Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SA Orthopaedic Journal
versão On-line ISSN 2309-8309
versão impressa ISSN 1681-150X
SA orthop. j. vol.9 no.2 Centurion Jan. 2010
CLINICAL ARTICLE
Paediatric septic arthritis in a tertiary setting: a retrospective analysis
HF VisserI; A VisserII; K GollerIII; R GollerIV; JM NelIV; CH SnyckersV
IMBChB(Pret) Senior Registrar, Department of Orthopaedic Surgery, University of Pretoria
IIMBChB(Pret), DTM+H, PG(Dip)TM Senior Registrar, Department of Clinical Pathology, University of Pretoria, National Health Laboratory Services, Tshwane Academic Division
IIIBPhysT(Pret) Physiotherapist, Newlands, Pretoria
IVMBChB(Pret) Senior Registrar, Department of Orthopaedic Surgery, University of Pretoria
VMBChB(Pret), Dip(PEC)SA, MMed(Orth)(Pret), FC(Orth)SA Consultant, Department of Orthopaedic Surgery, University of Pretoria
ABSTRACT
PURPOSE OF THE STUDY: Acute haematogenous septic arthritis is a relatively common condition in the paediatric population. Although Staphylococcus aureus is implicated as the most common causative agent, changes in resistance and the increasing importance of other pathogens have been reported. The purpose of this study is to establish the spectrum of aetiological organisms with their respective sensitivities, and to use this data to evaluate choices in empiric antimicrobial therapy.
DESCRIPTION OF METHODS: A retrospective analysis was performed on patients aged 12 years and younger, admitted for suspected septic arthritis, at a tertiary hospital in South Africa from June 2005 to March 2009. All patients with a clinical diagnosis of septic arthritis were included in this study.
SUMMARY OF RESULTS: A total of 44 patients from the age of 2 weeks to 12 years were included. Thirty-nine samples were submitted for microbiological investigation. Thirty of these samples yielded bacterial growth; five of these had two microorganisms. Gram-positive organisms were cultured in 79% of isolates. Staphylococcus aureus was cultured in 66% of all isolates. Only three samples were multi-drug resistant. Gram-negative organisms constituted 17% of isolates. Of note is the isolation of a single isolate of Haemophilus influenzae type B, signifying possible vaccine or vaccination failure.
CONCLUSION: Gram-positive organisms, in particular Staphylococcus aureus, are still the most prevalent aetiological agent. The current use of cloxacillin as empiric antibiotic therapy will cover 69% of all isolates in this setting; use of coamoxyclav as empiric therapy will increase cover to 80%. Although this 11% difference is seemingly small, opting for co-amoxyclav may significantly reduce morbidity.
Introduction
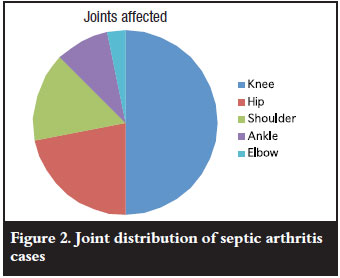
Acute haematogenous septic arthritis is a relatively common condition in the paediatric population, with an estimated incidence of 1 in 10 000, among children younger than 12 years of age.1 Staphylococcus aureus remains the most common causative organism, accounting for more than 50% of cases.2 Other important organisms include Groups A and B streptococci, Streptococcus pneumoniae and historically Haemophilus influenzae type B (now largely eliminated by vaccination).1 There is disagreement about the importance of Gram-negative bacilli in paediatric septic arthritis.3-4 Specific patient groups are susceptible to specific micro-organisms at different ages. Neonates are more susceptible to Group B streptococci. Patients with sickle cell anaemia are susceptible to Salmonella spp. Recently, Kingella kingae has been identified as an important pathogen in children under the age of 4 years5 (Table I). The increased incidence of methicillin-resistant Staphylococcus aureus (MRSA) complicates the selection of an antibiotic for early empiric therapy and also influences the clinical course of the disease.1
Knee and hip joints are most common affected in children, with ankles, elbows and sacro-iliac joints being less common. Multifocal infection has been reported in up to 9% of cases of septic arthritis.4
There are regular reports in the literature describing the spectrum of bacteria involved in septic arthritis.1-4,8-9 This has led us to perform the first retrospective analysis of the microbiology of septic arthritis in children at the Steve Biko Academic Hospital.
Materials and methods
This retrospective study included patients admitted to the Steve Biko Academic Hospital, Pretoria, South Africa, from June 2005 to March 2009 (46 months). All children below the age of 12 years with a clinical suspicion of septic arthritis were included.
