Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SA Orthopaedic Journal
On-line version ISSN 2309-8309
Print version ISSN 1681-150X
SA orthop. j. vol.9 n.3 Centurion Jan. 2010
CLINICAL ARTICLE
Tuberculosis of the sternum in patients with infective spondylitis
W HaynesI; S GovenderII
IMBBCh(Wits), FC(Orth)SA. Registrar. Department of Orthopaedic Surgery, King George Hospital, University of KwaZulu-Natal
IIMBBS, MD, FRCS. Professor and Head of Department. Department of Orthopaedic Surgery, King George Hospital, University of KwaZulu-Natal
ABSTRACT
OBJECTIVE: Tuberculous osteomyelitis of the sternum is rare. With the increased incidence of HIV seropositivity, atypical musculoskeletal manifestations of tuberculosis (TB) are becoming more common. The aim of this study was to review the clinical presentation, diagnosis, treatment and outcome of patients presenting with tuberculous involvement of the sternum.
METHODS: A retrospective chart review was performed on all patients with tuberculous involvement of the sternum, with specific reference to: age, gender, clinical presentation, HIV status, CD4 count, antiretroviral treatment, co-morbidities, diagnosis, management and outcomes.
RESULTS: Fourteen patients with sternal TB were reviewed at King George V Hospital from May 2006 to June 2008. The average age was 17.5 years. All 14 cases had infective spondylitis with associated sternal involvement. There were no cases of primary sternal TB. There were three cases of delayed diagnosis that presented with severe spinal and anterior chest wall deformities. The diagnosis was suspected clinically in seven patients and an incidental finding in the remaining seven. Sternal lesions on lateral radiographs were only picked up in two patients. MRI was diagnostic in all 14 patients. The disease was multifocal in all cases. Seven patients had multiple level, non-contiguous infective spondylitis. It was noted that all patients had a lesion in the dorsal spine. Six patients were HIV-positive, with average CD4 counts of 277 (110-696). None of the patients were on antiretroviral therapy prior to the diagnosis of sternal TB. The sternal component of the disease was managed non-operatively with antituberculous chemotherapy. Average follow-up was 14 months (3-22). All patients responded well to therapy.
CONCLUSION: Atypical manifestations of TB are becoming increasingly prevalent. Due to increased awareness among clinicians and improved imaging techniques, the diagnosis of sternal TB is easier to make and treatment with antituberculous chemotherapy appears to be an effective management strategy.
Introduction
The musculoskeletal manifestations of tuberculosis (TB) account for approximately 9% of all cases,1,2,3 with the incidence of sternal TB being reported as less than 1%.4 The dual epidemic of TB/HIV has led to an increased incidence of atypical extrapulmonary manifestations of TB.5 Early diagnosis and treatment is critical to reducing morbidity and mortality in these patients. The aim of this study was to review the clinical presentation, diagnosis, treatment and outcome of patients presenting with TB of the sternum.
Materials and methods
A retrospective chart review was carried out at King George V Hospital between 2006 and 2008. Of the patients presenting to the spinal unit with infected spondylitis, those who had sternal lesions as well as those with subclinical radiographic signs of suspected sternal TB were included in the study. Epidemiological and clinical data with specific reference to demographics, clinical presentation, HIV status, CD4 count, antiretroviral treatment, diagnosis, management and outcomes were gathered.
Past exposure to TB was noted. Details of the clinical examinations were recorded in all patients. Patients had haematological investigations including full blood count, erythrocyte sedimentation rate and liver function test. All patients received pre-test HIV counselling and consented to HIV testing. Following post-test counselling, CD4 counts were done on those patients who tested positive and antiretroviral therapy was commenced as per protocol.
Postero-anterior and lateral radiographs of the chest and MRIs of the spine were performed in all patients, as were MRIs of the spine.
The diagnosis of TB of the sternum was made on clinical as well as characteristic X-ray and MRI findings. All patients were commenced on empiric intense phase antituberculous treatment (rifampicin, isoniazid, pyrazinamide, ethambutol) for a minimum of 12 months.
