes 'the baseline characteristics that are most important to the surgeon for the purpose of planning a revision procedure and appropriately following the results.'
Despite poor reliability of the Paprosky classification on plain radiography, it remains useful because of its widespread use and the reconstruction guideline it provides. The classification serves as a guide only and it is important that the surgeon appreciates its limitations. Even with meticulous pre-operative planning, the final assessment of severity and location of bone loss is often made intra-operatively and reconstruction performed accordingly.
Pre-operative planning
Plain radiographs
Plain radiography is the most common, most cost-effective and possibly the most useful investigation for preoperative planning. It is not without limitations and generally underestimates osteolysis17
Three radiographic criteria are assessed on the AP radiograph for pre-operative classification according to the Paprosky system:
1. Superior migration of the hip centre. Superior migration of less than 2 cm is classified as a type II defect. Paprosky considers migration of more than 2 cm to be indicative of severe bone loss and major acetabular destruction, with loss of support structures. This is classified as type III defect. Some modifications of the original classification differentiate between type II and type III defects at 3 cm of superior migration.29,30 Pelvic discontinuity is not mentioned in the original classification. Once the acetabular component has migrated superiorly by more than 2 or 3 cm (type III defect), there is high risk of associated pelvic discontinuity because of the deficiency of the anterior and posterior columns at that level. In a later publication Paprosky et al31 state that pelvic discontinuity is unlikely if migration of the hip centre is less than 3 cm above the superior obturator line.
2. Ischial osteolysis is considered to be an indication of destruction of posterior support structures and is associated with type III defects. Type IIIA defects demonstrate moderate lysis and type IIIB defects severe lysis of the ischium. The classification does not clearly define moderate or severe osteolysis.
3. The teardrop is intact in type IIA and IIB but is obliterated in type IIC defects where the medial wall is absent. Type IIIA defects demonstrate incomplete destruction of the teardrop (medial wall of the teardrop present) and it is completely obliterated in type IIIB defects.
The position of the implant relative to the ilio-ischial line (Kohler's line) on the AP radiograph is preferred by some as a measure of medial migration because the teardrop may be absent if anatomy was distorted by the original pathology. The ilio-ischial line represents the posterior column and is usually disrupted in cases of pelvic discontinuity. With medial migration of the acetabular component the line becomes obscured and the disruption may not be appreciated. A 45° iliac oblique (Judet view) or 65° false profile view (Lequesne view) provides a better assessment of the posterior column and increases the sensitivity of diagnosis of pelvic discontinuity on plain films.32
A lateral radiograph of the hip is usually performed to assess the femur and femoral component. This view also provides additional assessment of the acetabular dome and position of acetabular component.
Templating
Templating is an important step in pre-operative planning of revision surgery. The requirement of unusual implants or sizes is often identified by templating. These implants may need to be specifically ordered.
Computerised tomography
CT scanning is the most sensitive and accurate modality for detecting and measuring acetabular peri-prosthetic bone loss.17,33 Some authors recommend routine CT scanning for pre-operative planning of revision THA or even as a screening tool in asymptomatic patients.i7 It is however a costly investigation and exposes the patient to additional radiation. It is reasonable to reserve CT scanning for assessing osteolysis of the anterior and posterior columns or for excluding pelvic discontinuity when it is not clearly defined on plain films.
3D model
A three-dimensional model pelvis can be created from CT images. This gives the surgeon the opportunity to pre-operatively match different cups or augments to available bone and to work out angles for screw fixation. As this handy tool becomes more cost-effective and readily available, it is likely to play an increasing role in pre-operative planning of acetabular reconstruction in the future.
Angiography
Angiography or CT angiography to identify the major pelvic arteries may be indicated in certain cases. Sporer30 recommends angiography or vascular consultation in all cases where the acetabular component has migrated medially to the ilio-ischial line. In our experience pre-operative angiography is only indicated in rare cases of catastrophic bone loss where an intra-pelvic approach will be required for retrieval of components. In these cases the surgery should be planned and performed in conjunction with a vascular surgeon. In the vast majority of cases of medial migration of the acetabular component, there is either remodelling of medial bone or fibrous tissue separating the component from the pelvic cavity. A conventional extensile approach will suffice in these cases and angiography is not required.
