Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SA Orthopaedic Journal
versão On-line ISSN 2309-8309
versão impressa ISSN 1681-150X
SA orthop. j. vol.11 no.1 Centurion Jan. 2012
CLINICAL ARTICLE
Is procalcitonin useful in diagnosing septic arthritis and osteomyelitis in children?
E Greeff MBChB(UP); MMed(Orth)(UFS)
Registrar, Department of Orthopaedics, University of the Free State, Bloemfontein
ABSTRACT
BACKGROUND: Early diagnosis of septic arthritis (SA) and osteomyelitis (OM) in children is essential to prevent long-term sequelae. The diagnosis for these orthopaedic emergencies can be difficult and challenging especially in infants. Standard blood tests used for diagnosis have a low specificity. Procalcitonin (PCT) is significantly elevated in bacterial infections and remains low in viral infections and inflammatory conditions. Good positive predictive values for PCT have been obtained in various studies used in paediatric infections but limited studies have examined the role in orthopaedic infections.
METHOD: All children under 14 years of age presenting with signs and symptoms of SA or OM from 1 June 2009 to 31 June 2010 were subjected to standard blood tests with the addition of PCT. The definitive diagnosis was made by clinical, surgical and microbiologic data obtained. A cut-off level of 0.2 ng/mL was used.
RESULTS: Thirty-three patients were included from which 12 were subdivided into the SA/OM group and 21 into an Other diagnosis group which acted as a control. Of the 12 patients in the SA/OM group, eight patients were diagnosed with SA and four with OM. In the SA/OM group, 11 from 12 patients had an increased PCT level compared to four in the Other diagnosis group. The calculated sensitivity of PCT was 92% with a confidence interval of 62-100%; the specificity was 81% with a confidence interval of 58-95%. In this study the sensitivity of CRP was 100% while the specificity 26%. The positive predictive value for PCT in this study was 73% and the negative predictive value was 94%. The accuracy for PCT in SA and OM in this study was 85%.
CONCLUSIONS: The calculated sensitivity and specificity in this study has proved that PCT testing can aid in the diagnosis of SA/OM in children by using 0.2 ng/mL as cut-off level. PCT is also more specific for bacterial infections in this study compared to CRP. Staphylococcus aureus is the most common organism isolated in this series with no resistant organisms seen. Further research is needed with larger numbers to conclusively prove that this specific cut-off for PCT is significant.
Key words: procalcitonin, septic arthritis, osteomyelitis, diagnosis, children
Introduction
Early diagnosis of septic arthritis (SA) and osteomyelitis (OM) in children is essential in preventing long-term sequelae from these infections, especially if the hip is involved. Acute SA can be difficult to diagnose in children especially neonates because the inflammatory response is blunted and signs such as fever, swelling, erythema and pain may be minimal or lacking. Infection at another site (e.g. umbilical catheter), irritability, failure to thrive, asymmetry of a limb position, or displeasure at being handled, may be the only finding in a neonate.1-5
The white blood cell count (WBC) is often normal but the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are usually elevated. The problem with the ESR is that it is non-specific and can be elevated in various conditions such as inflammatory diseases, rheumatic diseases, liver and kidney diseases, neoplasms, dysproteinaemias and anaemia. CRP can also be low in the early phase of acute systemic bacterial infections and is often still low in bacterial abscesses. CRP can also be elevated in viral infections, rheumatological diseases or tissue damage, for example, trauma and surgery. CRP thus reflects disease activity without a bacterial infection needing to be present. Levine et al confirmed that CRP is a better independent predictor of SA than ESR.6 Standard radiographs are generally negative initially but may show soft tissue swelling. Negative cultures are also common in early SA and OM. Ultrasound is inexpensive and convenient and can show intra-articular fluid and abscess formation. A bone scan can confirm the diagnosis 24 to 48 hours after onset in 90 to 95% of patients. MRI can show early inflammatory changes as well as joint fluid and intra- or subperiosteal abscesses. The last two tests are valuable in diagnosing infection but are expensive and time-consuming, possibly leading to a delay in treatment. Li et al confirmed that joint WBC is a better diagnostic test for SA compared to WBC and ESR; however, neither test was diagnostic.7 Aspiration of a suspected septic joint is helpful to confirm the diagnosis but is usually very painful and shouldn't be done routinely outside the operating theatre.8-12
Procalcitonin (a 116-aminoacid peptide) is a precursor of calcitonin. Procalcitonin (PCT) is significantly elevated in bacterial infections, but usually only slightly or moderately elevated in viral infections and non-infectious inflammatory diseases. PCT can be produced by several cell types and many organs in response to pro-inflammatory stimuli, in particular bacterial products without increasing calcitonin. PCT has the highest diagnostic accuracy where levels rise rapidly within 6-12 hrs after an infectious insult with systemic consequences compared to CRP only increasing after 12 hrs after onset of fever. Levels above 2 ng/mL are associated with increased likelihood of the presence of bacterial sepsis. If the level is between 0.5 and 2 ng/mL, a systemic infection cannot be excluded and the PCT should be repeated in 6-24 hrs. An increased level of PCT often correlates with the severity of the disease and mortality.
