Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SA Orthopaedic Journal
On-line version ISSN 2309-8309
Print version ISSN 1681-150X
SA orthop. j. vol.11 n.2 Centurion Apr. 2012
CLINICAL ARTICLE
Biting off more than you can chew: Microbiological flora isolated from human and animal bite wounds
Adele VisserI; Hilgaard F VisserII
IHead of Division Clinical Pathology, Department Medical Microbiology, University of Pretoria, National Health Laboratory Services, Tshwane Academic Division
IIOrthopaedic Surgeon, Life Eugene Marais Hospital
ABSTRACT
BACKGROUND: Bite wounds, from human and animal origin, can lead to significant complications if appropriate therapy is not undertaken timeously. A basic knowledge of the microbiological flora is essential for each clinical setting in order to be able to facilitate appropriate empiric antibiotic therapy.
MATERIAL AND METHODS: All patients admitted to the Steve Biko Academic Hospital over a 27-month period with histories of bite wound and taken to theatre for debridement were included in this study. All culture data was collected together with sensitivity profiles for all organisms isolated.
RESULTS: In total, 38 patients were included in this study, with 25 sustaining human bite wounds, 11 sustaining dog bites, and only two with snake bites. The most striking finding is the predominance of Streptococcus spp isolated from human bite wounds. Although a relatively rare finding, Salmonella spp was isolated from one of the patients who sustained a snake bite. A surprising fraction of isolates were resistant to Amox-Clav, with only marginally improved sensitivity rates to second generation cephalosporins and fluoroquinolones.
CONCLUSIONS: This study emphasises the importance of obtaining microbiological cultures on all patients admitted with bite wounds. This will not only assist in surveillance practices, but also provides the clinician with targeted therapy if the empiric antimicrobial should fail.
Key words: Animal bites, human bites, flora
Introduction
Bite wounds, from human and animal origin, can lead to significant complications if appropriate therapy is not undertaken timeously.1-5 An estimated 1% of all emergency room visits in the USA can be attributed to dog and cat bites alone.6 The risk of subsequent infection depends on certain factors, but varies from 20%6,7 in general to 40% in injuries inflicted on the hands.6,8,9 In addition, cat bite wounds are more prone to infection and complications like osteomyelitis and septic arthritis, as the teeth tend to penetrate deeper, with a relatively small drainage site.6,8,9 Human bite wounds can either be sustained during fighting ('clenched fist injury'), or by occlusive biting.10 Subsequent infection depends largely on the local flora found in the biting party's mouth.8
The clinical significance of bite wounds extends beyond the local complications as various systemic complications have also been ascribed to this type of injury. This includes systemic sepsis by Pasteurella multocida,11 Capnocytophaga canimorsuS6 or Salmonellosis,12 rat-bite fever13 and rabies,14 to name but a few.
For these reasons, bite injuries are exceedingly important as they can cause significant systemic complications and also impact greatly on functionality, particularly in the hand.6 It also emphasises the importance of a basic knowledge of human and animal normal mouth flora to ensure appropriate empiric antibiotic therapy.6,8,10
The aim of this study is to describe local epidemiology with regard to the types of bite injuries presenting to Casualty, the associated microbiological causes of infection and the appropriate antimicrobial therapy indicated.
Materials and methods
Patient population
All patients admitted to the Steve Biko Academic Hospital between January 2009 and March 2012 (27 months), with a history of human or animal bite wounds, and taken to theatre for debridement, were included in this study. Clinical data collected included the type of bite wound sustained, as well as patient demographics.
Microbiological sampling, isolation and sensitivity
All microbiological cultures performed were either on swab or tissue samples collected from all patients. The current practice at the Steve Biko Academic Hospital is to obtain multiple swab and/or tissue samples from a single patient during the initial debridement. This is irrespective of the number of bite wounds sustained. The aim is to produce a representative sample to the microbiology laboratory in order to optimise growth yield. These samples were subject to incubation following inoculation on blood-, chocolate- and MacConkey agars, for culture of Gram-positive, fastidious and Gram-negative organisms respectively.
Tissue samples were subject to anaerobic culture on 10% sheep blood agar. Each patient's organisms cultured were compared to samples from the same patient. Finally, sensitivity profiles of all organisms were obtained to evaluate the most appropriate choice of empiric therapy in this clinical setting.
Results
Patient population
In total, 38 patients were included in this study. Of these, the majority sustained human bite wounds (25 patients), followed by dog bites (11 patients). Only two cases of snake bites were admitted over this time, and no cat or rat bites were recorded. The majority of human bite wounds were sustained on the hands and fingers, with a history of fighting-associated injuries.