Surgical technique and implants
The aims of acetabular revision are to provide a functional, pain-free hip. It should be a long-term solution. This should be pursued while minimising morbidity and cost.
For revision of septic THA a two-stage procedure is recommended. Management of the septic THA is beyond the scope of this article and the surgical technique described here is for cases of aseptic loosening with bone loss.
Where possible deficient bone stock should be restored and further bone loss prevented. To recreate normal biomechanics of the hip, the centre of rotation should be restored. Often the position of optimal stability provided by remaining bone does not correlate with the optimal hip centre position. In these cases the surgeon needs to individualise the planned reconstruction according to the patient's requirements. For the younger patient as much bone as possible is preserved or restored and an optimal hip centre is aimed for. In the older, lower demand patient a sub-optimal hip centre may be accepted in favour of a less extensive procedure that still results in a stable implant (Figure 3).
Uncemented hemispherical cup
The majority of acetabular revisions can be performed with an uncemented hemispherical cup.1,2 Porous coating, porous metal or hydroxyapatite promotes biological fixation between host bone and the titanium shell. Initial stability is required to allow for bony on-growth or in-growth and this is achieved by a press-fit. Screws or spikes may be required for initial stability.
Cavitary (type IIA) defects and medial bone loss (type IIC) are filled with bone graft (Figure 4). Using the acetabular reamer in reverse is an elegant way to push bone graft into defects. Press-fit can be obtained if the acetabular rim is intact. A minimum of 50% host bone contact was traditionally recommended for using an uncemented hemispherical, and an anti-protrusio cage or roof reinforcement ring advised when there is less than 50% host bone contact.34,35
More recently hemispherical porous tantalum cups have been used in cases of less than 50% bone contact. Lakstein et aP6 reported reasonable early to mid-term results with hemispherical porous tantalum cups in 53 revision THA cases with 0%-50% (average 19%) host bone contact.
Contained medial defects can be filled with morcellised bone graft and the hip centre corrected with an uncemented hemispherical cup if a press-fit is achieved on an intact acetabular rim. If a press-fit is achieved in a medialised position, the hip centre can be corrected by using a lateralising cup or liner (Figure 5).
Segmental defects involving less than 30% (type IIB) of the acetabular rim can also be managed with a hemispherical cup.
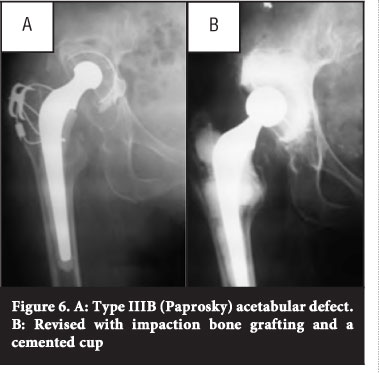
If a hemispherical cup is used for larger segmental defects or where remodelling of the acetabulum has occurred, it usually results in a change in the centre of rotation. This can be a subtle superior displacement of the hip centre when a large hemispherical cup is used for spherical remodelling (type IIIA) or a significant displacement in cases of oblong remodelling (type II B) or large superior segmental defects (type IIB) (Figure 6).
Cemented cup
With good results reported for uncemented acetabular revision, cemented acetabular revision has become less favoured over the last two decades, particularly in the USA37 In 1995 Raut et al38 published disappointing results with cemented acetabular revision at a mean follow-up of 5.5 years. The authors did comment, however, that similar results were published at the time for uncemented revisions. With improved cementing techniques and the use of cross-linked all-polyethylene (PE) cemented cups, excellent results have been achieved with cemented acetabular revision, particularly in Europe and the United Kingdom. Schreurs et at reported 75% survivorship at a minimum follow-up of 20 years following cemented acetabular revision with all re-revisions as an end-point. When septic re-revisions and re-revisions of well-fixed implants for PE wear were excluded, survivorship was 87%.