Most studies have only used PCT in other paediatric infections such as pneumonia and urinary tract infections and not SA or OM. Recent studies using PCT for these emergency orthopaedic infections suggested further work be done and a specific cut-off level determined for these infections.13-20
The aim of this study is to introduce PCT testing in the initial workup of paediatric orthopaedic infections, and to see:
- if it is useful in the diagnosis of orthopaedic infections
- whether it could replace the ESR or CRP
- what is the most accurate diagnostic level.
Patients and methods
A cross-sectional prospective study to determine PCT levels in all children younger than 14 years of age who presented at Pelonomi Hospital from 1 June 2009 to 31 June 2010 with signs and symptoms of acute OM or SA was performed. Thirty-three patients were included in the study. Standard workup included clinical examination, laboratory blood tests and X-rays. The laboratory tests were an FBC, CRP and ESR. The diagnosis was confirmed by clinical signs, the finding of pus at arthrotomy of the involved joint or aspiration and drilling of the bone in theatre and microscopy, culture, and sensitivity (MCS) of material (pus, tissue).
The doctors responsible for initial workup of these patients completed a short data sheet and added PCT to the standard blood tests; no extra blood was required. Blood samples were taken before antibiotic administration. PCT tests were performed with the BRAHMS PCT sensitive kit. The researcher followed these patients up noting the diagnosis and PCT level. The data sheets were collected weekly by the researcher and then analysed at the end of the study period.
The only additional expense was R300 for each PCT test. Sponsorship from Humor Diagnostics, the company supplying the reagents, was requested to offset these costs.
Approval for the study was obtained from the Ethics Committee of the University of the Free State. All patient data were recorded anonymously under a hospital number. Consent was obtained from the head of Pelonomi Hospital, Head of the Orthopaedic Department and the NHLS.
Results were analysed by the Department of Biostatistics UFS and summarised by means, standard deviations or percentiles (numerical variables) and frequencies and percentages (categorical variables). The sensitivity of PCT was calculated with a 95% confidence interval.
Results
PCT tests were done on all 33 cases using the BRAHMS PCT sensitive kit and subdivided into a SA/OM group and Other diagnosis group which acted as a control. The diagnosis of SA was made in eight patients and OM in four patients. The PCT results are summarised in Table I. In this study using PCT as a diagnostic aid for SA or OM the calculated sensitivity was 92% with a confidence interval of 62-100% (Table II). The specificity was 81% with a confidence interval of 58-95%. A cut-off value of 0.2 ng/mL was used in this study. The mean PCT value was 25.04 ng/mL in the SA/OM group ranging from 0.1 to 267 ng/mL. If a cut-off value of 0.5 ng/mL was used the sensitivity would change to 75% and the specificity to 90% and a cut-off of 0.1 ng/mL the sensitivity would change to 100% and the specificity to 76% in this study. The positive predictive value for PCT in this study was 73% with a confidence interval of 45-92%. The negative predictive value for PCT in this study was 94% with a confidence interval of 73-100%. The accuracy for PCT in SA and OM in this study was 85%.
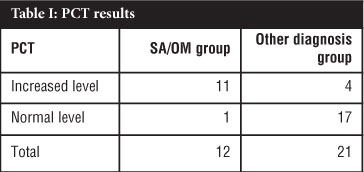
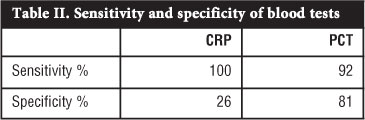
CRP levels were done in all 12 cases in the SA/OM group and all the test levels were elevated. Nineteen CRP tests were done out of 21 in the Other diagnosis group and 14 cases were elevated and five normal. The calculated sensitivity of CRP in this study was 100%, while the specificity was 26%.
In 11 of 12 cases in the SA/OM group, material (pus, tissue) was sent for MCS. In ten cases bacteria were isolated; Staphylococcus aureus was the most common organism and was cultured in eight cases. All of these, Staphylococci were sensitive to cloxacillin.
