Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Southern African Journal of Critical Care (Online)
versión On-line ISSN 2078-676X
versión impresa ISSN 1562-8264
South. Afr. j. crit. care (Online) vol.39 no.3 Pretoria nov. 2023
http://dx.doi.org/10.7196/SAJCC.2023.v39i3.1218
RESEARCH
The utility of brain natriuretic peptide as a prognosticating marker in critical care patients
A NaidooI; K de VasconcellosII
IMB ChB, FCP (SA); Department of Internal Medicine, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIMB ChB, DA (SA), FCA (SA); Department of Critical Care, King Edward VIII Hospital, Durban, and Discipline of Anaesthesiology and Critical Care, School of Clinical Medicine, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND. Brain natriuretic peptide (BNP) is an established biomarker of morbidity and mortality in cardiac failure. Data also suggest potential prognostic utility in non-heart failure cohorts. The utility of BNP in predicting intensive care unit (ICU) outcomes has not been well evaluated in a mixed critical care population in the South African (SA) context.
OBJECTIVE. To evaluate the ability of BNP to predict ICU mortality in a heterogeneous critical care population in SA.
METHODS. This was a retrospective observational study of 100 patients admitted to a multidisciplinary, closed, intensivist-run ICU in a tertiary academic hospital serving KwaZulu-Natal Province (1 January 2020 - 31 July 2022). Initial BNP was evaluated as a predictor of ICU mortality using univariate and multivariable analyses.
RESULTS. There was a statistically significant difference in BNP between survivors and non-survivors in the cohort of patients without heart failure. The median initial BNP in the non-heart failure cohort was 411 (interquartile range (IQR) 116 - 848) ng/L in non-survivors, and 150 (44 - 356) ng/L in survivors (p=0.028). The optimal cut-off for BNP was determined as 366 ng/L. A BNP >366 ng/L was an independent predictor of ICU outcome.
CONCLUSION. This study highlights the potential utility of BNP as a predictor of ICU mortality in a heterogeneous ICU population, with the greatest utility in patients without heart failure. Further studies are required to confirm this finding.
Keywords. Brain natriuretic peptide, ICU mortality, biomarker.
Brain natriuretic peptide (BNP) is a 32-amino acid peptide synthesised in the cardiac ventricles in response to stretch and increased wall tension.[1] Natriuretic peptides are involved in the regulation of sodium and water balance, blood volume and arterial pressure.[2]
BNP is a well-established prognostic biomarker in heart failure, and natriuretic peptides correlate with the haemodynamic changes and filling pressures in the heart.[3]
BNP has also manifested significant utility in outcome prediction in non-heart failure cohorts. This is probably best established in the perioperative literature, where an elevated BNP has been shown to be a powerful predictor of adverse events perioperatively.[4] This has resulted in BNP being included in international best practice guidelines on perioperative risk stratification.[5] The use of BNP in this context has allowed for the replacement of previous gold-standard tests, which were complex, costly or posed a risk to patients, with a single blood test. The addition of BNP to current risk stratification tools has resulted in superior performance compared with the standard tool alone.
BNP is elevated in response to cytokines, endotoxins and other inflammatory mediators. BNP levels are elevated in most shocked patients in the intensive care unit (ICU), which is not necessarily related to high cardiac filling pressures.[6] Studies suggest that elevated BNP is related to the severity of sepsis rather than the associated reversible sepsis-induced cardiomyopathy.[7] These findings indicate that BNP may thus also have clinical utility as a prognostic biomarker in critically ill patients with or without heart failure.
There are limited data on the use of BNP as a prognostic marker in critically ill patients in low- or middle-income countries. Critically ill patients in these settings may be younger, with a higher burden of trauma and communicable disease, but a lower burden of chronic cardiovascular disease. It is therefore important to evaluate the potential utility of BNP in these populations. Appropriate risk stratification of patients is important to determine appropriate levels of care, including monitoring, assisting with prognostication and counselling, and directing specific interventions. Current outcome prediction/risk stratification tools in ICU may be perceived as complex and time-consuming to perform, and have seldom been adequately evaluated in the South African (SA) critical care context. This is a hypothesis-generating study to determine whether BNP has potential utility as a predictor of outcome in a heterogenous population of critically ill patients in the SA context.