Dog bites were more common in female patients (male to female ratio of 3:8) and ages ranging from 6 to 76 years (mean 46 years). Human bite wounds were more common in males (male to female ratio of 2:1) with ages ranging from 6 to 58 years (mean of 36 years). As snake bites occurred in only two cases, demographic information in these patient groups was deemed to have limited significance.
Microbiological sampling
In 13 of the total 38 cases, only swab samples were submitted for microbiological analysis. In total, 12 cases had investigations involving sampling using tissue specimens. The remainder (13/38) were investigated using both swab and tissue specimens. Among these patients, correlation between swab and tissue samples could be demonstrated in less than 40% of cases.
Microbiological findings
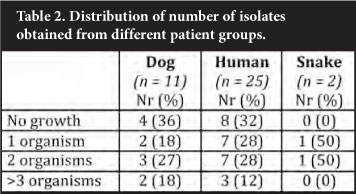
A variety of organisms was isolated from the samples submitted in the investigations of bite cases (Table 1). A variety of organisms was isolated from dog bite wounds. One patient cultured Aeromonas hydrophila from two swabs submitted, the origin of which is unknown. A likely explanation might be that the wound was irrigated with water contaminated with this organism, as its commensal nature in canine mouths has not been described. Although a similar wide range of organisms was found in human bites, the contribution by streptococci was notable. The number of snake bites was too small to draw any conclusions from; however, Salmonella spp was isolated from numerous samples submitted from a single patient. Isolation of a single isolate was an uncommon finding as most patient samples yielded multiple isolates per patient (Table 2). Of note, not a single anaerobic organism was isolated during this study period.
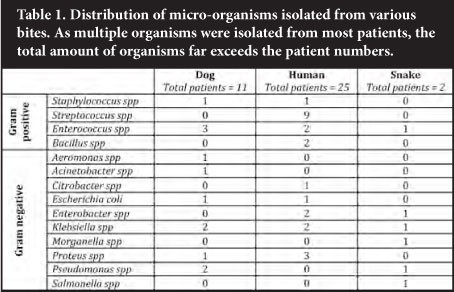
Sensitivity findings
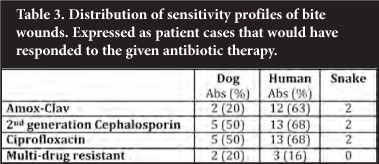
Snake and human bite wounds seemed to show the greatest degree of sensitivity. The majority of these cases (12/19 patients with positive cultures) would have responded to the recommended Amox-Clav combination. Should a fluoroquinolone or a cephalosporin have been used, this would have increased coverage minimally, not justifying this practice. The dog bites seemed to show an increase in resistance, with only 20% of culture-positive samples sensitive to the recommended Amox-Clav combination. Use of a second-generation cephalosporin or a fluoro-quinolone would again have only marginally improved coverage to 50% (Table 3).
Discussion
Bite wounds of varying origin, are a clinical entity the clinician is confronted with regularly.6,10 It has been estimated in US-based studies that management of dog bites alone costs as much as $100 million per annum, taking into account initial management and follow-up visits.1 Of note, this study did not consider loss of work-hours or functionality following injury. Timeous and appropriate therapy will improve outcome, and entails local debride-ment and antibiotic therapy.8 For this reason, a thorough knowledge of possible pathogens associated with specific exposures will be of value in deciding upon empiric antimicrobial therapy.
Human bites
Streptococci are the most commonly isolated organisms, contributing to approximately 80% of cases. In addition, Staphylococci and Eikanella corrodens are also considered important aerobic organisms in this setting.10,15 Anaerobic organisms also frequently contribute to infectious risk following bite wounds. These organisms include Fusobacterium spp, Peptostreptococcus spp and Prevotella spp (Table 4). Anaerobic organisms are typically isolated as part of mixed cultures and are present in as many as 60% of cases.8
Human bite wounds have also been associated with transmission of HIV, Hepatitis B virus, Hepatitis C virus and Herpes virus.16-18 Therefore, post-exposure prophylaxis should be considered in this setting.19
Cat bites
Cat bites are more prone to complicate due to the mechanism of injury. Aerobic organisms associated with infection include Pasteurella spp, Streptococci, Staphylococci and Moraxella spp. Anaerobic infection occurs commonly as part of mixed infection, and is associated with Fusobacterium spp, Bacteroides spp and Porphyromonas spp20 (Table 4).
Dog bites
The majority of dog bites are purulent with abscess formation occurring in approximately 12% of cases.8 Aerobic organisms typically isolated from these types of wounds are similar to cat bites, but Moraxella infections are rare as opposed to Neisserial infections. Anaerobes are also similar but also include Prevotella spp and Capnocytopaga spp20 (Table 4). Particular care should be taken in the case of Capnocytophaga spp infection in splenectomised patients, as they often complicate with systemic sepsis.21 Therefore, early treatment with Amox-Clav is essential to prevent these systemic complications.
