Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Southern African Journal of Critical Care (Online)
versão On-line ISSN 2078-676X
versão impressa ISSN 1562-8264
South. Afr. j. crit. care (Online) vol.39 no.3 Pretoria Nov. 2023
http://dx.doi.org/10.7196/SAJCC.2023.v39i3.1286
RESEARCH
Outcomes of traumatic brain injury patients in an adult intensive care unit of a South African regional hospital, without on-site neurosurgical service: A retrospective quantitative study on the neurological improvement at discharge
A SallieI; R WiseII, III
IMB ChB, DA (SA), FCA (SA); Discipline of Anaesthesiology, and Critical Care, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, South Africa
IIMB ChB, FCA(SA), Cert Crit Care (SA), MMed (Anaes), Dip Obst (SA), Dip PEC (SA); Faculty Medicine and Pharmacy, Vrije Universiteit Brussel (VUB), Belgium
IIIMB ChB, FCA(SA), Cert Crit Care (SA), MMed (Anaes), Dip Obst (SA), Dip PEC (SA); Adult Intensive Care Unit, John Radcliffe Hospital, Oxford University Hospitals Trust, Oxford, United Kingdom
ABSTRACT
BACKGROUND. Traumatic brain injury (TBI) is a major cause of mortality and disability. The South African (SA) province of Kwazulu-Natal faces challenges in providing appropriate care for TBI patients owing to limited resources and delayed access to healthcare services. We aimed to assess the outcomes of patients with TBI who were treated at a hospital without a neurosurgical unit (NSU).
OBJECTIVES. The primary objective was to compare the Glasgow Coma Scale (GCS) scores at admission and discharge from the intensive care unit (ICU) for patients with TBI receiving neuroprotection. Secondary objectives included analysing demographics and identifying predictive factors associated with GCS score improvement.
METHODS. This retrospective study analysed data from the already established ICU Integrated Critical Care Electronic Database. Data on patient demographics, mechanisms of injury and GCS scores were collected and analysed.
RESULTS. The analysis included 95 TBI patients, most of whom were young males. Interpersonal violence and transport-related trauma were the main causes of injury among patients. Approximately 63% of patients had a GCS score improvement >1 upon discharge from the ICU. Patients who received >12 hours of neuroprotection in the emergency department had significantly lower rates of improvement.
CONCLUSION. Sixty-three percent of TBI patients had improved GCS scores by >1 on discharge from the ICU, but outcomes varied. Delayed ICU admission from the emergency department of >12 hours might contribute to worse outcomes. Timely neuroprotection, improved access to neurosurgical care and better understanding of the factors affecting outcomes are needed.
Keywords: Traumatic brain injury, intensive care unit, neurological service, improvement.
Traumatic brain injury (TBI) is the leading cause of death and severe disability in survivors of severe blunt trauma. It remains a significant problem in sub-Saharan Africa where the incidence of TBI is 1.5 times higher than the global average.[1] Up to 50% of those with severe TBI have long-term complications, while up to 30% develop incapacitating long-term neurological deficits.[2] Trauma patients with TBI have a 10-fold higher mortality rate than those without TBI.[3]
Kwazulu-Natal Province (KZN) had an estimated population of 11.5 million in July 2020 (20% of South Africa's (SA) total population of 59.6 million).[4] Trauma constitutes ~25% of emergency cases in KZN public hospitals, where there are insufficient intensive care unit (ICU) beds and limited capacity for rehabilitation.[5,6,7] ICU beds are a precious resource, requiring careful triage due to their finite and limited number, particularly in the face of resource constraints at regional and tertiary public hospitals.[5,6,7]
Patients with severe TBI exclusively treated in hospitals without neurosurgical units (NSU) had a 26% higher mortality rate and a 2.15 increase in adjusted odds of death compared with those treated at an NSU.'31 The Brain Trauma Foundation's Prehospital Guidelines for the Management of TBI recommend that patients with suspected moderate-severe TBI be transported directly by the emergency medical services to a facility equipped with computed tomography (CT) scanning, timely neurosurgical care, and capacity to monitor intracranial pressure (ICP) and treat intracranial hypertension.[8]
Several functioning systems are required to enable this, including adequate staff, radiological equipment, training, and intensive care and theatre facilities. Unfortunately, KZN cannot facilitate this level of care, resulting in many patients with TBI being treated in regional hospitals. The outcome of these patents has not been well documented within the SA context.
