Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SA Orthopaedic Journal
versão On-line ISSN 2309-8309
versão impressa ISSN 1681-150X
SA orthop. j. vol.9 no.2 Centurion Jan. 2010
CLINICAL ARTICLE
Efficacy of an exercise programme on the functional capacity and disease activity in females with rheumatoid arthritis
Dr DC Janse van RensburgI; Dr L FletcherII; Prof M ViljoenIII; Ms C CoertzenIV; Mrs CC GrantVI; Dr DA RamagoleVII; Dr RM CollinsVII; Prof JA KerV
IMBChB, MMed, MSc, Section Sports Medicine, University of Pretoria
IIPhD,Department of Statistics, University of Pretoria
IIIPhD, PhD, Department of Physiology, University of Pretoria
IVBA(MBK) Hons, Department of Biokinetics, Sport and Leisure Sciences, University of Pretoria
VMBChB, MMed, MD, Faculty of Health Sciences, University of Pretoria
VIMSc, Section Sports Medicine, University of Pretoria
VIIMBChB, MSc, Section Sports Medicine, University of Pretoria
ABSTRACT
BACKGROUND: Rheumatoid arthritis (RA) is a chronic, immune-inflammatory disease of unknown aetiology affecting the synovial membrane of joints and surrounding tissues. Typically RA affects both large and small joints in a bilateral, symmetrical, poly-articular fashion. Degradation of bone, cartilage and muscle eventually lead to a reduction in physical function. The purpose of this study was to determine the efficacy of an endurance exercise programme on the fitness parameters (flexibility, strength and aerobic measurements), quality of life (visual analogue scale and health assessment questionnaire) and disease activity (DAS28[4] version with CRP) of female RA patients.
METHODS: Female RA patients were randomly allocated to the experimental group (EG) (n=19) and the control group (CG) (n=8). All participants went through a battery of tests before the intervention, and again after completion of the study. The 12 week training programme consisted of three 45-minute training sessions per week and included walking or aquatics, as well as stretches and isotonic strengthening exercises. The Mann-Whitney U test was used to compare measurements between groups. The Wilcoxon signed-rank test was used to compare baseline and post-intervention measurements within each group.
RESULTS: At the initiation of the study the CG and the EG were comparable for fitness, quality of life and disease activity. On completion of the training programme, statistically significant improvements at the 5% level of significance were seen between the EG and CG, in favour of the EG, for left lateral flexion (p=0.015) and the 1 mile walk test (p=0.011). Within the EG there were improvement of knee flexion left (p=0.026), knee extension (right p=0.011; left p=0.009), scratch test (right p=0.007; left p=0.01), chair sit and reach (right p=0.011; left p<0.001), strength parameters (p<0.05), 1 mile walk test (p<0.001), VO2 max (p=0.01) and DAS scores (p<0.001). Within the CG, improvements were shown for knee extension (right p=0.05; left p=0.013). Although their strength parameters improved significantly it was not in the same order as for the EG. The CG had a decline in their aerobic measurements but their HAQ score improved (p=0.03).
CONCLUSION: An endurance exercise programme, combined with isotonic strengthening exercise and stretching, improves the functional capacity, quality of life and disease activity of female patients with RA. Attention received during the study may have led to some placebo-induced improvements in control subjects, but not to the same extent as those involved in exercise programmes.