Rat and small rodent bites
Rat bites are associated with infection by two organisms, Spirillum minus and Streptobacillus moniliformis13 (Table 4).
These organisms not only cause local sepsis, but also a systemic triad of fever, rash and arthritis, with possible periods of relapse and remission. However, more serious complications like myocarditis, meningitis, amnionitis and disseminated abscess formation have also been described.22
Snake bites
Snake oral flora represents an array of aerobic and anaerobic organisms. Aerobic bacteria include Pseudomonas spp, Staphylococci and a range of Enterobacteriaciae (Escherichia coli, Morganella spp, etc.).23 Although not commonly isolated, Salmonella spp can also be found as a commensal in serpentine oral cavities.24 Anaerobes include organisms from the Clostridium species23 (Table 4).
Although tetanus prophylaxis is an important part of management of all patients sustaining bite wounds, it is of particular importance for snake bite victims. Various case reports have been published describing tetanus as a complication of these wounds.25,26
Microbial sampling
It is evident that both aerobic and anaerobic micro-organisms are well represented in these wound sites. In addition, most studies show multiple organisms isolated from single bite wounds, which reflect the oral flora.20 However, this study showed a notable absence of anaerobic organisms. This is most likely an analytical error, as anaerobes are unequivocally associated with bite wounds. These organisms are fastidious and culture techniques often fail. However, a change of empiric antibiotic choice would require more accurate means of evaluating flora, prior to undertaking this major step.
Anaerobes, as the name suggests, are extremely oxygen-sensitive, and demise rapidly upon exposure. For this reason, sampling and transport to the laboratory should maintain the anaerobic conditions as far as possible.27 Some controversy exists on how this is best performed but the practice of submitting tissue and swab samples does seem to show superior yield if combined with expedient process-ing.27 These samples should be placed in a sterile container with a small amount (1-2 ml) of saline to ensure the sample does not dry out. The aim is to maintain an area of anaerobic conditions within the sample to enable culture.
Prophylaxis and treatment
Current practice guidelines suggest that all bite wounds should prompt a thorough enquiry as to tetanus vaccination status. Although only snake bites are linked to cases of acquiring tetanus, good clinical practice suggests a global approach to all bite wounds.8
Rabies prophylaxis is indicated in not only bite wounds, but also licking or nibbling of uncovered skin. Species associated with possible transmission include dogs, cats, cattle, mongooses, foxes, jackals and rarely baboons. No cases of transmission has been reported for mice, rats or vervet monkeys.28 Although casualty officers often manage these cases, it is essential to be familiar with current guidelines as set forth by the National Institute of Communicable Diseases (fully reviewed in reference 28) (Figure 1).
Empiric antimicrobial therapy is often indicated together with a thorough debridement. Current practice guidelines advise on the use of Amox-Clav as the most appropriate choice in almost all bite wounds (Table 4). In the current study, however, only 20% of dog bites and 63% of human bites would have responded to this choice. It has to be considered that some of the patients may have been exposed to antimicrobials prior to presenting at Steve Biko Academic Hospital. This may have led to the associated sensitivity patterns noted, where an alarming proportion of patients seemed to harbour resistant strains. A thorough clinical and antibiotic history will therefore be of great value in this patient population.
In conclusion, bite wounds inflicted by humans and animals may cause a host of local and systemic effects, and aggressive initial therapy is essential to curb complications. In combination with this, microbiological examination of these wounds may assist the clinician in treatment should empiric antimicrobial therapy fail, and it will also assist in the assessment of resistance patterns in order to be able to then evaluate the possible need to change empiric antibiotic choices.
Declaration of transparency
This study has been approved by the Research Ethics Committee of the University of Pretoria, protocol number 58/2009. No benefits of any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Acknowledgements
Drs A Visser and HF Visser's work is supported by the Discovery Foundation.