Discussion
The early diagnosis of SA and OM is essential in avoiding severe complications.1,4,6,7,11,21,22,23 With the importance of early treatment of these patients in mind the diagnosis still remains a challenge. Various methods have been developed and researched to assist the doctor in making the diagnosis but have failed to attain high specificity.14,21 Procalcitonin is a marker of bacterial infection and has proven efficacy used in children with meningitis, pneumonia and urinary tract infections. Procalcitonin rises rapidly in bacterial infections compared to viral infections and other inflammatory conditions where PCT levels remain low.13,21 Previous studies comparing PCT and CRP in severe invasive bacterial infections reported higher sensitivities and specificities.20 Limited studies have examined the role of using procalcitonin in these orthopaedic emergencies to date and have struggled to find the ideal cut-off level.14
The research question was to find out if procalcitonin can aid in the diagnosis of SA and acute OM in children and our study has shown this test to be a useful marker in these infections. The calculated sensitivity of PCT in this study was 92% and the specificity 81%. The positive predictive value for PCT was 73% and the negative predictive value was 94% with an accuracy of 85%. These results are an improvement compared to previous stud-ies.13,14,21 PCT levels appeared more useful than CRP in diagnosing OM or SA in our patients (Table II).
Only four similar studies have been performed to date. The first study, by Butbul-Aviel,13 evaluated PCT values in 44 patients presenting to hospital with fever, limping and suspicion of SA or OM. In this study the calculated sensitivity for PCT was 43.5% and specificity of 100% in bone and joint infections.13 The low sensitivity in the Butbul-Aviel study was due to a specific test and cut-off level used in the study. They used a semi-quantitative rapid immunoassay (BRAHMS PCT-Q, Hennigsdorf, Germany) which is a bedside test that can be done in 30 minutes. This specific test has a low sensitivity with poor discrimination of values around threshold levels and can only be divided into four categories. The results are semi-quantitative and could only be divided into less than 0.5 ng/mL, 0.5 ng/mL-2 ng/mL, 2-10 ng/mL, and more than 10 ng/mL. In this study the authors could not evaluate specific PCT values with their calculated sensitivity and specificity and could only use 0.5 ng/mL as a cut-off level. The Butbul-Aviel study concluded that PCT seems to be a promising marker more for acute OM than for SA and that further research is needed.
Faesch et al21 evaluated PCT levels in 339 patients presenting in a paediatric emergency department for suspected OM or SA. All patients presenting with nontraumatic decreased active motion of a skeletal segment with or without fever were included. The ages ranged from 1 month to 14 years and were divided into three groups.
The first group had confirmed infection with a positive culture and the second presumed infection without positive culture but positive infective markers, purulent bone or joint fluid aspiration or positive scintigraphy. The third group was the non-infected patients with negative markers and normal X-rays and ultrasound. These groups were then compared to each other. Group 1 comprised eight patients, group 2 had 40 patients and group 3 had 291 patients. Comparing group 1 with group 3 the specificity of PCT used as marker of bacterial infection was 96.9%, the sensitivity 25%, the positive predictive value 18.2% and the negative predictive value 97.9%.21 Compared to the Butbul-Aviel study, the sensitivity of PCT was lower but the specificity similar. In the Faesch study a more sensitive PCT test was used where values can range from 0.06 to 50 ng/mL. The same test was used in our study. There are two limitations to the Faesch study. Children with a history of trauma were excluded from the study and as this often precedes OM, as in 25% of our cases, some OM cases may have been excluded distorting the results.1,4,22 Another problem is the very low incidence of positive bacteriological findings which raises some doubts about the accuracy of the investigation and the conclusions drawn.
Fottner et al14 examined PCT and CRP values in 33 patients who presented with symptoms of acute arthritis of unknown origin at their emergency department. The more sensitive PCT test was used, as in the study by Faesch with a cut-off level again of 0.5 ng/mL. Fifteen patients were diagnosed with SA on the basis of microbiological cultures. The calculated sensitivity of PCT in this series was 53.3% with a specificity of 100%. CRP levels in both septic and non-septic arthritis groups were not within normal limits and resulted in a specificity of 0%.14 The sensitivity in this study is similar to the previous two studies concluding that using 0.5 ng/mL as the cut-off level for PCT is not reliable to distinguish between septic and non-septic arthritis. However they found that lowering the cutoff level improved the diagnostic value of PCT, and by using a cut-off of 0.2 ng/mL they attained a sensitivity of 100% and a specificity of 94.4%. Another study by Hügle15 also used a lower cut-off and attained improved sensitivity and specificity for diagnosing joint infections. These findings were also proven in our study where 0.2 ng/mL was also used as the cut-off level for PCT with a much improved sensitivity of 92% and specificity of 81% compared to the previous studies. Using a cut-off level of 0.2 ng/mL for PCT has also achieved a sensitivity and specificity of up to 100% in the diagnosis of bacterial meningitis.17 These findings support the conclusion that by lowering the cut-off level of PCT to 0.2 ng/mL, a more accurate diagnosis of bone and joint infection can be made.