Methods
Study design and data source
This was a retrospective observational study of patients admitted to the study ICU, evaluating BNP as a prognostic biomarker between 1 January 2020 and 31 July 2022.
The primary outcome was ICU mortality. The severity of illness and organ dysfunction was described using the sequential organ failure assessment (SOFA) score. Clinical data, including data on comorbidities, were recorded from the patients' notes. Echocardiography data were recorded from formal bedside echocardiography reports (where available) by trained technologists.
Study site
The study ICU is a multidisciplinary, closed, intensivist-run ICU in a tertiary academic hospital serving KwaZulu-Natal Province.
Study population
Charts of all patients who were admitted to the ICU during the study period were reviewed. All adult patients were eligible for inclusion if they had a BNP performed within 72 hours of ICU admission. Patients were excluded if they were < 18 years old or had no BNP performed. A BNP was done at the request of the treating intensivist.
Data management and analysis
Data were collected from the patients' ICU charts using a Microsoft Excel spreadsheet (Microsoft Corp., USA) and subsequently analysed using SPSS version 28.0 (IBM Corp., USA). A total of 100 patients were included owing to the anticipated availability of hard copies of patient records. A p-value of <0.05 was taken as statistically significant.
Analysis was conducted on the entire cohort and the remaining cohort after patients with heart failure were excluded. The statistical plan included analysis of 4 BNP cut-off values: BNP <100 ng/L (cut-off at which the laboratory servicing the ICU deemed heart failure unlikely), BNP >500 ng/L (value above which the laboratory servicing the ICU deemed heart failure likely), BNP >80 ng/L (value at which major adverse cardiac events are deemed more likely after acute coronary syndrome) and the optimal cut-off calculated from the receiver-operator characteristic (ROC) curve and Youden's index. Because of the study findings of a significant association between BNP and ICU outcome in patients without heart failure, an additional BNP cut-off using the ROC curve and Youden's index was also evaluated in patients without heart failure.
The diagnostic performance (in terms of ICU mortality) of the above cut-off values was also evaluated in terms of sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, negative likelihood ratio and diagnostic accuracy. Multivariable analysis was performed for predictors of ICU outcome with binary logistic regression, using a backward stepwise (likelihood ratio) method. The five best-performing variables on univariate analysis (excluding variables possibly associated with significant collinearity), and the best-performing BNP cut-off for ICU mortality, were selected for inclusion in the multivariable analysis. This number of variables was chosen to allow for a variable-to-event ratio of ~5, to reduce the risk of overfitting.
Ethical approval for the study was obtained from the University of KwaZulu-Natal Biomedical Research Ethics Committee (ref. no. BREC/00002767/2021), King Edward VIII Hospital, Durban, and additional permission was obtained from the study hospital and the Provincial Department of Health before commencement.
Results
The baseline characteristics and ICU outcomes of the study population are shown in Table 1. The patients' ages ranged from 18 to 86 years. While sepsis was the primary diagnosis in 39% of patients, it was either the primary or secondary diagnosis in 80% of patients. The most common source of sepsis was abdominal in 42 (51.9%) patients, followed by community-acquired pneumonia in 14 (17.3%) and skin or soft-tissue infections in 11 (13.6%). Ten patients were known to be HIV positive, with 45 HIV negative; however, 45 patients had an unknown HIV status, and therefore the 10% HIV positivity rate for the cohort was possibly underestimated. While only 4 patients were known to have congestive cardiac failure on admission, a further 8 patients were diagnosed with heart failure on admission or during their ICU stay. It was hypothesised that the impact of an elevated BNP and the relevant cut-off values may differ between patients with primary cardiac failure and those with primarily other conditions. Therefore, where relevant, analyses were performed in the full cohort and in a cohort where the cardiac failure patients were excluded. This latter cohort of 88 patients is referred to as the non-heart failure cohort hereafter.
