Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Southern African Journal of Critical Care (Online)
versão On-line ISSN 2078-676X
versão impressa ISSN 1562-8264
South. Afr. j. crit. care (Online) vol.39 no.1 Pretoria Mar. 2023
http://dx.doi.org/10.7196/SAJCC.2023.v39i1.558
RESEARCH
Prevalence and independent predictors of in-hospital stroke among patients who developed acute alteration of consciousness in the medical intensive care unit: A retrospective case-control study
S Tongyoo; T Viarasilpa; M Vichutavate; C Permpikul
MD; Faculty of Medicine, Mahidol University; Siriraj Hospital, Bangkoknoi, Bangkok, Thailand
ABSTRACT
BACKGROUND: In-hospital stroke is a serious event, associated with poor outcomes and high mortality. However, identifying signs of stroke may be more difficult in critically ill patients
OBJECTIVES: This study investigated the prevalence and independent predictors of in-hospital stroke among patients with acute alteration of consciousness in the medical intensive care unit (MICU) who underwent subsequent brain computed tomography (CT
METHODS: This retrospective study enrolled eligible patients during the period 2007 - 2017. The alterations researched were radiologically confirmed acute ischaemic stroke (AIS) and intracerebral haemorrhage (ICH
RESULTS: Of 4 360 patients, 113 underwent brain CT. Among these, 31% had AIS, while 15% had ICH. They had higher diastolic blood pressures and arterial pH than non-stroke patients. ICH patients had higher mean (standard deviation (SD) systolic blood pressures (152 (48) v. 129 (25) mmHg; p=0.01), lower mean (SD) Glasgow Coma Scale scores (4 (3) v. 7 (4); p=0.004), and more pupillary abnormalities (75% v. 9%; p<0.001) than AIS patients. AIS patients were older (65 (18) v. 57 (18) years; p=0.03), had more hypertension (60% v. 39%; p=0.04), and more commonly presented with the Babinski sign (26% v. 9%; p=0.04). Multivariate analysis found that pupillary abnormalities independently predicted ICH (adjusted odds ratio (aOR) 26.9; 95% CI 3.7 - 196.3; p=0.001). The Babinski sign (aOR 5.1; 95% CI 1.1 - 23.5; p=0.04) and alkalaemia (arterial pH >7.4; aOR 3.6; 95% CI 1.0 - 12.3; p=0.05) independently predicted AIS
CONCLUSIONS: Forty-six percent of the cohort had ICH or AIS. Both conditions had high mortality. The presence of pupillary abnormalities predicts ICH, whereas the Babinski sign and alkalaemia predict AIS
Keywords: acute alteration of consciousness; clinical clues; factors; in-hospital stroke; medical ICU; patients; prevalence
In-hospital stroke is a serious event in critical care. The condition is associated with poor outcomes and high mortality.[1-3] Its main risk factors are patient age, underlying disease, unstable haemodynamics, infection, coagulopathy, metabolic alterations, and medications.[1] Early recognition of the condition is essential. Physical signs, such as altered consciousness, hemiparesis, seizures, and fixed pupils, have been reported to be common indicators that prompt diagnostic procedures.[1-4] However, identifying signs of stroke may be more difficult in patients with pre-existing alterations of consciousness or neurological impairment.[5-7]
Computed brain tomography (brain CT) is an essential tool for diagnosing in-hospital stroke -either intracerebral haemorrhage (ICH) or acute ischaemic stroke (AIS). However, moving critically ill patients to the CT suite is not without risk.[8] Identifying patients with a high probability of in-hospital stroke would facilitate their early management and prevent unnecessary movement. Although an abnormal finding on neurological examination is a major clue among general patients, its usefulness for intensive care unit (ICU) patients has not been investigated. Other clinical parameters, such as the baseline severity score, underlying conditions, medications received, and coagulation status, may help identify high-risk patients.
The purpose of this study was to determine the prevalence and independent predictors of in-hospital stroke among patients who developed acute alteration of consciousness in the medical ICU (MICU) and who underwent subsequent brain CT.