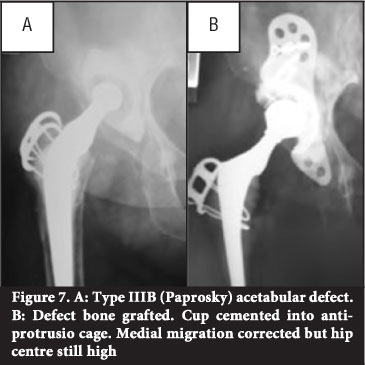
Impaction bone grafting with cemented acetabular revision is an effective way to restore lost bone (Figure 7). The technique described by Schreurs et aP9 involved morcellising cancellous bone with a rongeur to chips 0.5-1cm in diameter. Segmental medial wall defects are closed off with slices of cortico-cancellous bone. Cavitary defects are tightly impacted with the morcellised cancellous bone using specifically designed impactors and a mallet. The authors advise against the use of a bone mill to morcellise bone and reverse acetabular reaming for impaction. In their study autograft or fresh frozen allograft was used; no irradiated allograft was used.39
Autograft can be harvested from the iliac crest but this adds time and morbidity to the procedure and may not provide enough graft. Fresh allograft can be collected during primary arthroplasty procedures to be frozen in a bone bank, but this is not common practice in countries where there is a high incidence of communicable diseases. Gamma radiation treatment and freeze-drying of allograft reduces the risk of disease transmission and immunological rejection but also reduces osteoinductive, osteoconductive and osteogenic properties, as well as structural integrity.40
There is some concern that irradiated allograft might not incorporate as well as autograft or fresh frozen allograft. Disappointing results have been published where irradiated allograft was used for impaction bone grafting in femoral revisions.41 Emms et al42 have shown encouraging mid-term results with the use of irradiated allograft for impaction bone grafting and cemented acetabular revision but longterm results are not yet known.
Another concern with the technique is reported high failure rates in high-grade and uncontained deficiencies.6
Alternatives to autograft and allograft include xenograft and bone graft substitutes such as hydroxyapatite, calcium sulphate, polyhydroxyacids, glass-ionomer ceramics, absorbable ceramics and collagen matrices.43 Xenograft shows poor osseointegration and collagen matrices and polyhydroxyacids lack the required strength.43
Bone graft substitutes can be used in isolation or in combination with auto- or allograft. Encouraging results have been reported with combining fresh frozen allograft with calcium phosphate,44 hydroxyapatite45 or biphasic porous ceramic (80% tri-calcium phosphate, 20% hydroxyapatite).46 The volume of fresh frozen allograft is reduced and the authors argue that this reduces the risk of disease transfer or immune response.46
McNamara et al.47 combined irradiated allograft with calcium phosphate (1:1) for acetabular impaction bone grafting and report 100% survival at a minimum of 3.4 years.
Beswick et af& did a systematic review of the literature pertaining to the use of bone graft substitutes in revision THA. They concluded that 'the literature is deficient in both long term follow up of larger series and in randomised controlled trials comparing bone graft substitutes with allograft. In the context of allograft shortage, concerns over infection and immunological rejection, and costs, there is a need for appropriately designed randomised controlled trials comparing use of bone graft substitutes with established practice. In addition to prosthesis related outcomes, studies should explore the patient experience of revision hip replacement incorporating bone graft substitute material.
Structural support
In type III defects (superior migration of the acetabular component by >2 cm), a normal centre of rotation cannot be recreated with a hemispherical cup without augmentation. Superior structural support can be provided by bulk allograft or by metal augmentation.
Excellent bony in-growth has been shown with porous metal (tantalum).54 Modular augments, plates and cup-cage constructs offer great versatility for acetabular reconstruction. Early results of acetabular reconstruction using tantalum in cases of severe bone loss look promising55-58 Tantalum augments shaped as part of a hemisphere are available in various diameters and heights. Once the acetabular bone has been prepared using a reamer, a trial acetabular component and trial augments are placed in the cavity and optimal positioning is determined. Definitive augments are attached to bone with screws. Fenestrations in the augments allow for the placement of morcellised bone graft. A layer of cement should separate any two modular tantalum components. A modular cup with screw holes (with cement between the cup and augment) can then be used if the ideal orientation can be achieved. Screws are placed through the shell into host bone. An all tantalum revision cup can also be used and a polyethylene liner cemented into the shell. Additional screw holes can be created in the tantalum revision cup using a high speed burr if required.