Access to the appropriate level of hospital care is delayed for multiple reasons, including a shortage of emergency medical services staff and ambulances, incorrect triaging of patients due to inadequate training, transportation of patients to an inappropriate level of healthcare facility and delays in interhospital transfers of ventilated patients due to a shortage of advanced life support-trained staff and equipment.[5]
Primary healthcare facilities are not equipped with adequate diagnostic imaging and the advanced care required for TBI patients.[5] A CT scan of the brain is the gold standard to detect intracranial abnormalities caused by TBI, and district hospitals are not equipped with this technology.[5,9] These scans help identify abnormalities that might necessitate neurosurgical intervention and determine whether patients should be transferred to an NSU. They also help with prognostication and decisions regarding hospital and ICU admission.[9] Delayed access to CT imaging may contribute to delays in appropriate initial and definitive care.[5]
The centralisation of NSUs requires CT images to be transferred electronically to enable neurosurgeons to rapidly interpret the scan and make clinical decisions. When this function is unavailable or malfunctions, the interpretation of the CT scan is reliant on the staff at the referring hospital. Failing this, the Glasgow Coma Scale (GCS) scores would have to serve as the sole triage tool, which may lead to inappropriate transfers.[10]
Study site
The study was performed at Harry Gwala Regional Hospital (HGRH), formerly Edendale Hospital (EDH). This is a 900-bed regional hospital in Pietermaritzburg (KZN), with a geographically remote NSU (80 km away), located at Inkosi Albert Luthuli Central Hospital (IALCH) in Durban.[11] TBI patients who present to HGRH face many challenges owing to delays in initial treatment and subsequent transfer to the IALCH NSU, limited accessibility to urgent CT scans and delayed neurosurgical intervention. A single CT scanner is available at HRGH; however, there are staffing shortages, particularly in radiographers, radiologists, nurses and porters. Consequently, after-hours radiology is limited. There are times when the CT scanner is broken (with no backup CT scanner available) or the link for transmission of images to the referral centre is out of order.
Transportation to IALCH is often delayed following acceptance of a TBI patient, as it requires confirmation of an available ICU bed at the sole NSU referral centre in the KZN public health sector. A study performed at the study site in 2007 demonstrated that the average delay in transportation of TBI patients to the NSU was 7 hours.[11]
This study aimed to determine the differences in admission v. discharge GCS scores, following neuroprotection among TBI patients admitted into the adult ICU in a single SA regional hospital without an on-site NSU. Secondary objectives included assessing patient demographics and identifying factors that might contribute to a lack of improvement in the GCS score. We hypothesised that following neuroprotection in a regional hospital's adult ICU, TBI patients have minimal improvement in GCS scores (difference of 0 - 1) from admission to discharge from the ICU.
Method
Study design
This retrospective study analysed data from the Integrated Critical Care Electronic Database (iCED).[12] The database implemented in the Pietermaritzburg Metropolitan Critical Care system effectively addressed data collection needs and is a cost-efficient and therapeutically useful solution. It satisfies the criteria for a registry, facilitating research, system planning, and quality improvement in a developing nation.[12]
Ethics approval to maintain the database is obtained from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (UKZN) (ref. no. BCA 211/14). Referral, admission and discharge information are prospectively entered into the database by ICU doctors (interns, medical officers, registrars and consultants). Patient clinical details are transcribed from paper form to the database for every referral and admission, enabling the real-time input of clinical data. Upon discharge or death, a similar process is followed, except that a substantial portion of the record is free text.
Data from TBI patients admitted to the adult ICU for neuroprotection between 1 July 2015 (inception of database use at HGRH) and 29 February 2020 were included for analysis. The original end date would have been 30 June 2020 (5 years), but was adjusted to avoid the influence of the lockdown imposed by the SA government in response to the COVID-19 virus pandemic, and the possibility of COVID-19 infections among TBI patients which may have contributed to increased mortality risk.
Sample size
A sample size of 85 patients was required to estimate the proportion of patients with an improved outcome within a 15% margin, with a 95% probability.