Methods
We retrospectively reviewed the medical records of adult patients 18 years or older who were admitted to the MICU of Siriraj Hospital, a national university-based tertiary referral centre located in Bangkok, Thailand. The study period was 1 January 2007 to 30 June 2017. The patients underwent brain CT after the development of acute alteration of consciousness (defined by a Glasgow Coma Scale (GCS) score <10). The altered consciousness occurred >24 hours after MICU admission, with or without a focal neurological deficit. We excluded patients with a known diagnosis of cerebrovascular disease before admission to the MICU. At our ICU, brain CT was considered to identify intracranial causes for patients with new onset of altered consciousness (with or without focal neurological deficits) who did not recover after all metabolic causes had been corrected. However, patients were not candidates for CT if they had haemodynamic instability, were under resuscitation, had severe metabolic derangement, were receiving sedative or paralytic agents, or had signed an advance directive specifying no resuscitation. The decisions to send patients for CT scanning were made by attending physicians.
Data collection
Collected data were as follows: patient demographics; comorbid diseases; current use of antiplatelet or anticoagulation medications; MICU admission diagnosis; disease severity (assessed by Acute Physiology and Chronic Health Evaluation II (APACHE II) score[9] and Sequential Organ Failure Assessment score;[10] GCS score; blood pressure; and heart rate at the time of the alteration of consciousness event. Neurological examination findings (including abnormal pupil examination, hemiparesis, and Babinski sign) were also recorded. A pupillary abnormality was defined as a difference of >2 mm in the pupil diameter of each side or a dilated pupil (>5 mm) without response to light projection. Laboratory data collected were platelet count, international normalised ratio of prothrombin time, blood urea nitrogen, arterial pH, and serum lactate. In the case of patients receiving ventilatory support, peak inspiratory pressure (PIP) and positive end-expiratory pressure (PEEP) at the time of the event were also collected. Thirty-day mortality data of normal and abnormal brain CT finding groups were also recorded and compared. Diagnoses of AIS and ICH were made according to the findings of contrast CT imaging. Patients who had cardiac arrest before their CT scan were excluded.
Ethical considerations
Before this research began, its protocol was approved by the Siriraj Institutional Review Board of the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (approval number Si 451/2016). The requirement for written informed consent was waived owing to the retrospective, anonymity-preserved nature of the study. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed.
Statistical analysis
To identify independent predictors of AIS and ICH, we compared data collected from patients with abnormal brain CT findings (AIS or ICH) and those with normal brain CT findings. Continuous data were checked for normal distribution using the Kolmogorov-Smirnov test. Continuous data with a normal distribution were compared using Student's t-test, and the results are presented as mean (standard deviation (SD)). Continuous data with a non-normal distribution were compared using the Mann-Whitney U test, and those results are given as median and interquartile range (IQR). The tests used to compare categorical data were the x2 test and Fisher's exact test, and their results are presented as frequency and proportion. Univariate and multivariate analyses were performed to identify factors significantly and independently associated with AIS or ICH. The results of the multivariate analysis are shown as adjusted odds ratios (aORs) and 95% confidence intervals (CIs). A p-value <0.05 was deemed statistically significant. All data analyses were conducted with PASW Statistics for Windows, version 18 (SPSS Inc., USA).[11]
Results
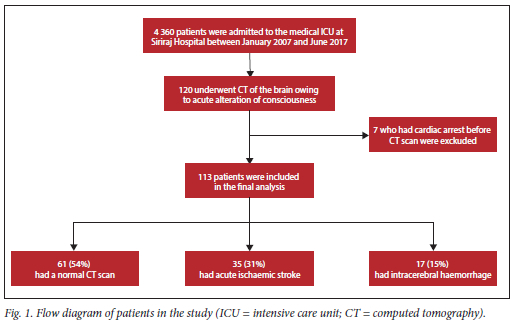
During the study period, 120 of the 4 360 patients admitted to the MICU of Siriraj Hospital underwent brain CT owing to acute alteration of consciousness. Seven patients who developed cardiac arrest before their CT study were excluded, leaving 113 patients for final analysis (Fig. 1). Of these, 35 (31%) had AIS, 17 (15%) had ICH, and 61 (54%) did not show new abnormalities. All patients were intubated and ventilated with assist-control mode ventilation before brain CT was performed.
