Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SA Orthopaedic Journal
On-line version ISSN 2309-8309
Print version ISSN 1681-150X
SA orthop. j. vol.9 n.3 Centurion Jan. 2010
CLINICAL ARTICLE
Histomorphometry and the management of metabolic bone disease
EJ Raubenheimer
MChD(UP), PhD(Medunsa), DSc(UP). Chief Specialist, Faculty of Health Sciences, University of Limpopo, Medunsa Campus
ABSTRACT
Static and dynamic histomorphometry provides reproducible data on bone morphology and metabolism. The series of selected abbreviated case reports presented in this manuscript illustrates the role of histomorphometry in the diagnosis and management of metabolic bone disease states.
Introduction
The application of histomorphometry to the diagnosis and management of metabolic bone diseases was covered by previous reviews.1-3 The technique involves the taking of a trans-iliac bone biopsy after labelling the skeleton with two cycles of tetracycline administration separated by at least 14 days and preparation of non-demineralised microscopic sections. Histomorphometric analyses provide quantifiable and reproducible data on the thickness of the cortical plates, volume and width of the trabecular bone, extent and rate of mineralisation of osteoid, and osteoclastic activity. The data obtained are correlated with clinical, radiological and biochemical findings. This manuscript is aimed at presenting abbreviated case reports that demonstrate the application of histomorphometry to the multi-disciplinary diagnosis and management of metabolic bone disease.
Case 1
A 13-year-old male patient presented with a history of intermittent atraumatic bone fractures for the past four years. The patient was bedridden for seven months and frequently complained about abdominal pain and diarrhoea. He received long-term prednisone and anti-inflammatory drugs for atypical juvenile dermatomyositis diagnosed at a rheumatology clinic. Urinary biochemistry, serum calcium and PTH concentrations were normal, alkaline phosphatase mildly increased and 25-hydroxyvitamin D (25(OH)D) was decreased. Celiac disease (a common cause for bone deficiency in patients with abdominal symptoms) was serologically excluded. Radiographs showed healing fractures of both femurs and diffuse osteopaenia. A transcortical trephine biopsy of the iliac crest was taken and processed according to the protocol for histomorphometry.1 The bone marrow showed an increase in mature eosinophils. Histomorphometry revealed severe thinning of the cortex and an increased trabecular bone volume. The latter was due to an increase in homogenous osteoid deposited by normal-appearing osteoblasts. The surface of the trabecular bone showed several empty Howship's lacunae, many of which were filled with osteoid. Eighty-seven per cent of bone surfaces were covered by osteoid (n=18.9%, SD 5%). Mineralisation fronts in the osteoid were focally broad and ill-defined. Tetracycline lines were present on 8% of bone surfaces (n=12.8%, SD2.3%) and were less than 4 microns apart (n=6.4µ, SD1µ) (Figure 1). Further examination for the cause of the bone marrow eosinophilia revealed intestinal parasitic infestation (Taenia solium). The significant decrease in cortical bone was undoubtedly the direct cause for the repeated fractures as cortical bone constitutes 85% of skeletal mass.4 Abundant osteoid was indicative of adequate osteoblastic recruitment and a lack of concomitant mineralisation activity, histomorphometric features of hypertrophic rickets. The normal PEI and homogenous nature of the osteoid argued against hypophosphatemic rickets in which the osteoid usually has a laminar appearance.5 Osteoblasts and mineralised bone had normal morphologies excluding a diagnosis of osteogenesis imperfecta.
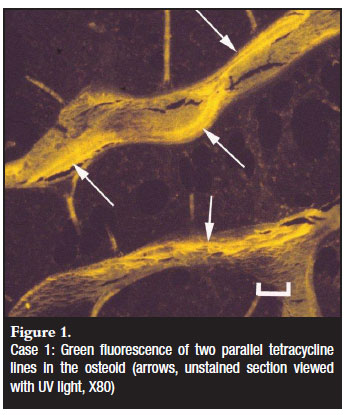
A deficiency of calcium at osteoblastic level was the principal cause for the reduced mineralisation of the wide osteoid seams (as reflected by the reduced distance between the tetracycline lines). The reasons for the unavailability of calcium at cellular level were multi-factorial. The low 25(OH)D concentration was the result of the lack of sun exposure and contributed to a reduced uptake of dietary calcium. Long-term corticosteroid therapy inhibited intestinal mineral uptake and reduced osteoblast mediation of mineralisation. Intestinal parasitic competition for dietary mineral added another parameter to the complex pathogenesis. The lack of loading of the skeleton due to the bedridden state of the patient was furthermore a potent stimulus for mineralised bone loss, a phenomenon particularly relevant to the growing skeleton.6 As some degree of mineralisation occurred during the period of hospitalisation (reflected by the broad mineralisation front of recent onset) a dietary deficiency before admission was also suspected. Treatment of the parasitic infestation, an alternate anti-inflammatory drug with a less deleterious effect on the skeleton, cholecalciferol administration and a mineral-supplemented diet were advised. In addition, restoration of physical activity provided a holistic approach to a protracted management regimen. Eight months after initiation of therapy a follow-up biopsy revealed restoration of bone volumes and mineralisation activities.