Data collection and management
The principal investigator was the single data collector, ensuring uniformity. All patients who met the inclusion criteria within the stipulated timeframe were included, eliminating the need for randomisation and blinding. Each patient data entry was reviewed to confirm inclusion and exclusion criteria (Table 1).
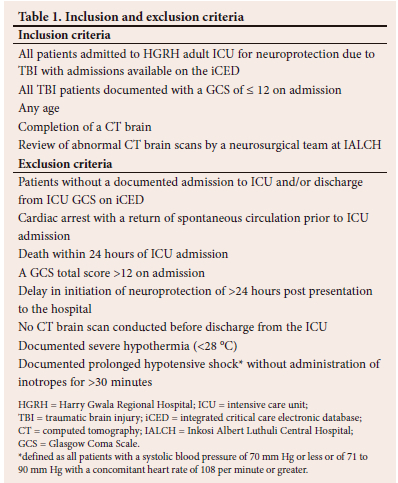
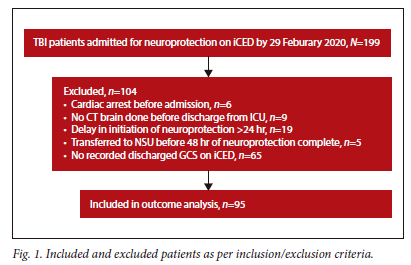
A total of 199 TBI patients were admitted for neuroprotection, but only 95 patient records were included in the analysis (Fig. 1).
Results
We analysed 95 TBI patient admission records. The patients were relatively young, with 76.8% (n=73/95) under 40 years of age. The male-to-female ratio was 5.7:1, with 85.2% (n=81/95) of patients being male. The main mechanisms of injury were interpersonal violence (38.9%; n=37/95) and transport-related injuries (57.9%; n=55/95). Sixty-three percent of TBI patients (n=60/95) had improved GCS scores by more than 1 upon discharge from the ICU. The 37% (n=35/95) of patients who did not have improved scores were divided into those with no improvement (34%; n=12/35) those with a GCS difference of 1 (8%; n=3/35) and those with a decreased GCS score (57%; n=20/35).
The only statistically significant factor for no or minimal improvement in GCS scores at discharge v. admission was the duration of neuroprotection >12 hours in the emergency department (ED) before being referred to the ICU. Almost half of the patients (48.6%) with >12 hours in the ED had no or minimal improvement v. only 25% in the <12 hours group (p=0.02). This could be an area of focus to allow for improved outcomes.
Nearly half of the patients (46%; 44/95) had an admission GCS score of 3; however, these might have been higher at referral. Many of these patients were admitted after sedation to facilitate CT imaging or ventilation in the ED. The residual effects of anaesthesia in patients admitted postoperatively may have also contributed to lower GCS scores; however, they only made up 11% (n=5/44) of those admitted with a GCS score of 3. Data on the amount of sedation received before admission is insufficient, but the difference between the postoperative and non-operative groups was assessed. The postoperative group had a no improvement rate of 41.2% (n=7/17), while the non-operative group showed a rate of 32.1% (n=25/78, p=0.47).
Out of 95 patients, 34.7% (n=33) presented with extracranial injuries and were labelled as non-isolated TBI patients (Table 3). The non-isolated TBI patients had a higher percentage of no change or decreased GCS scores than the isolated TBI group (38.7% v. 24.2%, p=0.15). Insufficient data points were available to assess if each polytrauma patient in this study had an abbreviated injury score of >3.
For the 63 patients that had a GCS score improvement >1, the average increase in GCS score was 5.86 with an average discharge GCS score of 12.
Discussion
Three large TBI databases guide protocols, identify prognostic variables and infer prognostic predictive models. '3,13-151 The most applicable to our setting is the Corticosteroid Randomisation After Significant Head Injury (CRASH-1) trial,[14] where 75% of patients were recruited from low-middle income countries (LMICs). It is the largest clinical trial conducted on TBI patients, with a sample size of 10 008.[14-16]
The CRASH-1 trial collaborators developed a prognostic model for predicting two outcomes in two settings (LMICs and high-income countries) (Fig. 2.).[14] The two predicted outcomes were death at 14 days and a Glasgow Outcome Scale (GOS) of 1 - 3 (death or severe disability) at 6 months post-TBI.'141 Predictive factors and their respective risks for the outcome can be obtained from their web-based calculator (www.crash2.lshtm.ac.uk/). By entering the varying predictors, the expected risk of death at 14 days and of death or severe disability at six months can be calculated.[14] This LMIC basic prognostic model could be used in an ED.