CRP was done in all the cases in the bone or joint infection group and all results were raised. CRP was also raised in 14 of 19 cases in the Other diagnosis group. This finding was also seen in the Fottner study and signifies the low specificity of CRP in diagnosing bone or joint infections.
The most common organism isolated was Staphy/ococcus aureus in 80% of cases in the bone or joint infection group and was sensitive to cloxacillin in all cases. No resistant organisms were seen. This finding correlates with current literature.1,9,21,22,24
There are a few limitations to this study. Further studies will need to be done with larger number of cases to improve the confidence interval of using PCT in bone and joint infections as well as to use the more accurate cut-off of 0.2 ng/mL. Another limitation is whether the human immunodeficiency virus (HIV) has an influence on PCT results. The one case of SA of the knee, known with HIV, had a normal PCT result. No conclusion can be made because not all the cases were tested for HIV but definite further research in this field is needed especially because there is such a high prevalence of HIV in Southern Africa.25 Also in the Other diagnosis group four patients had increased PCT levels of which two needed drainage of abscesses and two intravenous antibiotics to control soft tissue infections. This means that the false positive patients still needed acute surgical or medical management. PCT tests are not cheap and cost approximately R300 a test and $50 in the USA compared to other infective markers. No research has been done on the cost-effectiveness of PCT and it is speculated in one paper that PCT can reduce hospital admissions, theatre time and antibiotic usage.20
Certain recommendations can be made after considering the results of this study. PCT is more accurate in diagnosing bone and joint infections if a lower cut-off level of 0.2 ng/mL is used. This finding is proved in our study and in Fottner and Hügle's papers. In the literature much work has been done creating prediction rules to aid the doctor to distinguish between SA and transient synovitis of the hip. Since Kocher et a/23 started work in 1999 on clinical prediction rules, an evolution of these rules have been made, and Caird et a/26 in 2006 refined the rules. They used the following predictors to aid the diagnosis of SA:
- Temperature > 38.5 °C
- CRP > 2.0 mg/L
- ESR > 40 mm/hr
- Refusal to bear weight
- WCC > 12 000/mm3
The probability of SA when using these prediction rules is 16.9% for zero predictors, 36.7% for one predictor, 62.4% for two predictors, 82.6% for three predictors, 93.1% for four predictors, and 97.5% for five predictors.26 After PCT efficacy has been finally proven in bone and joint infections addition into these prediction rules can aid the diagnosis of these emergencies. PCT levels often correlate with the degree of microbial invasion and decrease rapidly with the addition of antibiotics.20 With a rapid PCT reaction to treatment in mind further research can be done to evaluate PCT effectiveness as a response marker to treatment.
Conclusions
Procalcitonin testing can aid in the early diagnosis of SA or acute OM in children by lowering the cut-off level from 0.5 ng/mL to 0.2 ng/mL. This finding was proven in our study with a sensitivity of 92% and a specificity of 81% as well as in Fottner and Hugle's research. Procalcitonin is more specific for bacterial infections compared to CRP and rises more rapidly than the latter.
Acknowledgements
Thank you to Prof JA Shipley for guidance and advice and to all my colleagues who participated in the study.