Results
Fourteen patients (nine male, five female) with sternal TB were included in the study. There was a history of previous pulmonary TB in five patients and a positive TB contact in six patients. The mean age was 17.5 years (4 to 45 yrs). All patients had infective spondylitis with associated sternal involvement. There were no cases of primary sternal TB. Only one patient presented with classic symptoms of fever and night sweats. Twelve patients gave a history of loss of weight. Four patients presented with clinical symptoms suggestive of sternal TB - these included anterior chest wall tenderness (n=3) and a healed anterior chest wall sinus in one patient. There were three cases of delayed diagnosis that presented with severe spinal and anterior chest wall deformity (Figure 1). The diagnosis was made as an incidental finding in the remaining seven patients. The majority of the patients (n=13) were from rural KwaZulu-Natal.

Sternal lesions on lateral radiographs were only noted in two patients (Figure 2). MRI performed in all 14 patients showed hypointense lesions on T1-weighted images and hyperintense lesions on T2-weighted images involving the sternal bone marrow (Figure 3). The manubrium was involved in nine patients and the body of the sternum in five patients. There was also evidence of peristernal soft tissue involvement and mediastinal lymphadenopathy in three of the patients.


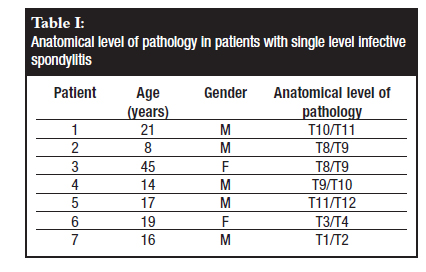
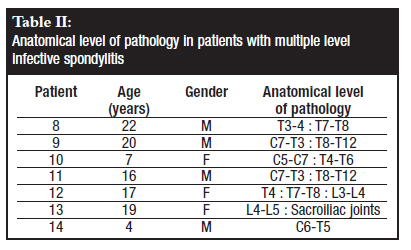
The disease was multifocal in all cases, presenting with single level infective spondylitis (Table I) in seven patients and multiple level, non-contiguous infective spondylitis in the other seven patients (Table II). Three patients were noted to have active pulmonary TB at the time of presentation. In our series it was noted that all patients with sternal TB had a lesion in the dorsal spine.
The ESR was raised in 86% (n=12) of the patients.
Six patients were found to be HIV-positive, with an average CD4 count of 277 cells/mm3 (110-695). None of the patients were on antiretroviral therapy prior to the diagnosis of sternal TB. Five patients were commenced on antiretroviral therapy following post-test counselling. One patient refused antiretroviral therapy.
The sternal component of the disease was managed nonoperatively with intense phase antituberculous chemotherapy. The patients were seen initially at 1-monthly intervals, for the first 3 months, followed by 3-, 6- and 12-monthly visits. Average follow-up was 14 months (3-22). Patients were assessed clinically, haematologically and radiologically. All patients responded well to therapy with no evidence of recurrence.
Discussion
Sternal TB is a rare manifestation of musculoskeletal TB, with an overall quoted incidence of less than 1%.4,6 Sternal TB was first reported in 1918 by Vaughn.7 Since then the literature is confined to isolated case reports1,4,7,8 and a series of 14 patients by Khan et al in 2007.4
There is an increased incidence in TB endemic countries.1 In a series of 980 patients from India, Tuli et al9 reported an incidence of sternal lesions of 1.4% in those patients with musculoskeletal TB. In a study from England and Wales, Davies et al10 reported an incidence of 0.05% in 4 000 patients with TB.
The pathogenesis of sternal TB is usually secondary to reactivation of latent loci or as a late complication of pulmonary TB.2,4,11 Seeding of tuberculous bacilli to the sternum occurs via haematogenous or lymphatic dissemination.12 Direct retrosternal extension from mediastinal lymph nodes is another mechanism by which the sternum may be involved.13 Rubenstein et al14 described a case of direct implantation following sternotomy for coronary artery bypass graft.
TB of the sternum has been described as occurring in an isolated primary form as well as presenting as part of a multifocal disease process.4,8,15 Radiological evidence of pulmonary involvement has been seen in up to a third of patients16 and has also been described as part of a disseminated multifocal picture,4 as is the case in our study. Sternal TB may follow BCG vaccination.17
Known risk factors for TB include: corticosteroid therapy, malnutrition, low socio-economic status, ethanol abuse, history of exposure to TB, HIV infection and immunocompromised states.1 With the increased incidence of HIV, atypical manifestations are becoming more common.5,18,25 The two diseases represent a deadly combination since they are more destructive together than either disease alone. TB is difficult to diagnose, and progresses faster, in patients who are HIVpositive.5 Despite the advent of highly active antiretroviral therapy (HAART), atypical TB is still more frequent than in the HIV-negative population. In our study it was noted that the majority of patients with multiple level involvement were HIV-positive (85.7%). Only one patient in the group of single level disease was HIV-positive.