Baseline characteristics
The mean (SD) age of the 113 patients was 59 (19) years, and 41% were men (Table 1). Compared with patients with normal CT findings, patients with AIS were significantly older (65.1 (17.5) v. 56.5 (18.4) years; p=0.03), more likely to have a prior history of hypertension (60% v. 39%; p=0.04), and more likely to be admitted to the ICU owing to acute respiratory failure (31% v. 10%; p=0.008). The AIS group had lower proportions of patients with malignancy (9% v. 32%; p=0.02) and septic shock (31% v.61%; p=0.006) than the normal imaging group. Other comorbid diseases, reasons for ICU admission, the use of antiplatelets or anticoagulants, and disease severity were not significantly different between patients with AIS and those with normal brain CT findings. There were also no significant differences in the baseline characteristics of patients with ICH and those with normal brain CT results.
Vital signs, neurological findings and laboratory findings at the time of event
The diastolic blood pressure (DBP) of patients with ICH or AIS was significantly higher than that of patients with normal brain CT findings (70 (SD 15) mmHg in patients with normal CT v. 84 (22) mmHg in patients with ICH (p=0.02) v.77 (16) mmHg in those with AIS (p=0.03)) (Table 2). Higher systolic blood pressure (SBP) was found only in patients with ICH (129 (25) mmHg in patients with normal brain CT v. 152 (48) mmHg in those with ICH (p=0.01) v.130 (22) mmHg in those with AIS (p=0.8)). There were no significant differences in the heart rates of the normal imaging, ICH and AIS groups.
Regarding neurological findings, patients with ICH had a significantly lower mean GCS score (4 (3) v. 7 (4); p=0.004) than those with normal CT imaging. The ICH group was also significantly more likely to have pupillary abnormalities (75% v. 9%; p<0.001). Additionally, a higher proportion of patients with AIS presented with the Babinski sign than those with normal CT imaging (26% v. 9%; p=0.04). However, there were no statistically significant differences in GCS scores or pupillary abnormalities. Lastly, more patients with both types of acute stroke had unilateral muscle weakness on examination during alteration of consciousness than those with normal CT. Again, the differences were not significant.
In terms of the laboratory results, the arterial pH values during altered consciousness in patients with ICH and patients with AIS were significantly higher than in those with normal brain imaging. This finding was despite non-significant differences in the PIP and PEEP values of the normal imaging and the two stroke groups (7.39 (SD 0.11) in patients with normal brain CT v. 7.46 (0.07) in those with ICH (p=0.02) v. 7.44 (0.09) in those with AIS (p=0.02)). The other laboratory results of the three groups were not significantly different.
Outcomes
The 30-day mortality rate of critically ill patients who developed altered consciousness, with or without new abnormal CT findings, was very high compared with overall MICU mortality (81% v. 28%; p<0.001). Mortality was significantly higher for patients with ICH than in those with normal imaging (100% v.75%; p=0.03). In contrast, there was no significant difference in mortality of patients with AIS and those with normal brain CT findings (80% v. 75%; p=0.61).
Predictors of in-hospital stroke
The results of univariate and multivariate analyses to identify factors that independently predict ICH and AIS are detailed in Tables 3 and 4, respectively. Factors identified as significant in the univariate analysis were entered into the multivariate analysis to identify those independently associated with ICH and AIS. Pupillary abnormality was found to independently predict ICH, with an aOR of 26.9 (95% CI 3.7 - 196.3; p=0.001) (Table 3). The presence of the Babinski sign (aOR 5.1; 95% CI 1.1 - 23.5; p=0.04) and alkalaemia (aOR 3.6; 95% CI 1.0 - 12.3; p=0.05) were found to independently predict AIS (Table 4).
Discussion
In this study, 113 critically ill patients with alteration of consciousness underwent brain CT. Almost half (46%) had an acute stroke. Of these, two-thirds had an AIS, and one-third had an ICH. The mortality rate among these in-hospital stroke patients was very high regardless of stroke type. Multivariate analysis revealed that a pupillary abnormality was the only independent predictive factor for ICH at the time of consciousness alteration. However, both alkalaemia and the presence of the Babinski sign were identified as independent predictors of AIS. Univariate analysis revealed that DBP and arterial pH measured at the time of consciousness alteration were significantly higher in patients with AIS than in those with normal CT findings.