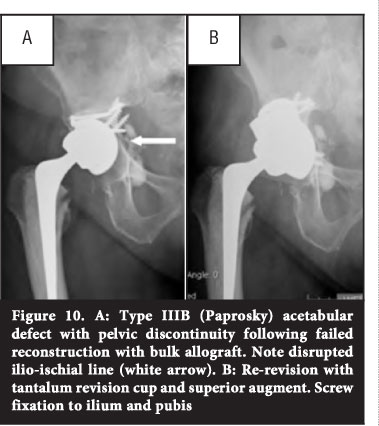
Pelvic discontinuity
Pelvic discontinuity occurs when the superior and inferior aspects of the hemipelvis are separated by a fracture through both columns.59 This can be due to severe bone loss or trauma. Reconstruction of severe acetabular defects with associated pelvic discontinuity is a challenging problem. Some success has been reported with acetabular anti-protrusio cages that span the defect and are secured to the ilium and ischium, combined with morcellised bone graft, but longterm results remain disappointing.60,61 Total acetabular transplant with bulk allograft combined with a cage is also associated with high morbidity and failure rates.30
In a pelvic discontinuity with the potential to heal, osteosynthesis can be performed by rigid fixation of the posterior column with a plate and screws construct.31 This will prevent distraction of the defect when a press-fit is sought with the acetabular component and augments.
Distal femur or femoral head allograft is shaped into a figure-of-seven and attached to the ilium with screws.49 A new acetabulum is then created in an anatomical position using an acetabular reamer. Both cemented and uncemented cups have been used with bulk allograft. Bulk allograft can also be combined with other devices such as a reinforcement ring, anti-protrusio cage or the Kerboull acetabular reinforcement device.50-52 The potential advantage of bulk allograft is the restoration of bone stock. It is however technically difficult and high complication and failure rates have been reported.51
Metal augmentation can be done by using modular porous metal augments or a dual-geometry or oblong acetabular component (Figure 8).53

Sporer and Paprosky31 describe a technique of stressing the acetabular bone intra-operatively to assess for pelvic discontinuity. If present, a distinction is made between an acute discontinuity with minimal gapping, and chronic discontinuity with sclerotic bone and a large amount of fibrous tissue. An acute discontinuity has the potential for healing, and the fracture is bone grafted and compressed. In chronic discontinuity with no potential for healing, the defect is distracted.
Early results with the use of tantalum acetabular components and augments are promising (Figure 9).55-58 If augments alone do not provide enough stability for the acetabular component, a cup cage construct can be utilised.62,63 The cup cage is implanted into the revision tantalum cup. Fixation with screws is through the cage and cup into available host bone, and through the iliac flange into the ilium. A smaller inferior flange is designed to spike into the ischium.

Salvage options
The saddle prosthesis is designed to be used in cases of total acetabular bone loss.64 It is a hemi-arthroplasty that consists of a femoral stem and a saddle shaped surface that articulates with the ilium. The implant has been refined from a mono-block design to the modular mark II saddle prosthesis with a conventional femoral stem and an additional artic-ulation.64 Poor functional outcomes have been reported with the use of saddle prostheses and they are rarely indicated outside of tumour surgery.65-67
Excision arthroplasty (Girdlestone procedure) is usually regarded as the final option for the failed THA. Most reported series were performed for salvage of septic cases. In these cases a stable pseudoarthrosis develops and reasonable outcomes have been reported68,69 Limb shortening and instability are the concerns if a Girdlestone procedure is performed in cases of severe bone loss.
Summary
Acetabular bone loss presents a challenge to the revision total hip arthroplasty surgeon. Today various implants and techniques are available to address this problem. Modern implants may provide better outcomes in the future. Some old techniques have stood the test of time and should not be forgotten.