TB of the sternum is more common in young adults,11,19 affecting more males than females. It has been diagnosed in patients as young as 9 months17 and as old as 82 years.11
In the early stages of the disease these patients present with subtle, non-specific symptoms, requiring a high degree of suspicion.2,18 Clinically patients may present with a tender anterior chest wall mass or a chronically draining sinus.4,8,20-22 The differential diagnosis of a sternal lesion includes: inflammatory conditions such as acute pyogenic osteomyelitis; sub-acute osteomyelitis; fungal, actinomycosis and parasitic infections; malignancy and sarcoidosis.18
Plain radiography is neither sensitive nor specific for picking up lesions in the sternum.2 On lateral radiographs one can appreciate soft tissue masses with areas of bone lysis and cortical sclerosis, usually occurring late in the disease process. Computed tomography of the chest provides additional information regarding anatomical localisation as well as the magnitude of the lesion, but does not offer much in terms of soft tissue involvement. MRI is the ideal imaging modality for sternal TB. It offers multi-planar capabilities and provides excellent spatial and contrast resolution.2 It is sensitive for determining site and extent of the lesion and the degree of soft tissue and bone marrow involvement. These lesions are hypointense on T1-weighted images, and hyperintense on T2-weighted images. They show enhancement on gadolinium contrast-enhanced MRI. Three-phase technetium bone scintigraphy is reported to have a high sensitivity and specificity for osteomyelitis but does not differentiate between TB and other infecting agents.11 The value of this investigation is as a screening tool, thus alerting the physician to unsuspected areas of increased uptake.
Definitive diagnosis can be made with histological and microbiological examination of sternal biopsy material.8,12,13 The absence of histological confirmation of diagnosis is a limitation of this review. In our environment we felt that the high incidence of TB together with classic X-ray and MRI findings, together with the patients' clinical response, were sufficient evidence to institute antituberculous therapy without a formal tissue diagnosis.
There is no consensus regarding optimal treatment of TB of the sternum. The multidrug antitubercular chemotherapy is the treatment of choice, with or without surgical debridement.4,23 Surgical debridement is indicated in those patients with large sequestrated bone fragments or in those who do not respond to antitubercular therapy. Some authors advise aggressive debridement with primary closure as an adjunct to chemotherapy so as to prevent recurrence or the formation of a chronic sinus.20,22,24 Fortunately we had none of these complications. It is recommended that for extrapulmonary TB, a minimum of 9 months of treatment be given.8,18 In susceptible organisms the initial intense phase is changed from four-drug therapy (rifampicin, isoniazid, pyrazinamide, ethambutol) to two-drug continuation phase therapy (rifampicin and isoniazid) after 2 months. However, in HIV-positive patients with extrapulmonary TB it is advised that ethambutol be added to the continuation phase.8 In our institution we treat all patients with intense phase therapy for a minimum of 12 months.
Conclusion
The dual epidemic of TB and HIV has led to an increase in atypical manifestations of TB. Together with increased awareness and improved imaging these lesions are being diagnosed more frequently. Early diagnosis and early antituberculous chemotherapy are essential in the successful management of this disease.