Case 2
An 8-year-old female from Lesotho presented with diplegia, progressively decreased mobility and failure to thrive. She sustained a fracture of her femur during normal playground activities. A skeletal radiological survey showed generalised osteopaenia. All bone metabolites fell within normal age-matched limits, and urinary metabolic studies were unremarkable. A transcortical iliac crest biopsy was performed according to protocol.1 Histomorphometry showed reductions of the inner cortical plate thickness and a mean trabecular bone width (Figure 2). All bone surfaces were smooth and no active osteoblasts were noted. No recently formed woven bone was present, excluding a possibility of a late manifestation of osteogenesis imperfecta. The inner plate of the cortical bone showed 35% of the surface area occupied by empty Howship's lacunae (n=5.1%, SD 0.6%). Scattered active osteoclasts were present. Recruitment of osteoblasts was zero as all bone surfaces as well as the Howship's lacunae were devoid of osteoblasts and osteoid. Mineralisation activity as reflected by tetracycline uptake was zero. The findings were in line with those of a recent onset negative shift in bone formation with increased bone resorption. Thyroid hormone biochemistry was requested and hypothyroidism due to an endemic goitre confirmed. The increase in Howship's lacunae with active osteoclasts is proof of the selective stimulatory effect of TSH acting via receptors on osteoclasts.7 Management of the case entailed the establishment of an euthyroidal state, prescription of a mineral-supplemented diet and step-wise reintroduction of physical activity. The case was lost for follow-up.

Case 3
A 19-year-old male presented with a history of renal arterial thrombosis as a child with chronic renal failure. Skeletal deformities had become progressively worse over the last three years on renal dialysis. Six months before the bone biopsy a successful renal transplant was performed. Bone scans revealed generalised osteopaenia. Renal function tests, PTH and calcium concentrations were normal, 25(OH)D low and phosphate excretion index determined on a 24-hour urine sample within upper normal limits. The patient had received corticosteroids and cyclophosphamide since the renal transplant. An iliac crest biopsy was performed according to the protocol for histomorphometry.1 The three main types of bone disease in patients with renal failure (renal osteodystrophy) are osteitis fibrosa (high turnover hyperparathyroid bone disease), osteomalacia and adynamic bone disease.8 Only histomorphometry can readily distinguish the three types of renal osteodystrophy.9 Although the renal transplant suspended the pathogenesis of skeletal resorption in this case, elements of all three microscopic entities of renal osteodystrophy were present. A universal feature of renal failure is hyperplasia of the parathyroids and a rise in serum PTH in response to hypocalcaemia. Foci of inactive tunnelling resorption and osteitis fibrosa, important features of previous high turnover hyperparathyroid bone disease, were evident (Figure 3). Parathyroidectomy was however not indicated due to a suspension of osteoclasts activity through the successful renal transplant. The volume of osteoid was increased and mineralised cortical bone reduced in width - histomorphometric data compatible with osteomalacia. Mineralisation activity was, for practical purposes, not demonstrable. If mineralisation of the osteoid could be induced, the Howship's lacunae would mineralise and disappear.

The lack of mineralisation activity was addressed through dietary mineral and cholecalciferol supplementation, and moderate skeletal loading with supervised biokinetic activity was advised. Factors that impacted on mineral metabolism in this case included physical activity and immunosuppressive therapy as discussed in Case 1. In renal dialysis patients, the potential toxic effect of aluminium on osteoblasts should also be considered as a potential factor hampering bone mineralisation. Stains for aluminium deposits in the bone marrow were however negative. Follow-up DXA measurements after one year showed a mild increase in bone mineral density. The patient refused a follow-up bone biopsy.