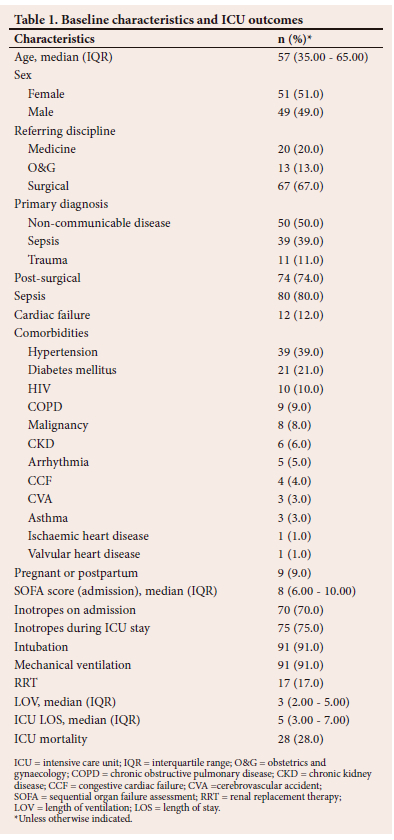
The initial BNP levels ranged from 1 ng/L to 6 961 ng/L, with a median of 189 (interquartile range (IQR) 55 - 585) ng/L. Initial BNP was performed on day 1 of ICU stay in 95 patients, day 2 in 2 patients and day 3 in 3 patients. Twenty-five patients had more than one BNP performed during the first 72 hours of their ICU stay. The associations between clinical and biochemical data and ICU mortality for the whole cohort are shown in Table 2. While initial and highest BNP values were greater in patients who died in ICU, it did not reach statistical significance when BNP was treated as a continuous variable. There was, however, a statistically significant difference in BNP between survivors and non-survivors in the non-heart failure cohort. Median initial BNP in the non-heart failure cohort was 411 (IQR 116 - 848) ng/L in non-survivors, and 150 (44 - 356) ng/L in survivors (p=0.028). The highest median BNP was 454 (141 - 848) ng/L in non-survivors, and 154 (45 - 450) ng/L in survivors (p=0.017).
ROC curve analysis for initial BNP and ICU mortality for the entire cohort revealed an area under the curve (AUC) of 0.60 (0.47 - 0.73) (p=0.122). The optimal BNP cut-off using Youden's index was 269 ng/L, followed closely by 366 ng/L, as seen in Table 3. ROC curve analysis for the non-heart failure cohort showed an AUC of 0.65 (0.52 - 0.78) (p=0.03), with an optimal cut-off of 366 ng/L. The AUC for the admission SOFA score was 0.71 (0.60 - 0.82) (p=0.001) for the entire cohort and 0.71 (0.59 - 0.82) (p=0.003) for the non-heart failure cohort. When comparing the ROC curves for BNP and SOFA score, despite the numeric differences, neither the entire cohort, nor the non-heart failure cohort, was statistically significantly different (p=0.199 and p=0.532, respectively). There was no statistically significant association between BNP as a continuous variable or at any cut-off and ICU mortality in the heart failure cohort. The median BNP in patients with heart failure who survived was 1 057 (430 - 1 541) ng/L, and 290 (11 - 448) ng/L in those with heart failure who died in the ICU (p=0.145).
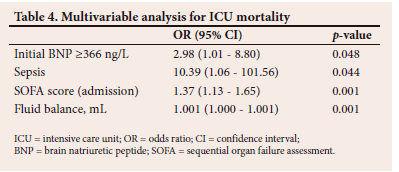
The results of the multivariable analysis are shown in Table 4. Inotropic support on admission and renal replacement therapy were no longer significantly associated with ICU mortality on multivariable analysis.