References
1. Jain VK, Singh Y, Shukla A, Mittal D. Tuberculous osteomyelitis of sternum: a case report. J Clin Diag Res 2007 June;3:163-7. [ Links ]
2. Shah J, Patkar D, Parikh B et al. Tuberculosis of the sternum and clavicle: image findings in 15 patients. Skeletal Radiol 2000;29:447-53. [ Links ]
3. Gopal R, Padmavathy S. Extra-pulmonary tuberculosis - a retrospective study. Indian J Tub 2001;53:225-6. [ Links ]
4. Khan SA, Varshney MK, Hasan AS, Kumar A, Trikha. Tuberculosis of the sternum. J Bone Joint Surg [Br] 2007; 89:817-20. [ Links ]
5. Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the hiv epidemic. Arch Intern Med 2003;163:1009-21. [ Links ]
6. Kalra P, Sharinia BK, Banerjee CK, Khosla VK. Sternal involvement in disseminated tuberculosis. J Assoc Phys India 1988;36:292-3. [ Links ]
7. Vaughn RT. Acute osteomyelitis of the sternum: woody phlegmon: osteotomy and drainage. Surg Clin North Am 1918;5:253-62. [ Links ]
8. McLellan DGJ, Phillips KB, Corbett CE, Bronze MS. Sternal osteomyelitis caused by mycobacterium tuberculosis: case report and review of the literature. Am J Med Sci 2000;319:250-4. [ Links ]
9. Tuli SM, Sinha GP. Skeletal tuberculosis "Unusual" lesions. Indian Journal of Orthopaedics 1969; 3:5-18. [ Links ]
10. Davies PDO, Humphries MJ, Byfield SP, et al. Bone and joint tuberculosis : a survery of notifications in England and Wales. J Bone Joint Surg [Br] 1984;66-B:326-30. [ Links ]
11. Dhingra VK, Rajpal S, Gupta UA, Aggarwal JK, Mandal AK, Chowdhury V. A young man with midline swelling over the front of chest. Indian J Tuberc 2005;52:93-8. [ Links ]
12. Sharma S, Juneja M, Garg A. Primary tubercular osteomyelitis of the sternum. Indian J Pediatr 2005;72:709-10. [ Links ]
13. Bohl J, Janner D. Mycobacterium tuberculosis sternal osteomyelitis presenting as an anterior chest wall mass. Pediatr Infect Dis J 1999;18:1028-9. [ Links ]
14. Rubinstien EM, Lehmann T. Sternal osteomyelitis due to Mycobacterium tuberculosis following coronary artery bypass surgery. Clin Infect Dis 1996;23:202-3. [ Links ]
15. Bajracharya S. Primary tubercular osteomyelitis of the sternum: report of two cases. Internet J of Orthopedic Surgery 2007;4:2. [ Links ]
16. Gopal K, Raj A, Rajesh MR, Prabhu SK, Geothe J. Sternal tuberculosis after sternotomy for coronary artery bypass surgery: a case report and review of the literature. J Thorac Cardiovasc Surg 2007;133(5):1365- 6. [ Links ]
17. Kato Y, Horikwana, Nishimura Y. Sternal tuberculosis in a 9month-old infant after BCG vaccination. Acta Paediatr 200;89:1495-7. [ Links ]
18. Tristano AG, Willson ML, Lopez A, De Abreu F. Sternal osteomyelitis caused by mycobacterium tuberculosis: case report and review of the literature. Infect Dis Clin Pract 2004;12:174-7. [ Links ]
19. Tasdan Y, Alikasifoglu M, Midilli K, Itler O. Chest wall abscess as an unusual presentation of childhood tuberculosis. Pediatr Infect Dis J 1998;17:85-6. [ Links ]
20. Ford SJ, Rathinam S, King J, Vaughan R. Tuberculous osteomyelitis of the sternum: successful management with debridement and vacuum assisted closure. Eur J Cardiothorac Surg 2005;28:645-7. [ Links ]
21. Sipsas NV, Panayiotakopoulos GD, Zormpala A. Sternal tuberculosis after coronary bypass graft surgery. Scand J Infect Dis 2001;33:387-8. [ Links ]
22. Banic A, Ris HB, Erni D, Striffler H. Free latissimus dorsi flap for chest wall repair after complete resection of infected sternum. Ann Thorac Surg 1995;60:1028-32. [ Links ]
23. Faure E, Souilamas R, Riquet M, et al. Cold abscess of the chest wall: a surgical entity? Ann Thorac Surg 1998;66:1174-8. [ Links ]
23. Sharlak AY, Gundes H, Gundes S, Alp M. Primary sternal tuberculosis: a rare unhealed case treated by resection and local rotational flap. Thorac Cardiovasc Surg 2001;49:58-9. [ Links ]
24. Cacho G,Yebra M, Berrocal E, Ruiz J. Tuberculous chest wall abscess in patients with AIDS. Clin Infect Dis 1993;16:727-8. [ Links ]
 Reprint requests:
Reprint requests:
Dr W Haynes
Department of Orthopaedics
Private Bag 7
4013 Congella Durban
Tel: (031) 260-4297
Email: willhaynes75@hotmail.com
The content and preparation of this paper is the sole work of the authors. No benefits of any form have been received from a commercial party related directly or indirectly to the subject of this article.