Case 4
A 62-year-old male patient presented with generalised skeletal osteopaenia and diffuse bone pains. The patient gave a history of hypercalcaemia with nephrocalcinosis one year prior to the diagnosis of the squamous cell carcinoma of the bronchus for which a pneumonectomy had been performed eight months previously. Mediastinal lymph nodes showed metastatic deposits. PET scans identified several foci of FDG uptake in the axial skeleton and metastatic deposits were suspected. The patient refused postoperative chemotherapy but gave consent for a bone biopsy for histomorphometry. Upon admission for the latter, serum calcium concentrations were found to be slightly elevated and 25(OH)D decreased. Albumin and alkaline phosphatase concentrations and urinary mineral biochemistry were within normal limits. Intact s-PTH concentration was low. A transcortical iliac crest biopsy was performed according to protocol the for bone histomorphometry. 1 The biopsy showed a moderate reduction of cortical and trabecular bone. No osteoblastic activity or demonstrable osteoid were present and mineralisation activity (tetracycline lines) could therefore not be demonstrated. Noteworthy was an increase in osteoclasts with foci of active tunnelling resorption (Figure 4) despite reduced PTH concentrations. The effect of PTHrP (parathyroid hormone-related protein, produced by the metastatic deposits of the squamous cell carcinoma) on bone was suspected. This was supported by the reduced PTH- and increased calcium concentrations. Uncoupling of osteoblast and osteoclast activities distinguishes PTHrP-induced bone loss from hyperparathyroidism and is an important histomorphometric characteristic of humeral hypercalcaemia of malignancy (HHM).10 HHM was most likely the cause for the past nephrocalcinosis of the patient. As in our case, relationships are frequently demonstrable between the effect of PTHrP on bone and HHM11,12 as well as 25(OH)D and PTH concentrations. Through the release of PTHrP, cancer cells stimulate osteoclast activity both at the site of the metastatic deposit as well as at distant skeletal sites leading to localised osteopaenia (manifesting as osteolytic metastases) and distant diffuse skeletal osteopaenia as in our case.

Management of the diffuse skeletal osteopaenia under these circumstances would include reducing the bulk of malignant cells through adjuvant therapy, as well as the administration of bisphosphonates. The latter group of drugs inhibits osteoclastic bone resorption, corrects HHM and improves quality of life in terminal cancer patients by reducing bone pains and preventing the development of new osteolytic lesions.13 Recent cases presented at the 2010 annual meeting of the American Association of Orthopedic Surgery meeting in New Orleans indicate unusual fractures in patients on bisphosphonates for more than 4 years. These reports are the first on long-term bisphosphonate therapy and may introduce recommendations for periodic drug cessation in order to prevent fractures. Chemotherapy and radiotherapy with the administration of corticosteroid drugs may impact negatively on all parameters of bone metabolism and complicate the management of the skeletal deficiencies of the cancer patient. Despite progression of the malignancy, two-year follow-up bone scans indicated stabilisation of skeletal mineral density and no new skeletal metastatic deposits.
Case 5
A 6-month-old baby presented with a history of multiple fractures since birth. All biochemical parameters were within normal limits and osteogenesis imperfecta was suspected. A transcortical iliac crest biopsy was taken three days after two cycles of tetracycline administration 14 days apart. The cortical bone showed an irregular thickness varying between 60-400 µm (n=750 µm at age 6 months). The trabecular bone volume was normal. The volume of woven bone in both the cortex and trabeculae was high at the expense of lamellar bone. Osteoblasts were spindle-shaped, resembled fibroblasts and deposited bone that failed to show lamellar transformation (Figure 5). No Haversian systems were present in the cortices.

The osteocyte lacunae were large and an increased number of osteocytes per unit area was counted. The microscopic features were compatible with a diagnosis of osteogenesis imperfecta. Osteogenesis imperfecta involves all tissues with a type I collagen content and may manifest over a wide age range. The mode of inheritance, severity and clinical manifestations depends on the effect of the gene mutation on the collagen molecule.14,15 Management of children who survive is focused on all possible ways of increasing bone mass and includes bisphosphonates and hormone replacement after menopause.
Case 6
A 13-year-old patient, ill for the past three years and diagnosed with chronic lung disease, presented with fractures of the left humerus and femur. The patient had received treatment for tuberculosis in the past although all tests for the infection were negative. Bone scans showed generalised osteopaenia with increased metaphyseal uptake in several bones suggestive of leukaemia. Blood smears however showed plasmacytoid lymphocytes only. Serum and urine metabolic screening provided no additional information.
A transcortical iliac crest biopsy was performed as prescribed by the regimen for histomorphometry. Collections of uni-nuclear histiocytes with round cytoplasmic inclusions were present in the marrow spaces. Silver stains showed numerous yeast cells (Figure 6). A diagnosis of disseminated histoplasmosis was suggested and mycological confirmation recommended. The patient demised before antifungal therapy was commenced. This case illustrates an unexpected finding for a metabolic bone clinic.

Case 7 A 15-year-old male patient presented with abnormal exostosis in the external ear canal and thickening of the temporal bone with progressive deafness. All biochemical and haematological parameters were within normal limits, except for a mild increase in alkaline phosphatase. The patient received two cycles of tetracycline for dynamic bone histomorphometry1 and biopsies were taken of the iliac crest, temporal bone and an exostosis of the occiput. A fragment of each biopsy was submitted for transmission electron microscopy. The sections of the bone taken from the iliac crest showed grossly thickened cortices (1 863 µm), increased trabecular bone volumes (32.4%), cartilage inclusions (Figure 7), elevated parameters of osteoid deposition and a normal population of osteoclasts per linear bone surface. No Haversian systems were noted. Biopsies from the other sites showed thick lamellar bone with small marrow spaces. An exhaustive search with the transmission electron microscope revealed two osteoclasts, both without cytoplasmic ruffled borders. The findings were compatible with those reported for osteopetrosis.16 Osteopetrosis, from a clinical view point, is a heterogeneous group of diseases and subsequently various classification systems exist. Because bone formation is normal or even excessive and bone resorption suppressed, consensus is that the primary defect lies with the osteoclast lineage.