This article is the sole work of the author. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
1. Dlabach JA, Park AL. Infectious arthritis. In: Canale ST, Beaty JH. Campbell's Operative Orthopaedics.llth edition. Philadelphia: Mosby Elsevier; 2008. p. 723-50. [ Links ]
2. Staheli LT. Practice of Pediatric Orthopedics. 2nd edition. Philadelphia: Lippincott Williams and Wilkins; 2006. [ Links ]
3. Chapman MW Chapman's Orthopaedic Surgery. 3rd edition. Philadelphia: Lippincott Williams and Wilkins; 2001. [ Links ]
4. Dabov GD. Osteomyelitis. In: Canale ST, Beaty JH. Campbell's Operative Orthopaedics.llth edition. Philadelphia: Mosby Elsevier; 2008. p. 695-702. [ Links ]
5. Min-Kyu N, Ka Em K, Chung-Hung W Septic arthritis and acute osteomyelitis in early infancy. Clinical Neonatology 1999;6(2):9-13. [ Links ]
6. Levine M, McGuire K, McGowan K, Flynn J. Assessment of the test characteristics of C-reactive protein for septic arthritis in children. J Pediatric Orthop 2003;23(3):373-77. [ Links ]
7. Li SF, Cassidy C, Chang C, Gharib S, Torres J. Diagnostic utility of laboratory tests in septic arthritis. Emerg Med J 2007;24(2):75-77. [ Links ]
8. Siegenthaler W. Differential diagnosis in Internal Medicine. 1st English edition. New York:Thieme; 2007. [ Links ]
9. Mathews CJ, Coakley G. Septic arthritis: current diagnostic and therapeutic algorithm. Curr Opin Intern Med 2008;7(5):532-37. [ Links ]
10. Mathews CJ, Kingsley G, Field M, Jones A, Weston VS, Phillips M, et al. Management of septic arthritis: a systemic review. Postgrad Med J 2008;84(991):265-70. [ Links ]
11. Jung ST, Rowe SM, Moon ES, Song EK, Yoon TR, Seo HY. Significance of laboratory and radiologic findings for differentiating between septic arthritis and transient synovitis of the hip. J Pediatric Orthop 2003;23(3):368-72. [ Links ]
12. Bonhoeffer J, Haerberle B, Schaad BS, Heininger U. Diagnosis of acute haemotogenous osteomyelitis and septic arthritis: 20 years' experience at the University children's hospital Basel. Swiss Med Wkly 2001;131:575-81. [ Links ]
13. Butbul-Aviel Y, Koren A, Halevy R, Sakran W Procalcitonin as a diagnostic aid in osteomyelitis and septic arthritis. Pediatr Emerg Care 2005;21(12):828-23. [ Links ]
14. Fottner A, Birkenmaier C, Von Schulze P, Bernd W, Volkmar J. Can serum procalcitonin help to differentiate between septic and nonseptic arthritis? Arthroscopy 2008;24(2):229-33. [ Links ]
15. Hügle T, Schuetz P, Mueller B, Laifer G, Tyndall A, Regenass C. Serum procalcitonin for discrimination between septic and non-septic arthritis. Clin Exp Rheumatol 2009;26(3):453-56. [ Links ]
16. BRAHMS. Guide for the clinical use of procalcitonin (pct) in diagnosis and monitoring of sepsis. [ Links ]
17. Carrol ED, Thomson APJ, Hart CA. Procalcitonin as a marker of sepsis. Int J Antimicrob Agents 2002;20(1):1-9. [ Links ]
18. Meisner M. Procalcitonin: A new, innovative infection parameter. Biochemical and clinical aspects. 3rd edition. New York:Thieme 2000. [ Links ]
19. Maniaci V, Dauber A, Weiss S, Nylen E, Becker KL, Bachur R. Procalcitonin in young febrile infants for the detection of serious bacterial infections. Pediatrics 2008;122(4):701-10. [ Links ]
20. Van Rossum AM, Wulkan RW, Oudesluys-Murphy AM. Procalcitonin as an early marker of infection in neonates and children. Lancet Infect Dis 2004;4(10):620-30. [ Links ]
21. Faesch S, Cojocaru B, Hennequin C, Pannier S, Glorion C, Lacour B, et al. Can procalcitonin measurement help the diagnosis of osteomyelitis and septic arthritis? A prospective trial. Ital J Pediatr 2009;35(1):1-6. [ Links ]
22. Stans AA. Osteomyelitis and septic arthritis. In: Morrissy RT, Weinstein SL. Lovell and Winter's Pediatric Orthopaedics. 6th edition. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 440-91. [ Links ]
23. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am 1999 Dec; 81(12):1662-70. [ Links ]
24. Visser HF, Visser A, Goller K, Goller R, Nel JM, Snyckers CH. Paediatric septic arthritis in a tertiary setting: A retrospective analysis. SAOA. 2010;9(2):92-96. [ Links ]
25. UNAIDS Global report 2010 [homepage on the internet]. World Health Organization 2009. Available from:http://www.unaids.org/globalreport/HIV_prevalence_map.htm. [ Links ]
26. Caird MS, Flynn JM, Leung YL, Millman JE, d'Italia JG, Dormans JP. Factors distinguishing septic arthritis from transient synovitis of the hip in children. A prospective study. J Bone Joint Surg Am 2006 Jun;88(6):1251-57. [ Links ]
 Reprint requests:
Reprint requests:
Dr E Greeff
Postnet Suite 273
Private Bag X87
Bryanston 2021
Cell: 082 927 0568
Email: egreeff@lantic.net