The priorities when planning the reconstruction are to provide a stable implant, to restore bone stock and to optimise the biomechanics of the hip. The patients functional demands and co-morbidities should be considered as well as the cost-effectiveness of the planned reconstruction.
No benefits of any form have been received from a commercial party related directly or indirectly to the subject of this article.
References
1. Hallstrom BR, Golladay GJ, Vittetoe DA, Harris WH. Cementless acetabular revision with the Harris-Galante porous prosthesis. Results after a minimum of ten years follow-up. J Bone Joint Surg Am. 2004; 86:1706-11. [ Links ]
2. Templeton JE, Callaghan JJ, Goetz DD, Sullivan PM, Johnson RC. Revision of a cemented acetabular component to a cementless acetabular component. A ten to fourteen year follow-up study. J Bone Joint Surg Am. 2001; 83:1706-11. [ Links ]
3. Schreurs BW, Bolder SBT, Gardiniers JWM, Verdonschot N, Sloof TJJH, Veth RPH. Acetabular revision with impacted morsellised cancellous bone grafting and a cemented cup: A 15- to 20-year follow-up. J Bone Joint Surg Br. 2004; 86-B:492-97. [ Links ]
4. Schreurs BW, Keurentjes JC, Gardeniers JWM, Verdonschot N, Slooff TJJH, Veth RPH. Acetabular revision with impacted morsellised cancellous bone grafting and a cemented acetabular component: A 20- to 25-year follow-up. J Bone Joint Surg Br. September 2009 91-B:1148-53. [ Links ]
5. Berry DJ, Muller ME. Revision arthroplasty using an antiprotrusio cage for massive acetabular bone deficiency. J Bone Joint Surg Br. 1992; 74:711-15. [ Links ]
6. Van Haaren EH, Heyligers IC, Alexander FGM, Wuisman PIJM. High rate of failure of impaction grafting in large acetabular defects. J Bone Joint Surg [Br] 2007; 89-B:296-300. [ Links ]
7. Paprosky WG, Perona PG, Lawrence JM. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J Arthroplasty 1994; 9:33. [ Links ]
8. D'Antonio JA. Periprosthetic bone loss of the acetabulum. Classification and management. Orthop Clin North Am 1992; 23:279. [ Links ]
9. Saleh KJ, Holtzman J, Gafni A, et al. Development, test reliability and validation of a classification for revision hip arthroplasty. J Orthop Res 2001;19:50. [ Links ]
10. Engh CA, Glassman AH. Cementless revision of failed total hip replacement: an update. Instr Course Lect 1991; 40:189. [ Links ]
11. Gross AE, Allan DG, Catre M, et al. Bone grafts in hip replacement surgery. The pelvic side. Orthop Clin North Am 1993;24:679. [ Links ]
12. Gustilo RB, Pasternak HS. Revision hip arthroplasty with titanium ingrowth prosthesis and bone grafting for failed cemented femoral component loosening. Clin Orthop Relat Res 1988;111. [ Links ]
13. Campbell DG, Garbuz DS, Masri BA, et al. Reliability of acetabular bone defect classification systems in revision total hip arthroplasty. J Arthroplasty 2001;16:83. [ Links ]
14. Gozzard C, Blom A, Taylor A, et al. A comparison of the reliability and validity of bone stock loss classification systems used for revision hip surgery. J Arthroplasty 2003; 18:638. [ Links ]
15. Johanson NA, Driftmier KR, Cerynik DL, Stehman CC. Grading acetabular defects. The need for a universal and valid system. J Arthroplasty 2010; 3:25 [ Links ]
16. Bonshahi AY, Gambhir AK. Evaluation of a painful total hip replacement. Orthopaedics and Trauma. 2009 23(5):301-306. [ Links ]