The GOS (Table 4) predicts mortality (GOS 1) v. survival (GOS 2 - 5), and unfavourable (GOS 1 - 3) v. favourable (GOS 4 - 5) outcomes.[17,18] The GOS has five categories:[14,17,18]
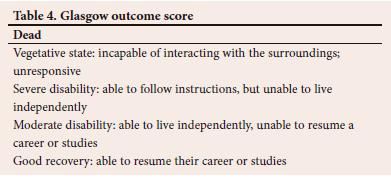
Prognostic models employing statistical, clinical and data-based models aim to predict outcomes more accurately than clinical bedside predictions. The use of computer-based outcome prediction for TBI patients influences early decisions regarding management.[14] These facilitate a more ethical distribution of limited resources, with increased therapeutic interventions for those predicted to have a good outcome while reducing their use for those with a poor prognosis. These models have been internally validated, but the external validation was done in comparison to the high-income countries cohort only.[14]
The CRASH-1 basic model includes age, GCS score, pupil reactivity and the presence of major extracranial injury. Information can be entered into a web-based calculator. Older age (>40 years), low GCS score, absent pupil reactivity and the presence of major extracranial injury (defined as an abbreviated injury score of >3) predicted a worse prognosis.[14]
TBI patients older than 40 years old are predicted to have a worse neurological prognosis.[14] However, in our cohort, no statistical difference was noted between the older and younger groups, where no GCS score improvement was observed in 36.4% (n=8/22) v. 32.9% (n=24/73, p=0.76) of patients in the >40 years v. patients <40 years group, respectively.
Pooled data from the International Mission for Prognosis and Analysis of Clinical Trials in TBIs (IMPACT) project and CRASH-1 trial showed a mortality rate of 51% with a GCS score of 3. Higher GCS scores demonstrated a mortality rate that declined progressively to 3% when the GCS score was 15. Similarly, there was a decline in the rate of unfavourable outcomes at 6 months post-injury, decreasing from 70% at a GCS score of 3 to 12% at a GCS score of 15.[15] There is insufficient data to determine the GCS at the scene prior to hospital transfer or admission.
Injuries in LMICs are mainly violence or transport-related.[5,14] This is mirrored in SA data,[5] with similar findings in the current study. The CRASH study also identified that patients from LMICs were younger, more likely to be male and had less severe TBI compared with patients from high-income countries.[14] Trauma patterns in Pietermaritzburg previously demonstrated an almost 4:1 male-to-female ratio.[19] There is no suggestion of any association between sex and neurological prognosis.[20,21] The patient population in this study had expected demographics in keeping with the CRASH study.
A recent study completed at HGRH showed the average time between ICU decision to accept the patient and the actual admission was just over 6 hours (this included all ICU referrals including non-surgical patients).[7] These delays and lengthy transfer times to the NSU may contribute to increased morbidity and mortality in patients with severe TBI.[10,11] Of the TBI patients admitted to the ICU, 46.3% (44/95) experienced a similar delay of more than 6 hours. The three most common reasons for delayed admission to the ICU were waiting for a neurosurgery review or opinion, unavailability of ICU beds (including no beds due to ICU staffing constraints) and the necessity for emergency surgery. These delays may contribute to worse outcomes as seen in our study, where 40.9% (18/44) of the delayed group had no or minimal change in GCS versus 27.5% (14/51) in the non-delayed group (p=0.17).
Prolonged neuroprotection >12 hours in the ED before an ICU referral was the only significant secondary finding contributing to a worse outcome. The ICU and ED follow the same neuroprotection protocol goals based on the Brain Trauma Foundation guidelines. Delays between the presentation to the ED and the referral to the ICU may occur for several reasons and require scrutiny to untangle the complex intertwined systems that need to be negotiated in a busy ED. Sufficient senior staff, adequate number of porters and transfer equipment, timely access to radiology services, rapid communication and assessment of CT scan findings, appropriate level of nursing care and rapid response from the ICU, are all important factors contributing to the appropriate triage of TBI patients.