References
1. Weiss H, Friedman D, Coben J. Incidence of dog bite injuries treated in emergency departments. JAMA. 1998; 279: 51-53. [ Links ]
2. Mason M. Human bite infections of the hand. Surg Gynecol Obstet. 1930; 51: 591-625. [ Links ]
3. Lauer E, White W, Lauer B. Dog bites: a neglected problem in accident prevention. Am J Dis Child. 1982; 136: 202-204. [ Links ]
4. Elliot D, Tolle S, Goldberg L, miller J. Pet-associated illness. N Engl J Med. 1985; 313: 985-95. [ Links ]
5. Cummings P. Antibiotics to prevent infection in patients with dog bite wounds: a meta-analysis of randomized trials. Ann Emerg Med. 1994; 23: 535-40. [ Links ]
6. Oehler R, Velez A, Mizrachi M, Lamarche J, Gompf S. Bite-related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009; 9: 439-47. [ Links ]
7. Fleisher G. The management of bite wounds. N Engl J Med. 1999; 340: 138-40. [ Links ]
8. Stevens D, Bisno A, Chambers H. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005; 41: 1373-403. [ Links ]
9. Callaham M. Controversies in antibiotic choices for bite wounds. Ann Emerg Med. 1988; 17: 1321-30. [ Links ]
10. Talan D, Abrahamian F, Moran G, Citron D, Tan J, Goldstein E. Clinical presentation and bacteriologic analysis of infected human bites in patients presenting to emergency departments. Clin Infect Dis. 2003; 37: 1481-89. [ Links ]
11. Migliore E, Serraino C, Brignone C, Ferrigno D, Cardellicchio A, Pomero F, et al. Pasteurella multocida infection in a cirrhotic patient: case report, microbiological aspects and a review of literature. Adv Med Sci. 2009; 54(1): 109-12. [ Links ]
12. Kolker S, Itsekzon T, Yinnon A, Lachish T. Osteomyelitis due to Salmonella enterica subsp. arizonae: the price of exotic pets. Clin Microbiol Infect. 2011; 18: 167-70. [ Links ]
13. Dendle C, Woolley I, Korman T. Rat-bite fever septic arthritis: illusrative case and literature review. Eur J Clin Microbiol Dis. 2006; 25: 791-97. [ Links ]
14. Blumberg L, Jong Gd, Thomas J, Archer B, Cengimbo A, Cohen C. Outbreaks in South Africa 2004-2011, the Outbreak Response Unit of the NICD, and the visio of an inspired leader. South Afr J Epidemiol Infect. 2011; 26(4): 195-97. [ Links ]
15. Goldstein E, Citron D, Wield B. Bacteriology of human and animal bite wounds. J Clin Microbiol. 1978; 8: 667-72. [ Links ]
16. Vidmar L, Poljak M, Tomazic J, Seme K, Klavs I. Transmission of HIV-1 by human bite. Lancet. 1996; 347: 1762. [ Links ]
17. Dusheiko G, Smith M, Scheuer P. Hepatitis C virus transmitted by human bite. Lancet 1990; 336: 503-504. [ Links ]
18. Davis L, Weber D, Lemon S. Horizontal transmission of hepatitis B virus. Lancet. 1989; 1: 889-93. [ Links ]
19. Visser A, Visser H, Richter K. Post-exposure prophylasis (PEP): A practical guide. SAOJ. 2009; Autumn: 60-65. [ Links ]
20. Talan D, Citron D, Abrahamian F, Moran G, Goldstein E. Bacteriologic analysis of infected dog and cat bites. N Engl J Med. 1999; 340: 85-92. [ Links ]
21. Ensor C, Russell S, Wittstein I, Conte J. Capnocytophagia canimorsus sepsis in an asplenic heart transplant candidate with left ventricular assist system. Prog Transplant. 2011; 21(2): 121-23. [ Links ]
22. Roughgarden J. Antimicrobial therapy of ratbite fever. A review. Arch Intern Med. 1965; 116: 39-54. [ Links ]
23. Garg A, Sujatha S, Garg J, Acharya N, Parija SC. Wound infections secondary to snake bite. J Infect Dev Ctries. 2009; 3(3): 221-23. [ Links ]
24. Kolker S, Itselzon T, Yinnon A, Lachish T. Osteomyelitis due to Salmonella enterica subsp. arizonae: the price of exotic pets. Clin Microbiol Infect. 2012; 18: 167-70. [ Links ]
25. Habib A. Tetanus complicating snake bite in northern Nigeria: clinical presentation and public health implications. Acta Trop. 2003; 85(1): 87-91. [ Links ]
26. Suankratay C, Wilde H, Nunthapisud P, Khantipong M. Tetanus after white-lipped green pit viper (Tremersurus albolabirs) bite. Wilderness Environ Med. 2002; 13(4): 25661. [ Links ]
27. Pellizzer G, Strazzabosco M, Presi S, Furlan F, Lora L, Benedetti P, et al. Deep tissue biopsy vs. superficial swab culture monitoring in the microbiological assessment of limb-threatening diabetic foot infection. Diab Med. 2001; 18(10): 822-27. [ Links ]
28. Blumberg L, Weyer J, Frean J, Ogunbanjo G. Rabies: an evidence-based approach to management SA Fam Pract. 2007; 49(7): 35-40. [ Links ]
 Reprint requests:
Reprint requests:
Dr A Visser
Email: adele.vis@gmail.com