Associations between clinical and biochemical parameters and an elevated BNP are shown in Table 5.
Of the 26 patients with abnormal echocardiography findings (of 48 patients who had echocardiography performed), 19 were in the non-heart failure cohort. Despite not being in clinical heart failure, patients with abnormal echocardiography results were significantly more likely to have a BNP >366 ng/L, with 33.3% (7 of 21) of patients with a BNP <366 ng/L having abnormal echocardiography findings and 66.7% (12 of 18) of patients with a BNP >366 ng/L having an abnormal echocardiogram (p=0.038). The echocardiography abnormalities ranged from left ventricular (LV) systolic dysfunction to regional wall motion abnormalities and tricuspid regurgitation with elevated pulmonary artery systolic pressures.
On logistic regression analysis of the primary diagnosis, patients with sepsis were significantly more likely to have an elevated BNP (>366 ng/L) when referenced to those with trauma (odds ratio (OR) 9.5 (1.11 - 81.51)) (p=0.04).
Discussion
Elevated BNP is a well-established prognostic marker in cardiac failure and perioperative medicine.[2,8,9] Our study aimed to evaluate the potential utility of BNP as a prognostic biomarker in a heterogenous cohort of critically ill patients admitted to a multidisciplinary ICU in SA.
The study population comprised predominantly surgical patients, with 74% being admitted in the postoperative period, compared with studies in high-income countries where acute coronary syndromes and pneumonia were the most common indications for ICU admission.[10] Eighty per cent of patients in our study had sepsis as a primary or secondary diagnosis, which is in line with that in other centres.[11] Ninety-one per cent of patients admitted to ICU in this study required ventilation, and 75% required inotropic support. Only 12% of patients were in cardiac failure. The study cohort therefore predominantly comprised surgical patients, with a high incidence of sepsis and multi-organ failure. The incidence of trauma and medical admissions was lower than usual for the study ICU, due to the impact of the COVID-19 pandemic. The median age of 57 years was higher than in previous studies in the study ICU, possibly reflecting the reduction in trauma admissions and a possible selection bias whereby BNP was performed in older patients.[12]
As described in the results section, both initial and highest BNP values were numerically greater in patients who died in ICU, although this does not reach statistical significance when BNP is treated as a continuous variable for the entire cohort. There was however a statistically significant increase in mortality in patients with an elevated BNP using various cutoff values, ranging from 269 ng/L to 400 ng/L. There was a statistically significant difference in BNP levels between survivors and non-survivors when patients with heart failure were excluded, with initial and highest BNP being significantly elevated in non-survivors and with an elevated mortality in those with BNP levels above all cut-offs from 269 ng/L to 500 ng/L. While there was no statistically significant association between BNP and ICU outcome in patients with heart failure, the median BNP was unexpectedly higher in those with heart failure who survived their ICU stay. There are a number of potential explanations for these findings. Because of the small sample size, the lack of significance in the overall cohort and in the heart failure cohort is due to a type II statistical error. While this appears likely for the whole cohort, given the significant associations seen at specific cut-off values, the reasons for the findings in the heart failure cohort are less clear and potentially more complex. These may simply be due to the erroneous diagnosis of heart failure by the treating clinicians. However, given the experience of the ICU team and the ready availability of advanced cardiovascular assessment tools, it seems unlikely. Patients with heart failure and low natriuretic peptide levels are a well-described subset of heart failure patients.[13,14] We hypothesise that these patients may have characteristics that predispose them to adverse ICU outcomes, e.g. obesity, diastolic dysfunction and acute heart failure. In a large study of 30 487 patients at Vanderbilt University Medical Center, it was found that BNP was the strongest predictor of death in non-heart failure patients. In this cohort, tachycardia, indicating increased sympathetic activity, was significantly associated with circulating BNP levels.[15] In patients with heart failure, the BNP is elevated owing to ventricular wall stretch secondary to congestion. Diuresis can rapidly improve the congestive heart failure. With regard to causes for an elevation of BNP in non-cardiac failure, the mechanism is more complex and multifactorial and may be less amenable to treatment. Consequently, patients with decompensated chronic heart failure may paradoxically show a better response to therapy than those with an elevation in BNP due to other causes or than patients with heart failure who do not have an elevated BNP.