17. Egawa H, Powers CC, Beykirch SE, Hopper RH Jr, Engh CA Jr, Engh CA. Can the volume of pelvic osteolysis be calculated without using computed tomography? Clin Orthop Relat Res. 2009;467:181-87. [ Links ]
18. Nishii T, Sakai T, Takao M, Yoshikawa H, Sugano N. Ultrasound Screening of Periarticular Soft Tissue abnormality Around Metal-on-Metal Bearings. J Arthroplasty. 2012;27:895-900. [ Links ]
19. Cahira JG, Tomsa AP, Marshalla TJ, Wimhurstb J, Nolan J. CT and MRI of hip arthroplasty. Clin. Rad. 2007;62:1163-71. [ Links ]
20. Sabah SA, Mitchell AWM, Henckel J, Sandison A, Skinner JA, Hart AJ. Magnetic Resonance Imaging Findings in Painful Metal-On-Metal Hips a Prospective Study. J Arthroplasty. 2011;26:71-76. [ Links ]
21. Temmerman OPP, Raijmakers PGHM, David EFL, Pijpers R, Molenaar MA, Hoekstra OS, Berkhof J, Manoliu RA, md, Teule GJJ, Heyligers IC. A comparison of radiographic and scintigraphic techniques to assess aseptic loosening of the acetabular component in a total hip replacement. J Bone Joint Surg. 2004; 86-A:2456-63. [ Links ]
22. Merkel KD, Brown ML, Dewanjee MK, Fitzgerald RH. Comparison of indium-labeled-leukocyte imaging with sequential technetium-galliumscanning in the diagnosis of low-grade musculoskeletal sepsis a prospectivestudy. J Bone Joint Surg. 1985; 67-A:465-76. [ Links ]
23. Kraemer WJ, Saplys R, Waddell JP, Morton J. Bone scan, gallium scan, and hip aspiration in the diagnosis of infected total hip arthroplasty. J Arthroplasty. 1993;8:611-16. [ Links ]
24. Oswald SG, Van Nostrand D, Savory CG, Callaghan JJ. Three-phase bone scan and indium white blood cell scintigraphy following porous coated hip arthroplasty: a prospective study of the prosthetic tip. J Nucl Med. 1989;30:1321-31. [ Links ]
25. Scher DM, Pak K, Lonner JH, Finkel JE, Zuckerman JD, Di Cesare PE. The predictive value of indium-111 leukocyte scans in the diagnosis of infected total hip,knee, or resection arthroplasties. J Arthroplasty. 2000;15:295-300. [ Links ]
26. Glithero PR, Grigoris P, Harding LK, Hesslewood SR, McMinn DJW. White cell scans and infected joint replacements. Failure to detect chronic infection. J Bone Joint Surg Br. 1993;75-B:371-74. [ Links ]
27. Parvizi J, Ghanem E, Menashe S, Barrack RI, Bauer TW. Periprosthetic infection: What are the diagnostic challenges? J Bone Joint Surg. 2006;88-A:138-47. [ Links ]
28. Parvizi J, Ghanem E, Newberg A, Zhuang H, Alavi A. FDG-PET imaging can diagnose periprosthetic infection of the hip. ClinOrthop Relat Res. 2008:466;6:1338-42. [ Links ]
29. Deirmengian GK, Zmistowski B, O'Neil JT, Hozack WJ. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am. 2011;93:1842-52. [ Links ]
30. Sporer SM. How to do a revision total hip arthroplasty: Revision of the acetabulum. J Bone Joint Surg. 2011;93-A:1359-66. [ Links ]
31. Sporer SM, Paprosky WG. Acetabular revision using a trabecular metal acetabular component for severe acetabular bone loss associated with a pelvic discontinuity. J Arthroplasty. 2006;21(6 suppl 2):87-90. [ Links ]
32. Wendt MC, Adler MA, Trousdale RT, Mabry TM, Cabanela ME. Effectiveness of false profile radiographs in detection of pelvic discontinuity. J Arthroplasty. Article in press. [ Links ]
33. Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84:609-14. [ Links ]
34. Boscainos PJ, Kellett CF, Maury AC, Backstein D, Gross AE. Management of periacetabular bone loss in revision hip arthroplasty. Clin Orthop Relat Res. 2007;465:159-65. [ Links ]