Introduction
Rheumatoid arthritis (RA) is a chronic, immune-inflammatory disease of unknown aetiology affecting the synovial membrane of joints and surrounding tissues.1-4 Typically RA affects both large and small joints in a bilaterally symmetrical polyarticular fashion. The most common joints involved are the small joints of the hands and feet followed by the wrists and ankles. The affected joints are swollen, warm, tender and painful on movement.1
RA causes various physical impairments in those affected by the disease.5 Some of these may include: an inhibition of muscle contraction as result of joint effusion; myositis; muscle atrophy due to decreased activity levels which leads to a decrease in muscle strength; a loss of joint motion; and reduced aerobic capacity secondary to systemic effects and reduced activity levels. Persons with RA, especially those severely affected by the disease, are in general 33% to 55% weaker than their healthy counterparts.6
Although there is no cure for RA, much can be done to manage the condition. Four major treatment approaches are recognised in the management of RA, including medication, physical exercise, joint protection and lifestyle changes, and surgical intervention.7
According to the American College of Sports Medicine the primary objectives of exercise therapy in patients with RA is (1) to preserve or restore range of motion (ROM) and flexibility around affected joint(s); (2) to increase muscle strength and endurance to build joint stability; and (3) to increase aerobic capacity in order to enhance psychological state and decrease the risk of cardiovascular disease.2
A comprehensive exercise programme for RA patients is said to include aerobic exercise at a moderate intensity for three to five days a week, isometric or isotonic strength training exercises three days a week, as well as stretching exercises at least once daily.8 Several papers suggest that exercise may improve functional capacity (i.e. quality of life and fitness parameters),9-12 and have an effect on disease activity.13-17
The objective of this study was to measure the effect of an endurance training programme on the fitness parameters, quality of life and disease activity of females suffering from RA.
Materials and methods
Study design
The study was conducted at the University of Pretoria, and ethical clearance was obtained from the Ethical Committee, Faculty of Health Sciences. All participants signed an informed consent document. A prospective analytical pre-post group comparison was adopted. Participants were selected based on the inclusion and exclusion criteria (Table I).

A random sample, equally allocated to a control group (CG) and an experimental group (EG) consisting of two subsets (Aquatic exercise and Land exercise) was planned. The final sample consisted of 37 patients who were randomly allocated to the EG (n=25) and the CG (n=12) by drawing a card stating the group they were in. In this article the two experimental groups are reported together as the aim was to assess the effect of exercise on RA patients. Nineteen subjects from the EG and eight from the CG completed the study.
Intervention
The EG was required to train three times per week, 45 minutes at a time, for 12 weeks. The training was done under supervision of a biokineticist (specialised in physical training programmes). The CG received no intervention and was asked to continue with their sedentary lifestyles. The programme consisted of a warm-up phase (5 min), aerobic exercise (walking or aquatics for 20 min), strength training (10 min) and flexibility training (10 min). Aerobic exercise intensity was started at 60% of the heart rate maximum and was individually tailored to a maximum of 80%. Strength training in week 1 was 50% of the one repetition maximum and this was gradually increased to 80% in week 12.
Measurements
Participants were tested at baseline and after 12 weeks (post-intervention). Measurements included:
• Physical parameters: height (cm); weight (kg); body mass index (BMI)
• Flexibility parameters: wrist flexion and extension (degrees); knee flexion and extension (degrees); hip flexion and extension (degrees); lateral flexion (sideways bend [cm]); chair sit and reach (cm); scratch test (cm)
• Strength parameters: grip strength (kg); leg strength (kg); arm curls (s); sit to stand test (s)
• Fitness parameters: Rockport 1 mile walk test (min); VO2max Relative (ml/kg/min)
• Quality of life measures: Health Assessment Questionnaire (HAQ);18 Visual Analogue Scale for pain (VAS)19
• Disease activity scores: DAS28(4) CRP version20
Results
Collected data were captured and analysed by using SPSS Statistics 17.0. The non-parametric Mann-Whitney U test was used to compare the EG with the CG before and after intervention. The difference between the post-intervention and baseline measurements were used to assess whether the intervention had an effect. This method was used to control for initial differences between the two groups at baseline. To compare baseline measurements and post-intervention measurements within each group the Wilcoxon signed-rank test was used. Significant differences at the 5% and 10% level are reported.
Baseline characteristics for the two groups are shown in Table II.
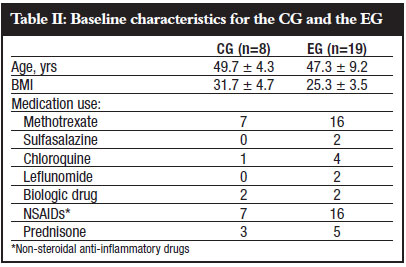
A description of the measurements that changed significantly, as well as the direction that indicates an improvement, are explicated in Table III.
Firstly the functional parameters (flexibility, strength and fitness) will be reported on, whereafter the quality of life (HAQ and VAS) and disease activity parameters (DAS) will follow. Mean values are displayed in the tables. The graphs display the 95% confidence intervals around the mean.