There are possibly multiple mechanisms whereby BNP is elevated in critically ill patients without overt heart failure and why this subgroup of patients may have an increased mortality. These mechanisms may include haemodynamic and non-haemodynamic factors. In terms of haemodynamic factors, an elevated BNP may identify a patient with a pre-existing, undiagnosed cardiac condition; or indicate a patient with a previously normal heart that has now been subjected to cardiac strain due to the severity of their critical illness and consequent pressure overload, volume overload or ischaemia; or indicate a combination of both the vulnerable heart and an acute insult. All three subgroups of patients could be hypothesised to be at increased risk of mortality. Non-haemodynamic mechanisms are however also likely to be important determinants of BNP and outcome in the critically ill patient. Sepsis causes peripheral vasodilation, autonomic dysregulation, oxygen free-radical production, mitochondrial dysfunction and production of myocardial depressants, such as tumour necrosis factor alpha and interleukin-1ß, which result in myocardial dysfunction. Myocardial dysfunction caused by sepsis comprises reversible systolic and diastolic dysfunction, which leads to myocardial ischaemia and decreased ejection fraction. Septic shock is a stimulus for the release of BNP, and its elevation is independent of cardiogenic shock.[13,15] The clearance of BNP is impaired in septic shock owing to impaired neutral endopeptidase 24.11 enzymatic activity. This enzyme activity was markedly reduced in septic shock patients and was not altered in those with severe sepsis and cardiogenic shock.[16] This could be an explanation for the high enzyme levels in the cohort, as many patients were in septic shock. Sepsis was independently associated with ICU mortality in this study. It was also associated with an elevated BNP when evaluated as a primary diagnosis but not when all patients with sepsis (primary or secondary diagnosis) were included. The procalcitonin was also significantly higher in those with an elevated BNP than in those without an elevated BNP, making a link between infection (or inflammation) and a rise in BNP likely. Sepsis and an elevated BNP were independent predictors of ICU mortality on multivariable analysis. Overall, these findings suggest that, while there is an association between sepsis and BNP, the increased mortality seen with elevated BNP in this ICU cohort is not exclusively explained by this association. Of interest, while an increase in fluid balance was associated with increased ICU mortality, there was no difference in fluid balance between those with and without elevated BNP levels. This finding suggests that the association between an elevated BNP and increased ICU mortality is not due to increased fluid therapy in ICU non-survivors.
On assessing individual associations with an elevated BNP, the statistically significant variables included cardiac-specific parameters such as an elevated troponin I (p<0.001), ischaemic changes on electrocardiogram (ECG) (p=0.42), abnormal echocardiogram (p=0.02) and impaired ejection fraction (p=0.041). Interestingly, as mentioned above, the only non-cardiac parameter that was statistically significant was an elevated procalcitonin (p=0.035), a specific biomarker for sepsis. Septic shock causes both systolic and diastolic dysfunction, manifesting as biventricular dysfunction and reduced ejection fraction by myocardial depression from cytokines involving nitric oxide depressant.[17] This would explain the ischaemic changes on ECG and the reduced ejection fraction on echocardiogram, as well as elevated troponin I in the cohort with septic shock without heart failure. Furthermore, there was an association between abnormal echocardiographic parameters and an elevated BNP in the non-heart failure cohort. This association suggests that an elevated BNP may have utility in identifying patients with undiagnosed chronic cardiac conditions, or patients with acute cardiac dysfunction due to their critical illness. Whatever the underlying precipitant of the rise in BNP, the association with other markers of cardiac dysfunction suggests that BNP is possibly a surrogate marker of a final common pathway of cardiovascular dysfunction that links the severe physiological, and particularly haemodynamic, stressors of critical illness with mortality.