35. Gross AE. Restoration of acetabular bone loss 2005. J Arthroplasty. 2006;21(4 Suppl 1):117-20. [ Links ]
36. Lakstein D, Backstein D, Safir O, Kosashvili Y, Gross AE. Trabecular metal cups for acetabular defects with 50% or less host bone contact. Clin Orthop Relat Res. 2009;467:2318-24. [ Links ]
37. Gaffey JL, Callaghan JJ, Pedersen DR, Goetz DD, Sullivan PM, Johnson RC. Cementless acetabular fixation at fifteen years. A comparison with the same surgeon's results following acetabular fixation with cement. J Bone Joint Surg Am. 2004; 86:257-61. [ Links ]
38. Raut VV, Sidney PD, Wroblewski BM. Cemented revision for aseptic acetabular loosening. J Bone Joint Surg Br. 1995;77:357-61. [ Links ]
39. Schreurs BW, Sloof TJJH, Buma P, Gardeniers JWM, Huiskes R. Acetabular reconstructions with impacted morsellised cancellous bone graft and cement: a 10- to 15-year follow-up of 60 revision arthroplasties. J Bone Joint Surg [Br] 1998; 80-B:391-95. [ Links ]
40. Graham SM, Leonidou A, Aslam-Pervez N, Hamza A, Panteliadis P, Heliotis M, Mantalaris A, Tsiridis E. Biological therapy of bone defects: the immunology of bone allotransplantation. Expert Opin Biol Ther. 2010;10:885-901. [ Links ]
41. Hassaballa M, Mehendale S, Poniatowski S, Kalantzis G, Smith E, Learmonth ID. Subsidence of the stem after impaction bone grafting for revision hip replacement using irradiated bone. J Bone Joint Surg [Br] 2009;91-B:37-43.
42. Emms NW, S. C. Buckley SC, Stockley I, Hamer AJ, Kerry RM. Mid- to long-term results of irradiated allograft in acetabular reconstruction. A follow-up report. J Bone Joint Surg [Br] 2009;91-B:1419-23.
43. Whitehouse M, Blom A. The use of ceramics as bone substitutes in revision hip arthroplasty. Materials 2009;2:1895-907. [ Links ]
44. Timperley A, Ashcroft P, Dunlop D, Hua J. Use of calcium phosphate bone graft substitute for impaction grafting for revision hip arthroplasty: A randomised clinical study. J Bone Joint Surg Br 2011 vol. 93-B no. Supp II 151. [ Links ]
45. Aulakh TS, Jayasekera N, Kuiper J, Richardson JB. Longterm clinical outcomes following the use of synthetic hydroxyapatite and bone graft in impaction in revision hip arthroplasty. Biomaterials 30 (2009) 1732-38. [ Links ]
46. Blom AW, Wylde V, Livesey C, Whitehouse MR, Eastaugh-Waring S, Bannister GC, Learmonth ID. Impaction bone grafting of the acetabulum at hip revision using a mix of bone chips and a biphasic porous ceramic bone graft substitute. Good outcome in 43 patients followed for a mean of 2 years. Acta Orthopaedica 2009; 80 (2): 150-54. [ Links ]
47. McNamara I, Deshpande S, Porteous M. Impaction grafting of the acetabulum with a mixture of frozen, ground irradiated bone graft and porous synthetic bone substitute (Apapore 60). J Bone Joint Surg Br May 2010 vol. 92-B no. 5 617-23. [ Links ]
48. Beswick A, Blom AW. Bone graft substitutes in hip revision surgery: A comprehensive overview. Injury 2011 (42) S40-S46. [ Links ]
49. Jasty M, Harris WH. Salvage total hip reconstruction in patients with major acetabular bone deficiency using structural femoral head allografts. J Bone Joint Surg [Br] 1990;72-B:63-67.
50. Kawanabe K, Akiyama H, Onishi E, Nakamura T. Revision total hip replacement using the Kerboull acetabular reinforcement device with morsellised or bulk graft. Results at a mean follow-up of 8.7 years J Bone Joint Surg [Br] 2007; 89-B:26-31.