Study limitations
The data was obtained from a single centre, which restricts generalisation.
Database entries could be influenced by human error and contributed to the 65 record exclusions with no recorded discharge GCS scores. We could not avoid patients being admitted to ICU with a lower GCS due to the practice of sedation and endotracheal intubation of restless patients with a GCS score <8 to facilitate CT imaging.
Due to the patients' low GCS and lack of collateral family history, the patient's baseline functional status, existing neurological compromise, and other co-morbidities are commonly unknown on admission. The ED at HGRH is well equipped (ventilators and monitors), and consequently, many TBI patients underwent neuroprotection for prolonged periods in the ED and possibly transferred directly from the ED to the NSU. Some TBI patients referred to the ICU were possibly never admitted due to bed shortages.
The iCED has a data entry point for whether the patient's pupils are reactive or not, but this is not always completed. If the patient has non-reactive pupils, it does not allow for the option of whether one or both pupils are non-reactive. This limits the predictive value that the pupil score could provide. The GOS score would be the preferred outcome scale for future prospective studies in these patients, but there would have to be a buy-in from the various departments to standardise their data collection, preferably onto an online database. For example, the CT brain reports by radiologists should include the Marshall grading criteria.
Supervision of neuroprotection protocols was performed by on-site medical staff, as part of the responsibility of the intensivist in charge. Data regarding compliance was not available in the database as it is not designed for this detail.
Conclusion
Sixty-three percent of TBI patients improved their GCS by more than 1 upon discharge from the ICU. Among the 37% of patients that did not show improvement: 34% (n=12/35) showed no improvement, 8% (n=3/35) had a GCS difference of 1, and 57% (n=20/35) had a decreased GCS score.
The only statistically significant factor for no or minimal GCS improvement at discharge v. admission was the duration of neuroprotection >12 hours in the emergency department before being referred to ICU. This could be an area of focus for improved outcomes.
A prospective study with a 6-month follow-up may help validate the web-based prognostic score derived from the basic CRASH-1 trial prognostic tool in an LMIC setting and may assist with better allocation of resources.
Declaration. The BREC of the UKZN has given full ethical approval (number: BREC/00001013/2020). The most recent renewal of approval was issued on 28 March 2023.
Acknowledgements. The authors would like to thank Catherine Connolly from the Department of Biostatistics, University of Kwa-Zulu Natal for her assistance with statistical analysis. Thank you to all the doctors who contributed to the data collection on iCED. A special acknowledgement goes to Dr Nikki Allorto for the design of iCED and for teaching us how to export the data to MS Excel.
Author contributions. AS and RW conceived and planned this study. AS collected and analysed the data. AS and RW discussed the results. AS wrote the manuscript, RW provided critical feedback and helped shape the research, analysis and anuscript.
Funding. None.
Conflicts of interest. None.
References
1. Murray CJ, Lopez AD, Jamison DT. The global burden of disease in 1990: summary results, sensitivity analysis and future directions. Bulletin of the World Health Organization 1994;72(3):495-509. [ Links ]
2. Rizoli S, Petersen A, Bulger E, et al. Early prediction of outcome after severe traumatic brain injury: a simple and practical model. BMC Emerg Med 2016;16(1):32. https://doi.org/10.1016/s1073-5437(08)70092-5 [ Links ]
3. Patel HC, Bouamra O, Woodford M, King AT, Yates DW, Lecky FE. Trends in head injury outcome from 1989 to 2003 and the effect of neurosurgical care: an observational study. Lancet 2005;366(9496):1538-1544. https://doi.org/10.1016/s0140-6736(05)67626-x [ Links ]
4. Mid-year population estimates 2020 [press release]. http://www.statssa.gov.za/?p=13453 (accessed November 2023). [ Links ]