The overall incidence of acute stroke, comprising AIS and ICH, during MICU stay was previously reported tobe 1% to 4%.[4, 12] This range is comparable with the rate of 1.2% (52 of 4 360 patients) found in our study. However, the incidence of in-hospital stroke among critically ill patients with consciousness alteration was 46%, which is very high. This specific measurement parameter has not previously been reported. Additionally, the proportion of ICH in our study (33%) was higher than the 10% rate reported by other research.[13]
When we focus on comparing patients with AIS and those with normal CT findings, a significantly higher proportion of AIS patients were initially admitted with respiratory failure, and a significantly lower proportion of that group had septic shock. These results differed from those of earlier studies, which reported that cardiovascular disease and cardioembolism were significantly more common among patients with AIS.[13,14] The difference may be explained by our cardiac patients being admitted to our centre's cardiac care unit. Our study data were also taken from patients admitted to our centre's MICU. Our enrolled patients had a low proportion of cardiac disease. In addition, clinical examination revealed a significantly higher DBP and a significantly higher proportion of patients presenting with the Babinski sign among patients with AIS than those with normal imaging findings. In the multivariate analysis, the Babinski sign was also independently associated with in-hospital stroke. Although the sign has high specificity for identifying corticospinal tract dysfunction, its sensitivity is low.[15] The presence of the Babinski sign confirms the pathology of the pyramidal tract, but its absence does not exclude this condition.
Furthermore, abnormal pupils, hemiparesis, and the Babinski sign are late manifestations of cerebrovascular accident and stroke rather than premonitory signs. We did not assess the stroke-in-evolution of our patients owing to their characteristics and the study design. The patients enrolled in this study were critically ill medical patients; all required mechanical ventilator support and 54% were in shock. Inadequate tissue perfusion during ICU stay causes alteration of consciousness. This prevents neurological examination detection of the ongoing neurological defects that indicate a stroke in evolution.
This study did not include information about a scoring system to predict the risk of embolic stroke. This is because we enrolled critically ill medical patients. As such patients generally have a low risk of embolic stroke, calculating a score to predict embolic stroke was not routine clinical practice during the study period. Instead, we have provided data on the underlying diseases considered high risk for stroke (hypertension, diabetes mellitus, and atrial fibrillation; Table 1).
ICH patients had significantly higher SBP and DBP, lower GCS scores, higher arterial blood pH, and more abnormal pupil examinations. Abnormal pupils were the only condition that was independently associated with ICH in the multivariate analysis. This result is consistent with the findings of another study.[1] An increase in both blood pressure parameters of ICH patients has also previously been reported and was correlated with poor outcomes.[16,17] Our research results showed significantly higher SBP and DBP among ICH patients than among those with normal brain CT findings. The univariate analysis identified that an SBP >140 mmHg and a DBP >70 mmHg were significant predictive factors associated with the presence of ICH. However, neither parameter was identified by the multivariate analysis model as an independent predictive factor associated with ICH.
The lower GCS scores and the appearance of abnormal pupils can be explained by increased intracranial pressure effects on the oculomotor nerve and nucleus and by the pathways of sympathetic and parasympathetic fibres in the brainstem close to the reticular activating system.[18,19] However, the two-point difference in GCS might not help to differentiate ICH from metabolic encephalopathy in our study population. This is because all patients in our study cohort had a severely decreased level of consciousness (GCS <8). Therefore, an abnormal pupil examination finding is a positive sign of ICH in this setting.
The laboratory results revealed that more patients with in-hospital stroke (AIS or ICH) had alkalaemia (arterial pH >7.4) than patients with normal brain CT findings. This finding was despite using the same level of PIP and PEEP during mechanical ventilation. Alkalaemia was also independently associated with AIS in the multivariate analysis. Abnormal breathing in patients with central nervous system diseases (e.g. Cheyne-Stokes respiration or central hyperventilation) can cause acute respiratory alkalosis and alkalaemia.[20,21] However, we were unable to determine whether the alkalaemia resulted from acute respiratory alkalosis that developed after the onset of in-hospital stroke. This is because there were no significant differences in the values of the arterial partial pressure of carbon dioxide and serum bicarbonate of the normal brain CT group versus the AIS group or the ICH group (Table 1).