Functional parameters - between groups
At baseline the CG and EG were comparable, except for knee flexion. The EG had significantly better knee flexion, cf. Table IV.
Comparing the two groups post-intervention, right lateral flexion deteriorated for both groups, but significantly less so for the EG. Significant improvements were found in favour of the EG for left lateral flexion, chair sit and reach left, handgrip strength right and the 1 mile walk test (Table IV and Graph 1).

Functional parameters - within groups
The CG showed significant improvements for knee extension; however, hip extension and lateral flexion declined significantly from baseline to end of study. (Table Va and Graph 2a).
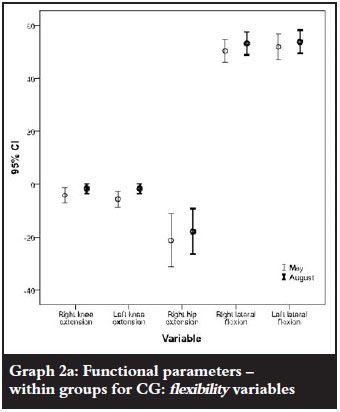
The strength parameters, including hand grip, leg strength, arm curls and sit to stand test also improved significantly from baseline to study completion in the CG (Table Vb and Graph 2b).

Within the EG the following flexibility parameters improved significantly post-intervention: knee flexion left, knee extension, hip flexion right, chair sit and reach and scratch test; however, as for the CG, hip extension declined significantly (Table VIa and Graph 3a).

The strength parameters that improved were hand grip, leg strength, arm curl test and sit to stand test (Table VIb and Graph 3b).

Both aerobic parameters i.e. the 1 mile walk test and the VO2maxRelative, also improved significantly from baseline to end of study in the EG (Table VIc and Graph 3c).

Disease activity parameters - between groups
The CG and EG did not differ at baseline with regard to their VAS, HAQ and DAS28(4) measurements. At completion of the study however, the EG improved significantly more than the CG with respect to DAS scores, while the CG rated themselves significantly better than the EG group did according to the HAQ (Table VII and Graph 4).


Disease activity parameters - within groups
The CG had significant improvements in the HAQ and VAS. The EG improved significantly in the DAS28(4) and VAS (Table VIII and Graph 5).

Discussion
The results of this study, with a compliance rate of 77%, indicated significant improvements for flexibility, strength and aerobic capacity measured after a 12-week aerobic exercise intervention. Previous research indicates that exercise compliance for RA patients are on average between 50% and 95% depending on accessibility of the exercise, intensity, duration, cost, and comfort involved for the patient.21 RA is the second-most common joint disease, causing various physical impairments either as result of the disease or due to inactivity.5,8 Patients with RA are hesitant to get involved in any form of exercise because of fear of pain and disability. These fears are unfounded as research has shown that regular and controlled exercise for those whose disease is under control decreases joint pain and stiffness and improves joint mobility, strength and aerobic capacity without exacerbating pain or disease activity in persons with RA.3,21,22
The flexibility findings of this study support the findings of previous studies done on patients with RA.13,23,24 The results showed significant improvements in flexibility of all the major joints for the EG whereas the flexibility of the CG remained relatively unchanged for the duration of the study.
Strength improved for both the CG and EG, even though the control group did not participate in a training programme. This is not an uncommon occurrence as a study by Bykerk and Keystone, showed similar results.25 According to Häkkinen, the improvements in the CG can be explained by the learning effects of repeated physical testing and variation in symptoms.10
The EG's strength improved by an average of 33% compared to the 27% improvement in the CG, indicating that the exercise intervention did have a positive effect on the patients' strength. The average improvements in the EG for hand grip strength, leg strength, arm curl test and sit to stand test respectively were 29%, 20%, 40% and 44%. These results are in agreement with studies done by Van den Ende et al., Häkkinen et al. and Stenstrom that similarly showed improvements in muscle strength ranging between 16% and 35%.13,24,26
Exercise programmes with the specific purpose of improving aerobic fitness have attracted only minimal attention in this population. Previous studies on the effect of aerobic exercise in RA patients made use of cycling, aquatics or aerobic dance.22,24,26 Studies done by Iversen et al, Minor et al, and Van den Ende et al showed significant improvements in aerobic capacity (20%) following 12 weeks of dynamic exercise of medium to high intensities.6,23,24 In keeping with these studies our results showed an average improvement of 14% in VO2max and 12% in 1 mile walk time in the EG, confirming that the intervention was successful in improving aerobic fitness. In contrast to these improvements, the CG showed a decrease in aerobic capacity of 30% over the same time period.