5. Hardcastle T, Oosthuizen G, Clarke D, Lutge E. Trauma, a preventable burden of disease in South Africa: review of the evidence, with a focus on KwaZulu-Natal. Health Systems Trust 2016:179-189. https://www.hst.org.za/publications/South%20African%20Health%20Reviews/15%20Traum a%20a%20preventable%20burden%20of%20disease%20in%20South%20 Africa%20Review%20of%20the%20evidence%20with%20a%20focus%20on%20KZN.pdf [ Links ]
6. Gordon K, Allorto N, Wise R. Analysis of referrals and triage patterns in a South African metropolitan adult intensive care service. S Afr Med J 2015;105(6):491-495. https//doi.org/10.7196/samj.9007 [ Links ]
7. Singh M, Maharaj R, Allorto N, Wise R. Profile of referrals to an intensive care unit from a regional hospital emergency centre in KwaZulu-Natal. Afr J Emerg Med 2021;11(4):471-476. https://doi.org/10.1016/j.afjem.2021.07.006 [ Links ]
8. Lulla A, Lumba-Brown A, Totten AM, et al. Prehospital Guidelines for the Management of Traumatic Brain Injury - 3rd Edition. Prehospital Emerg Care 2023:1-32. https://doi.org/10.1080/10903127.2023.2187905 [ Links ]
9. Jacobs B, Beems T, Stulemeijer M, et al. Outcome prediction in mild traumatic brain injury: age and clinical variables are stronger predictors than CT abnormalities. J Neurotrauma 2010;27(4):655-668. https://doi.org/10.1089/neu.2009.1059 [ Links ]
10. Zulu BMW, Mulaudzi TV, Madiba TE, Muckart DJJ. Outcome of head injuries in general surgical units with an off-site neurosurgical service. Injury 2007;38(5):576-583. https://doi.org/10.1016/j.injury.2007.01.002 [ Links ]
11. Alexander T, Fuller G, Hargovan P, Clarke D, J Muckart D, Thomson S. An audit of the quality of care of traumatic brain injury at a busy regional hospital in South Africa. S Afr J Surg 2009;47(4):120-122, 124-126. https://pubmed.ncbi.nlm.nih.gov/20141069/ [ Links ]
12. Allorto NL, Wise RD. Development and evaluation of an integrated electronic data management system in a South African metropolitan critical care service. Southern Afr J Anaesthesia Analg 2015;21(6):173-177. https://doi.org/10.1080/22201181.2015.1115607 [ Links ]
13. Butcher I, McHugh GS, Lu J, et al. Prognostic Value of Cause of Injury in Traumatic Brain Injury: Results from The IMPACT Study. J Neurotrauma 2007;24(2):281-286. https://doi.org/10.1089/neu.2006.0030 [ Links ]
14. MRC CRASH Trial Collaborators. Perel P AM, Clayton T, Edwards P, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ 2008;336(7641):425-429. https://doi.org/10.1136/bmj.39461.643438.25 [ Links ]
15. Brennan PM, Murray GD, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 1: The GCS-Pupils score: an extended index of clinical severity. J Neurosurg 2018;128(6):1612-1620. https://doi.org/10.3171/2017.12.jns172780 [ Links ]
16. Edwards P, Arango M, Balica L, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet 2005;365(9475):1957-1959. https://doi.org/10.1016/s0140-6736(05)66552-x [ Links ]
17. Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics. PLoS Med 2008;5(8):e165. https://doi.org/10.1371/journal.pmed.0050165 [ Links ]
18. Sobuwa S, Hartzenberg HB, Geduld H, Uys C. Predicting outcome in severe traumatic brain injury using a simple prognostic model. S Afr Med J 2014;104(7):492-494. https://doi.org/10.7196/samj.7720 [ Links ]
19. Moodley NB, Clarke DL, Aldous C. Current trauma patterns in Pietermaritzburg. S Afr J Surg 2015;53:37-39. https://pubmed.ncbi.nlm.nih.gov/28240482/ [ Links ]
20. Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma 2007;24(2):329-337. https://doi.org/10.1089/neu.2006.0035 [ Links ]
21. Mushkudiani NA, Engel DC, Steyerberg EW, et al. Prognostic Value of Demographic Characteristics in Traumatic Brain Injury: Results from The IMPACT Study. J Neurotrauma 2007;24(2):259-269. https://doi.org/10.1089/neu.2006.0028 [ Links ]
 Correspondence:
Correspondence:
A Sallie
allison.c.e@gmail.com
Accepted 24 November 2023
Contribution of the study
This study explores the outcomes of patients with TBI admitted to a non-neurosurgical ICU. Factors contributing to a worse outcome are identified, highlighting the need for adequate numbers of ICU beds and prompt admission from the emergency department.