We reviewed all microbiological investigations performed on joint fluid, pus or tissue. We also recorded the findings of laboratory markers for infection (white cell count, C-reactive protein and erythrocyte sedimentation rate) on admission. We were unable to determine whether patients infected with MRSA acquired their infections from the community or from a health care setting. We also included patients from whom coagulasenegative Staphylococcus were cultured because this organism can be pathogenic in certain patients.
Results
Patient demographics
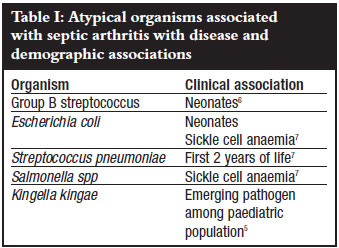
During the study period, a total of 44 patients from age 2 weeks to 12 years (Figure 1) were admitted with acute onset joint swelling, and were subsequently taken to theatre. The majority of patients were male (25/44) with a male to female ratio of 1.3:1, similar to international studies.10
Clinical findings
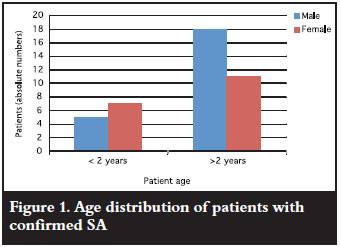
The knee was the most commonly affected joint followed by the hip, shoulder and elbow (in that order) (Figure 2). Two patients had septic arthritis in more than one joint. One had both ankles affected and the other the hip and shoulder affected. Less than 7% of the cohort was tested for HIV. We did not find a seasonal variation in the occurrence of septic arthritis.
Microbiological data
Material was submitted for bacterial culture from 39 of the 44 patients. Bacteria were isolated from 30 patients. Five of these had two organisms. Staphylococcus aureus was by far the most common isolate. Three of these were resistant to all the β-lactam antibiotic class (so-called methicillin resistance), as well as all other classes, except the glycopeptides (vancomycin). Gram-negative bacilli (GNB) isolated included Pseudomonas aeruginosa, Escherichia coli and Enterobacter cloacae (Figure 3).

Streptococcus pneumoniae is typically sensitive to penicillin, but one isolate was resistant, requiring clindamycin as therapy. One case of Haemophilus influenzae was also isolated in 2008, possibly indicating failure to vaccinate, as this is included as part of the National Vaccination Schedule. Gram-negative organisms included Pseudomonas aeruginosa, Eschericiae coli and other Enterobacteriaceae.
Despite formal departmental guidelines, eight joints did not undergo formal arthrotomy and were only aspirated. Only five of these aspirates were subsequently submitted for bacterial culture, of which four grew bacteria. This high positive yield suggests that in any patient with acute onset swollen joint in whom the diagnosis of septic arthritis is suspected, a formal arthrotomy should be performed.
Laboratory data
C-reactive protein (CRP) was determined in 29 of the 30 cases with positive cultures. The level was elevated in 26 cases (90%) of these. CRP levels varied, with five cases (17%) between 10 to 50 mg/ml, 14 cases (48%) 50-200 mg/ml and seven cases (24%) above 200 mg/ml. In the culture-negative group, 72% also had elevated CRP levels.
The white cell count determination was performed in 27 of 30 cases and was elevated in 48% of cases, with 41% being above 13 x 109/L and 7% above 30 x 109/L. In the culture-negative group, none of the patients had levels exceeding 13 x 109 cells per litre. ESR was found to be highly variable in both culture-positive and culture-negative groups. Of note, none of the comparative data on CRP, ESR or WCC was statistically significant, and should be interpreted with care.
Discussion
Paediatric septic arthritis requires urgent treatment. A delay in diagnosis is associated with significant morbidity2 and even potentially mortality.3,11-13 There are various underlying risk factors, including immunosuppression. In the South African setting, human immunodeficiency virus (HIV-1) infection remains a significant pathology,14 with an estimated incidence of 17.6%15 of the general population being infected. This incidence may be even higher in certain settings. As much as 50% have been reported positive in a healthy paediatric population admitted for Kangaroo mother care.16
The incidence of septic arthritis in the HIV-1 positive people is however, reportedly similar to that of HIV-1 negative people, but infection is usually associated with atypical organisms.17
Gram-positive organisms were isolated in 79% of isolates, with Staphylococcus aureus in 66% of cases. Methicillin-resistant Staphylococcus aureus was isolated in 10% of cases and was only susceptible to glycopeptides and rifampicin. It is important to mention that rifampicin should never be utilised as monotherapy in this setting, and is exclusively utilised as a combination treatment.18 In this study, we indicate a rate of 10% MRSA isolation, which is in keeping with findings among our adult population. 19 In our investigation we could not determine whether these organisms were truly community-acquired or health care-associated cases. A major concern is the increasing incidence of resistant bacteria in septic arthritis. In the Netherlands, as many as 72% of aetiological organisms have been found to be resistant.8 This makes the selection of antibiotics for empiric treatment very difficult. Some authors advocate the use of vancomycin for first-line use.20 Others caution that the widespread use of vancomycin as first-line antibiotic is associated with increased morbidity and mortality.21-23 Although use of vancomycin is probably not indicated as yet in this setting, it should be considered in patients not responding to the initial antimicrobial therapy.