Results on the activities of daily living (HAQ) are difficult to explain, but are similar to previous publications. The improvement of disease activity is also in support of some previous studies.11-13,15,16 Although the EG had marked improvement in their fitness parameters and disease activity scores, they did not improve significantly in their own assessment of activities of daily living. This might be because the inclusion criteria stipulated controlled disease and stable medication when the study started (HAQ scores were already low).
With improvements in flexibility, strength and aerobic fitness it can thus be concluded that the EG's functional capacity and disease activity improved as a result of a relatively short but well-controlled endurance exercise programme of 12 weeks. In agreement with Christie et al. exercise therapy should be considered the cornerstone of the multidisciplinary treatment approach of stable grade 1 or 2 RA.3
Although this study demonstrated that endurance exercise improves function and disease activity in RA patients, future research on the effect of exercise in female RA patients should aim for a larger study sample and a more individualised exercise programme in an attempt to decrease the SD between and within the groups for the tested parameters. Additionally the duration of intervention could be increased as very few studies lasted longer than 12 weeks thus the long-term effects of exercise on RA patients are not well known.22 The long-term influence of an exercise programme after cessation of the intervention will also be informative.
Acknowledgements
I wish to acknowledge the biokineticists Natania Fourie and Anneke de Beer for their involvement with the training, Brenda Weder for the typing of the document and Maureen Brassel for helping with the literature search.
References
1. O'Dell JR. Rheumatoid arthritis: The clinical picture. In: Koopman WJ, Moreland LW (eds). Arthritis and Allied Conditions: Textbook of Rheumatology. 15th ed. Philadelphia: Lippincott, Williams & Wilkins 2005;1165-94. [ Links ]
2. Armstrong L, Balady GJ, Berry MJ. ACSM Guidelines for Exercise Testing and Prescription 7th. ed. Philadelphia, Pennsylvania: Lippincott, Williams and Wilkins 2006;105-7. [ Links ]
3. Christie A, Jamtvedt G, Dahm KT, Moe RH, Haavardsholm EA, Hagen KB. Effectiveness of nonpharmacological and nonsurgical interventions for patients with rheumatoid arthritis: an overview of systematic reviews. Phys Ther 2007 Dec 87;12:1697-715. [ Links ]
4. Gaudin P, Leguen-Guegan S, Allenet B, Baillet A, Grange L, Juvin R. Is dynamic exercise beneficial in patients with rheumatoid arthritis? Joint Bone Spine 2008 Jan;75(1):11-17. [ Links ]
5. Hicks JE. Rehabilitation strategies for patients with rheumatoid arthritis. J Muskuloskelet Med 2000;17(4):991. [ Links ]
6. Iversen MD. Physical therapy for management of RA: rehabilitation measures are most effective when started early. J Muskuloskelet Med 2002;19(9):352-58. [ Links ]
7. Giannini MJ, Protas EJ. Exercise response in children with and without juvenile rheumatoid arthritis: a case-comparison study. Phys Ther 1992 May;72(5):365-72. [ Links ]
8. Millar AL. Action plan for arthritis: Your guide to pain-free movement. Human Kinetics 2003;31-49. [ Links ]
9. Gossec L, Pavy S, Pham T, Constantin A, Poiraudeau S, Combe B, et al. Nonpharmacological treatments in early rheumatoid arthritis: clinical practice guidelines based on published evidence and expert opinion. 2006 Jul;73(4):396-402. [ Links ]
10. Häkkinen A. Effectiveness and safety of strength training in rheumatoid arthritis. Curr Opin Rheumatol 2004 Mar;16(2):132-7. [ Links ]