Because of the factors presented above, BNP represents an attractive parameter to identify patients at risk of adverse ICU outcomes. In this regard, it showed moderate predictive ability in terms of ICU outcome, with an AUC of 0.65, which compared reasonably well with that of the SOFA score of 0.71. These data suggest that, while BNP cannot be used in isolation for prognostication decisions, it is potentially a useful tool to identify high-risk patients in a general ICU population. Further studies are required to confirm and refine the findings of our study. Of particular interest and importance would be a study evaluating the prognostic enrichment ability of BNP when used as an adjunct to established mortality prediction scores, e.g. the SOFA score. Patients with elevated BNP may benefit from enhanced attention to haemodynamic optimisation and may represent a subset of patients that should be specifically included in future interventional studies, e.g. goal-directed therapy, anticoagulation and/or anti-inflammatory therapies.
Study limitations
The limitations of this study include that it was performed at a single clinical site and the small sample size, which limit its generalisability. Neither the diagnosis of heart failure nor that of sepsis was standardised, but was based on clinical diagnoses from the treating physicians. It is likely that this resulted in a cohort of older patients with more cardiac risk factors than is the norm for the study ICU. This situation is indicative of common clinical practice and therefore indicated the utility of BNP in a real-world setting. Generalisability is therefore potentially limited and the results of this study may not apply to a young cohort of trauma patients, as seen in many ICUs in SA. This was a retrospective observational study and the decision to perform a BNP in a specific patient was not standardised or systematically recorded. Therefore, the patients who had a BNP performed may have differed systematically from those in whom it was not performed, resulting in selection/spectrum bias. The performance characteristics of a test may thus be falsely inflated when compared with the true performance in an unselected cohort. BNP may be increased in a number of comorbid diseases, e.g. renal failure, chronic obstructive pulmonary disease, and hyperthyroidism, which were not routinely documented or excluded.
Based on the abovementioned limitations, we believe that the findings of the study should be treated predominantly as hypothesis generating.
Conclusion
BNP is an established marker of morbidity and mortality in cardiac failure. This study has shown that an elevated BNP is associated with increased ICU mortality in an SA cohort of critically ill patients, particularly in those without a diagnosis of heart failure. An initial BNP of >266 ng/L, sepsis, the admission SOFA score and fluid balance were significant predictors of ICU mortality on multivariable analysis. Further prospective studies evaluating BNP as a prognostic marker in non-cardiac critically ill patients, and its utility as an addition to strengthen established outcome prediction scores, should be performed.
Declaration. None.
Acknowledgements. We are grateful to the administrative and clinical staff of King Edward VIII Hospital's ICU for the design and maintenance of the ICU patient data base.
Author contributions. AN developed the study concept and design, acquired patients and/or analysed the data, interpreted the data and prepared the manuscript. KdV developed the study concept and design, analysed and interpreted the data and prepared the manuscript. Both authors approved the final version of the article.
Funding. None.
Conflicts of interest. None.