51. Perka C, Ludwig R. Reconstruction of segmental defects during revision procedures of the acetabulum with the Burch-Schneider anti-protrusio cage. J Arthroplasty 2001;16:568. [ Links ]
52. Saleh KJ, Jaroszynski G, Woodgate I, Saleh L, Gross AE. Revision total hip arthroplasty with the use of structural acetabular allograft and reconstruction ring: a case series with a 10-year average follow-up. J Arthroplasty. 2000; 15:951-58. [ Links ]
53. Civinini R, Capone A, Carulli C, Villano M, Gusso MI. Acetabular revisions using a cementless oblong cup: five to ten year results. Int. Orth. 2008;32:189-93. [ Links ]
54. Bobyn JD, Stackpool GJ, Hacking SA, Tanzer M, Krygier JJ. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg [Br] 1999;81-B:907-14.
55. Levine B, Della Valle CJ, Jacobs JJ. Applications of porous tantalum in total hip arthroplasty. J Am Acad Orthop Surg 2006;14:646-55. [ Links ]
56. Lingaraj K, Teo YH, Bergman N. The management of severe acetabular bone defects in revision hip arthroplasty using modular porous metal components. J Bone Joint Surg [Br] 2009;91-B:1555-60.
57. Davies JH, Laflamme GY, Delisle J, Fernandes J. Trabecular metal used for major bone loss in acetabular hip revision. J Arthroplasty 2011;26:1245-50. [ Links ]
58. Sporer SM, Paprosky WG. Acetabular revision using a trabecular metal acetabular component for severe acetabular bone loss associated with a pelvic discontinuity. J Arthroplasty. 2006;21:87-90. [ Links ]
59. Berry DJ. Identification and management of pelvic discontinuity. Orthopedics 2001; 24:881. [ Links ]
60. Muller M, Berry DJ. Revision arthroplasty using an anti-protrusio cage for massive acetabular bone deficiency. J Bone Joint Surg [Br] 1992;74-B:711-15.
61. Goodman S, Saastamoinen H, Shasha N, et al. Complications of ilioischial reconstruction rings in revision total hip arthroplasty. J Arthroplasty 2004; 19:436. [ Links ]
62. Sporer SM, Paprosky WG. The treatment of pelvic discontinuity during acetabular revision. J Arthroplasty 2005; 20:79-84. [ Links ]
63. Kosashvili Y, Backstein D, Safir O, et al. Acetabular revision using an anti-protrusion (ilio-ischial) cage and trabecular metal acetabular component for severe acetabular bone loss associated with pelvic discontinuity. J Bone Joint Surg [Br] 2009; 91:870. [ Links ]
64. Nieder E, Elson RA, Engelbrecht E, Kasselt MR, Keller A, Steinbrink K. The saddle prosthesis for salvage of the destroyed acetabulum. J Bone Joint Surg [Br] 1990; 72-B:1014-22. [ Links ]
65. Cottias P, Jeanrot C, Vinh TS, Tomeno B, Anract P. Complications and functional evaluation of 17 saddle prostheses for resection of periacetabular tumors. J Surg Onc 2001; 78:90-100. [ Links ]
66. Donati D, D'Apote G, Boschi M, Cevolani L, Benedetti MG. Clinical and functional outcomes of the saddle prosthesis. J Orthopaed Traumatol 2012; 13:79-88. [ Links ]
67. Benevenia J, Cyran FP, Biermann JS, Patterson FR, Leeson MC. Treatment of Advanced Metastatic Lesions of the Acetabulum Using the Saddle Prosthesis. Clin Orthop Relat Res 2004; 426:23-31. [ Links ]
68. Ahlgren S, Gudmundsson G, Bartholdsson E. Function after removal of a septic total hip prosthesis. A survey of 27 Girdlestone hips. Acta Orthop Scand 1980; 51:541-45. [ Links ]
69. Schroder J, Saris D, Besselaar PP, Marti RK. Comparison of the results of the Girdlestone pseudarthrosis with reimplantation of a total hip replacement. Int Orthop 1998: 22;215-18. [ Links ]
 Reprint requests:
Reprint requests:
Dr C Reid
Email: cecilreid@mweb.co.za