11. Van Den Ende CH, Vliet Vlieland TP, Munneke M, Hazes JM. Dynamic exercise therapy for rheumatoid arthritis. Br J Rheumatol 1998;37(6):677-87. [ Links ]
12. Flint-Wagner HG, Lisse J, Lohman TG, Going SB, Guido T, Cussler E, et al. Assessment of a sixteen-week training program on strength, pain, and function in rheumatoid arthritis patients. J Clin Rheumatol 2009 Jun;15(4):165-71. [ Links ]
13. Stenstrom CH. Home exercise in rheumatoid arthritis functional class II: goal setting versus pain attention. J Rheumatol 1994 Apr;21(4):627-34. [ Links ]
14. Lee EO, Kim JI, Davis AH, Kim I. Effects of regular exercise on pain, fatigue, and disability in patients with rheumatoid arthritis. Fam Community Health 2006 Oct-Dec;29(4):320-7. [ Links ]
15. Lyngberg K, Danneskiold-Samsoe B, Halskov O. The effect of physical training on patients with rheumatoid arthritis: changes in disease activity, muscle strength and aerobic capacity. A clinically controlled minimized cross-over study. Clin Exp Rheumatol 1988 JulSep;6(3):253-60. [ Links ]
16. Neuberger GB, Aaronson LS, Gajewski B, Embretson SE, Cagle PE, Loudon JK, et al. Predictors of exercise and effects of exercise on symptoms, function, aerobic fitness, and disease outcomes of rheumatoid arthritis. Arthritis Rheum 2007 Aug 15;57(6):943-52. [ Links ]
17. Häkkinen A, Häkkinen K, Hannonen P. Effects of strength training on neuromuscular function and disease activity in patients with recent-onset inflammatory arthritis. Scand J Rheumatol 1994;23(5):237-42. [ Links ]
18. Pincus T, Summey JA, Soraci SA Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum 1983 Nov;26(11):1346-53. [ Links ]
19. Callahan LF, Pincus T. A clue from a self-report questionnaire to distinguish rheumatoid arthritis from noninflammatory diffuse musculoskeletal pain. The PVAS:D-ADL ratio. Arthritis Rheum 1990 Sep;33(9):1317-22. [ Links ]
20. Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995 Jan;38(1):44-8. [ Links ]
21. Westby MD, Li L. Physical therapy and exercise for arthritis: Do they work? Geriatrics Aging 2006;9(9):624-30. [ Links ]
22. Metsios GS, Stavropoulos-Kalinoglou A, Veldhuijzen van Zanten JJ, Treharne GJ, Panoulas VF, Douglas KM, et al. Rheumatoid arthritis, cardiovascular disease and physical exercise: a systematic review. Rheumatology (Oxford) 2008 Mar;47(3):239-48. [ Links ]
23. Minor MA, Hewett JE, Webel RR, Anderson SK, Kay DR. Efficacy of physical conditioning exercise in patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1989 Nov;32(11):1396-405. [ Links ]
24. van den Ende CH, Hazes JM, le Cessie S, Mulder WJ, Belfor DG, Breedveld FC, et al. Comparison of high and low intensity training in well controlled rheumatoid arthritis. Results of a randomised clinical trial. Ann Rheum Dis 1996 Nov;55(11):798-805. [ Links ]
25. Bykerk VP, Keystone EC. What are the goals and principles of management in the early treatment of rheumatoid arthritis? Best Pract Res Clin Rheumatol 2005 Feb;19(1):147-161. [ Links ]
26. Häkkinen A, Sokka T, Kotaniemi A, Kautiainen H, Jappinen I, Laitinen L, et al. Dynamic strength training in patients with early rheumatoid arthritis increases muscle strength but not bone mineral density. J Rheumatol 1999 Jun;26(6):1257-1263. [ Links ]
27. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988 Mar;31(3):315-324 [ Links ]
 Correspondence:
Correspondence:
Dr DC Janse van Rensburg
Section Sports Medicine University of Pretoria
P O Box 12651 0028 Hatfield
Tel: (012) 420-6057 Fax: (012) 362-3369
E-mail: vrensburg@sport.up.ac.za
No benefits of any form have been received from a commercial party related directly or indirectly to the subject of this article.