Haemophilus influenzae has been largely eradicated by the use of Hib vaccine. For this reason it is now a relatively rare cause of septic arthritis. Our single case may be due to failure of the vaccine or failure to vaccinate.
Gram-negative organisms constituted 17% of our isolates. Although none of these showed resistance, this finding has important implications on the choice of empiric therapy. Cloxacillin is widely used as first-line empiric antibiotic in septic arthritis,24 but has very little efficacy in Gram-negative organisms. Use of a β-lactam with additional clavulanic acid as empiric therapy will increase Gram-negative cover. In this clinical setting, use of co-amoxyclav as empiric therapy will increase antimicrobial cover from 69% to 80%.
Clinical diagnosis of septic arthritis remains problematic. Various laboratory investigations can be used to assist in the diagnosis of septic arthritis. The wide variety of these tests reflects the difficulty in finding reliable diagnostic criteria, for example Kocher et al,25 Eich et al,26 and Luhmann et al.27
Use of the white cell count (WCC), C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) may be useful in monitoring, but should not be used to establish a definitive diagnosis of septic arthritis, as normal levels have been described in severe infection.28.29 In the current study, the CRP level showed better correlation with infection, as levels were elevated in 90% of cases of confirmed infection with levels exceeding 200 mg/l showing increased sensitivity. The WCC was only elevated in 50% of cases; however, levels above 30 x 109 cells/litre showed a better correlation with infection. ESR was of little use. This re-emphasises the point of taking all parameters into consideration when establishing a diagnosis of septic arthritis.
The yield of microbiological culture on synovial fluid may vary depending on the nature of the organism, the technique of collection and culture, and conversely, the microbiological repertoire expected. Sampling technique is of the utmost importance; the optimal sample type is still not universally standardised as controversy exists. It is, however, accepted that pus swabs render a lower culture yield as compared to other techniques as they are more likely to become contaminated, may inhibit the growth of certain fastidious organisms and may facilitate bacterial adherence, thereby decreasing the inoculum concentration and decreasing the culture yield.30 In the vast majority of cases, routine culture of pus collected in a container neat or with a small amount of saline, should be adequate.30 Certain organisms seem to have more specific growth requirements, and various authors have suggested the use of aerobic blood culture bottles to facilitate growth,5,28,31 but this has been refuted by others.30 Recent reports on the importance of Kingella kingae, a fastidious Gram-negative bacillus usually grouped with the so-called HACEK organisms as a significant pathogen in both septic arthritis and osteomyelitis,5 may put more emphasis on more individualised culture practices.
Investigation of the synovial fluid, other than microbiological culture can be most helpful in offsetting inaccurate findings on microbiological cultures (total white cell count, differential white cell count, biochemistry, including synovial glucose levels taken in conjunction with blood glucose,as well as Gram-staining) and may contribute to diagnosis, provided that the choice in tests is made with due care. Joint fluid WCC, biochemistry and Gram stains may assist the establishment of a diagnosis.30 More experimental work including lactate dehydrogenase (LDH)32 and tumour necrosis factor-alpha (TNF-α) levels33 have not yielded promising findings, and is not indicated as part of routine investigation.28
Conclusions
Septic arthritis is an orthopaedic emergency. By recognising trends within specific population groups, an efficient protocol can be established to facilitate early case identification and prompt management, and so reduce morbidity and mortality. Collaboration with the local microbiology department could determine the contribution of more fastidious organisms in causing septic arthritis.
Surveillance should be performed on all confirmed cases of septic arthritis within each institution to establish sensitivity profiles for the aetiological organisms. This information can then be utilised in establishing a targeted protocol on empiric antimicrobials. It should be a continual effort, as the resistance trends are ever-changing.
References
1. Arnold S, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orth 2006;26(6):703-8. [ Links ]
2. Weston V, et al. Clinical features and outcome of septic arthritis in a single UK health district 1982-1991. Ann Rheum Dis 1999;58:214-9. [ Links ]
3. Ryan M, et al. Bacterial joint infections in England and Wales: analysis of bacterial isolates over a four year period. Br J Rheumatol 1997;36:370-3. [ Links ]
4. Goergens E, et al. Acute osteomyelitis and septic arthritis in children. J Paediatr Child Health 2005;41:59-62. [ Links ]
5. Yagupsky P. Kingella kingae: from medical rarity to an emerging paediatric pathogen. Lancet Infect Dis 2004; 4(6):358. [ Links ]
6. Dessi A, et al. Osteo-articular infections in newborns: diagnosis and treatment. J Chemother 2008;20(5):542-50 [Abstract] [ Links ].