References
1. Nakagawa Y, Nishikimi T, Kuwahara K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides 2019;111:18-25. https://doi.org/10.1016/j.peptides.2018.05.012 [ Links ]
2. Calzetta L, Orlandi A, Page C, et al. Brain natriuretic peptide: Much more than a biomarker. Int J Cardiol 2016;221:1031-1038. https://doi.org/10.1016/j.ijcard.2016.07.109 [ Links ]
3. Mueller C, Maisel A, Mebazaa A, et al. The use of B-type natriuretic peptides in the intensive care unit. Congest Heart Fail 2008;14(4):43-45. https://doi.org/10.1111/j.1751-7133.2008.tb00011.x [ Links ]
4. Rodseth RN, Biccard BM, Manach YL, et al. The prognostic value of pre-operative and post-operative B-type natriuretic peptides in patients undergoing noncardiac surgery: B-type natriuretic peptide and N-terminal fragment of pro-B-type natriuretic peptide. A systematic review and individual patient data meta-analysis. J Am Coll Cardiol 2014;63(2):170-180. https://doi.org/10.1016/j.jacc.2013.08.1630 [ Links ]
5. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017;33(1):17-32. https://doi.org/10.1016/jxjca.2016.09.008 [ Links ]
6. Januzzi JL, Morss, Tung R, et al. Natriuretic peptide testing for the evaluation of critically ill patients with shock in the intensive care unit: A prospective cohort study. Crit Care 2006;10(1):37. https://doi.org/10.1186/cc4839 [ Links ]
7. Papanikolaou J, Makris D, Mpaka M, et al. New insights into the mechanisms involved in B-type natriuretic peptide elevation and its prognostic value in septic patients. Crit Care 2014;18(3):94. https://doi.org/10.1186/cc13864 [ Links ]
8. Doust J, Lehman R, Glasziou P. The role of BNP in heart failure. Am Fam Phys 2006;74(11):1893-1900. [ Links ]
9. Shang C. B-type natriuretic peptide-guided therapy for perioperative medicine? Open Heart 2014;1:e000105. https://doi.org/10.1136/openhrt-2014-000105 [ Links ]
10. Wilhelms SB, Wilhelms DB. Emergency department admissions to the intensive care unit - a national retrospective study. BMC Emerg Med 2021;21:122. https://doi.org/10.1186/s12873-021-00517-0 [ Links ]
11. Mullins PM, Goyal M, Pines J. National growth in intensive care unit admissions from emergency departments in the United States from 2002 to 2009. Acad Emerg Med 2013;20:479-486. https://doi.org/10.1111/acem.12134 [ Links ]
12. De Vasconcellos K, Skinner DL. Hyperchloraemia is associated with acute kidney injury and mortality in the critically ill: A retrospective observational study in a multidisciplinary intensive care unit. J Crit Care 2018;1:45-51. https://doi.org/10.1016/j.jcrc.2018.01.019 [ Links ]
13. Bachmann KN, Gupta DK, Xu M, et al. Unexpectedly low natriuretic peptide levels in patients with heart failure. J Am Coll Cardiol: Heart Failure 2021;9(3):192-200. https://doi.org/10.1016/j.jchf.2020.10.008 [ Links ]
14. Sakane K, Kanzaki Y, Tsuda K, et al. Disproportionately low BNP levels in patients of acute heart failure with preserved vs. reduced ejection fraction. Int J Cardiol 2020;327:105-110. https://doi.org/10.1016/j.ijcard.2020.11.066 [ Links ]
15. Kakoullis L, Giannopoulou E, Papachristodoulou, et al. The utility of brain natriuretic peptides in septic shock as markers for mortality and cardiac dysfunction: A systematic review. Int J Clin Pract 2019;73(7):133-174. https://doi.org/10.1111/ijcp.13374 [ Links ]
16. York MK, Gupta DK, Reynolds CF, et al. B-type natriuretic peptide levels and mortality in patients with and without heart failure. J Am Coll Cardiol 2018;71(19):2079-2088. https://doi.org/10.1016%2Fj.jacc.2018.02.071 [ Links ]
17. Kandil E, Burack J, Sawas A, et al. B-type natriuretic peptide: A biomarker for the diagnosis and risk stratification of patients with septic shock. Arch Surg 2008;43(3):242-246. https://doi.org/10.1001/archsurg.2007.69 [ Links ]
 Correspondence:
Correspondence:
A Naidoo
avanitar@gmail.com
Accepted 16 October 2023
Contribution of the study
The study is a retrospective, observational study conducted in multidisciplinary, closed, intensivist-run ICU at a tertiary academic hospital. It showed an elevated BNP is associated with increased ICU mortality, particularly in those without a baseline diagnosis of heart failure. This identifies the need for further prospective studies evaluating BNP as a prognostic marker in non-cardiac critically ill patients, and its utility as an addition in pre-existing ICU outcome prediction scores.