The complications include bone marrow displacement with pancytopaenia, pathological fractures and pressure-induced neural atrophy with, among other symptoms, neuralgic pains and conductive hearing loss. Repopulation of the bone marrow with compatible bone marrow stem cells promises a future for the management of this chronic incapacitating disorder.
Conclusion
The cases demonstrate the multi-factorial pathogenesis of metabolic bone diseases where co-morbidities impact on the primary bone disease state. Histomorphometry is the only modality that accounts for all variables and accurately reflects the status of bone metabolism on cellular level.
References
1. Raubenheimer EJ. Part I: Metabolic bone disease: histomorphometry as a diagnostic aid. SA Orthopaedic Journal 2008;7(4):19-23. [ Links ]
2. Raubenheimer EJ. Part II: Metabolic bone disease: Recent developments in the pathogenesis of rickets, osteomalacia and age-related osteoporosis. SA Orthopaedic Journal 2009; 8(1): 38-42. [ Links ]
3. Raubenheimer EJ. Part III: Metabolic bone disease: Recent developments in the pathogenesis of endocrine-, drug-, genetic-, renal-, HIV-, and malignancy- induced bone disease. SA Orthopaedic Journal 2009; 8(3): 18-23. [ Links ]
4. Shao YY,Wang L, Ballock RT. Thyroid hormone and the growth plate. Rev Endocr Metab Disord 2005; 7(4):265-71. [ Links ]
5. Jowsey J. Metabolic Diseases of Bone. Philadelphia: W B Saunders Company; 1977:172-191. [ Links ]
6. Smith R, Wordsworth PW. Clinical and Biochemical Disorders of the Skeleton. Oxford: Oxford University Press, 2005; 154. [ Links ]
7. Galliford TM, Murphey E,Williams AJ, Bassett JH,Williams GR. Effects of thyroid status on bone metabolism: a primary role for thyroid stimulating hormone or thyroid hormone? Minerva Endocrinol 2005;30(4):237-46. [ Links ]
8. Goodman WG, Coburn JW, Slatopolsky E. Renal osteodystrophy in adults and children. In: Favus M (ed.) Primer on the metabolic bone disease and disorders of mineral metabolism, 4th edn. (1999). Philadelphia, Lippincott, Williams and Wilkens, 347-63. [ Links ]
9. Dahl E, Nordal KP, Halse J. Chronic renal failure: diagnostic measures before parathyroidectomy. Scan J Urol Nephrol 1994;28:291-4. [ Links ]
10. Roberts MM, Stewart AF. Humeral hypercalcemia of malignancy. In: Favus M (ed.) Primer on the metabolic bone disease and disorders of mineral metabolism, 4th edn (1999). Philadelphia, Lippincott, Williams and Wilkins, 203-7. [ Links ]
11. Bundred NJ, Radcliffe WA,Walker RA et al. Parathyroid hormone-related protein and hypercalcemia in breast cancer. Br Med J 1991;303:1506-9. [ Links ]
12. Kohno N, Kitazawa SW, Fukase M, et al. The expression of parathyroid hormone-related protein in human breast cancer with skeletal metastases. Surg Today 1994;24:215-20. [ Links ]
13. Greenspan SL, Bhattacharya RK, Sereika SM, Brufsky A, Vogel VG. Prevention of bone loss in survivors of breast cancer: A randomized, double- blind, placebo-controlled clinical trial. J Clin Endocrinol Metab 2007;92(1)131-6. [ Links ]
14. Spranger J, Cremin B, Beighton P. Osteogenis imperfecta congenital. Features and prognosis of a heterogenous condition. Pediatr Radiol 1982;12:21-7. [ Links ]
15. Smith R, Wordsworth PW. Clinical and Biochemical Disorders of the Skeleton. Oxford: Oxford University Press; 2005:265-289. [ Links ]
16. Helfrich MH, Aronson DC, Everts V et al. Morphological features of bone in osteopetrosis. Bone 1991;12:411-19. [ Links ]
 Reprint requests:
Reprint requests:
Dr EJ Raubenheimer
Tel: +27 12 521 4838
Email: ejraub@ul.ac.za
The content of the article is the sole work of the author. No benefits of any form have been or are to be received from a commercial party related directly or indirectly to the subject of the article.