7. Bradley J, et al. Pediatric pneumococcal bone and joint infections. Pediatrics 1998;102:1376-82. [ Links ]
8. Kaandorp C, et al. The outcome of bacterial arthritis: a prospective community-based study. Ann Rheum Dis 1997;62:327-31. [ Links ]
9. Dubost J, et al. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis 2002;61:267-9. [ Links ]
10. Caksen H, et al. Septic arthritis in childhood. Pediatrics International 2000;42:534-40. [ Links ]
11. Goldenberg D, Cohen A. Acute infectious arthritis. A review of patients with nongonococcal joint infections (with emphasis on therapy and prognosis). Am J Med 1976;60:369-77. [ Links ]
12. Ispahami P, et al. Septic arthritis due to Streptococcus pneumoniae in Nottingham, United Kingdom, 1985-1998. Clin Infect Dis 1999;29:1450-4. [ Links ]
13. Kaandorp C, et al. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis 1997;56:470-5. [ Links ]
14. Arfaj A. A prospective study of the incidence and characteristics of septic arthritis in a teaching hospital in Riyadh, Saudi Arabia. Clin Rheumatol 2008;27:1403-10. [ Links ]
15. Dohmen P. Antibiotic resistance in common pathogens reinforces the need to minimise surgical site infections. J Hosp Infect 2008;70(S2):15-20. [ Links ]
16. Visser A, Delport S, Venter M. Molecular epidemiological analysis of a nosocomial outbreak of respiratory syncytial virus associated pneumonia in a kangaroo mother care unit in South Africa. J Med Virol 2008;80(4):724-32. [ Links ]
17. Reveille J. The changing spectrum of rheumatic diseases in human immunodeficiency infection. Semin Arthritis Rheum 2000;30:147. [ Links ]
18. Forrest G, Tamura K. Rifampin combination therapy for nonmycobacterial infections. Clin Microbiol Rev 2010;23(1):14-34. [ Links ]
19. Nel J, et al. Adult septic arthritis in a tertiary setting: A retrospective analysis. SAOA 2009;8(3):53-8. [ Links ]
20. Sanford J. The Sanford Guide to Antimicrobial Therapy 2009. 39 ed. Sanford Guide, ed. D. Gilbert, et al. 2009, Sperryville: Antimicrobial Therapy Inc. [ Links ]
21. Khatib R, et al. Impact of initial antibiotic choice and delayed appropriate treatment on the outcome of Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 2006;25:181-5. [ Links ]
22. Stevens D. The role of vancomycin in the treatment paradigm. CID 2006;42(Suppl 1):S51-57. [ Links ]
23. Stryjewski M, et al. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. CID 2007;44(15 January):190-6. [ Links ]
24. Gibbon C. South African Medicines Formulary. 7th ed. South African Medicines Formulary, ed. C. Gibbon, et al. 2005, Cape Town: Health and Medical Publishing Group. [ Links ]
25. Kocher M, Zurakowski D, Kasser J. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg 1999;81(2):1662-70. [ Links ]
26. Eich G, et al. The painful hip: evaluation of criteria for clinical decision-making. Eur J Pediatr 1999;158(11):923-8. [ Links ]
27. Luhmann S, et al. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am 2004; 84A(5):956-62. [ Links ]
28. Mathews C, et al. Management of septic arthritis: a systematic review. Postgrad Med J 2008;84:p265-70. [ Links ]
29. Kang S, et al. The management of septic arthritis in children: systematic review of the English language litreature. J Bone Joint Surg 2009;91(9):1127-33. [ Links ]
30. Wilson M, Winn W. Laboratory diagnosis of bone, joint, soft-tissue, and skin infection. Clin Infect Dis 2008;46:453-7. [ Links ]
31. Essen,Rv, Holitta A. Improved method of isolates bacteria from joint flids by the use of blood culture bottles. Ann Rheum Dis 1986;45:454. [ Links ]
32. Shmerling R, et al. Synovial fluid tests. What should be ordered? JAMA 1990;264:1009. [ Links ]
33. Jeng G, Wang C, Liu S. Measurement of synovial tumor necrosis factor-alpha in diagnosing emergency patients with bacterial arthritis. Am J Emerg Med 1997;15:626. [ Links ]
 Correspondence:
Correspondence:
HF Visser
Private Bag X1 Suite 22 Queenswood 0121
Cell: +2782 333 3772
Email: adele@up.ac.za
This article is the sole work of the authors. No benefits of any form have been received from a commercial party related directly or indirectly to the subject of this article although a grant has been applied for.