As mentioned, critically ill patients with acute alteration of consciousness, whether with a new structure or not, had very high mortality. New brain lesions (AIS or ICH) increase the severity of illness, whereas those with normal scans require further investigation. The majority (61%) of the patients with normal brain scans had septic shock, which is associated with high mortality. In addition, consciousness alteration without structural lesions is characterised as metabolic encephalopathy or acute delirium. Possible underlying pathophysiologies are neuroinflammation, blood-brain barrier disruption, dysregulation of cerebral blood flow, and impaired cerebral autoregulation.[22-26] Delirium has also been reported to be independently associated with mortality in ICU patients.[27-32]
The strengths of our study are that we reported the characteristics and outcomes of a cohort of critically ill patients who experienced in-hospital stroke, and performed a multivariable analysis to determine the independent factors associated with ICH and AIS, separately. Also we identified the common neurological signs, which included pupillary abnormalities and the Babinski sign, as the predictive factors associated with stroke in ICU. This finding could be of benefit for physicians practising in resource-limited areas, especially in low-middle-income countries, in term of patient selection to perform further neurological imaging for definite diagnosis.
Study limitations
This study has some limitations. The incidence of in-hospital stroke might have been underestimated since diagnoses were based on the results of brain CT, which has a lower sensitivity to detect early ischaemic injury than magnetic resonance imaging.[22] Furthermore, this retrospective study included only comatose patients who underwent brain CT. Consequently, cases of acute stroke that developed in patients who had focal neurological deficits but without decreased levels of consciousness might not have been recognised. Moreover, we had no data on cranial nerve abnormalities other than the results of examinations of the pupils, e.g. facial palsy or abnormal eye movements. Such abnormalities also help to identify central nervous system pathology.[19] In addition, there were no data records specific to functional outcomes after discharge for patients who survived or of the exact aetiology of coma among patients who did not have new brain imaging abnormalities. In addition, this was a single-centre study with a small sample size. Therefore, its generalisability and power to detect significant differences and associations are potentially limited. More studies with a prospective design or a large multicentre cohort study should be performed to minimise the limitations of our study.
Conclusion
Our results revealed that almost half of the critically ill patients who developed decreased levels of consciousness in the MICU and who underwent brain CT had an in-hospital stroke. Mortality was high among these comatose patients regardless of stroke diagnosis. Some bedside clinical signs and laboratory results provided critical diagnostic clues. The presence of pupillary abnormalities (independently associated with ICH), the Babinski sign (independently associated with AIS), or alkalaemia (independently associated with AIS) increases the likelihood of in-hospital stroke in critically ill comatose patients. Brain imaging should be performed when one of these features is present.
Declaration. Researchers may contact the corresponding author for data sharing requests after approval of a planned analysis protocol. The anonymised participant data will be made available within 3 months of the publication of the article. The study protocol and statistical analysis plan are available in the Appendix.
Acknowledgements. The authors gratefully acknowledge Ms Khemajira Karaketklang of the Department of Medicine, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand for assistance with the statistical analyses. The authors are also indebted to Mr David Park for the English-language editing of this paper.
Author contributions. ST had full access to all study data and accepts full responsibility for the integrity of the data and the accuracy of the data analyses. ST designed the study, performed data analysis and interpretation, and drafted and revised the manuscript. TV performed data interpretation, and drafted and revised the manuscript. MV assisted in the study design and performed data collection. CP assisted with data interpretation and critically reviewed the manuscript. All authors read and approved the final manuscript, and they individually and collectively agree to be accountable for all aspects of the work.
Funding. The trial was funded by Siriraj Critical Care Research Funding.
Conflicts of interest. None.
References
1. Wijdicks EF, Scott JP. Stroke in the medical intensive-care unit. Mayo Clin Proc 1998;73(7):642-646. https://doi.org/10.1016/S0025-6196(11)64887-8 [ Links ]
2. Oppenheim-Eden A, Glantz L, Eidelman LA, Sprung CL. Spontaneous intracerebral hemorrhage in critically ill patients: Incidence over six years and associated factors. Intensive Care Med 1999;25(1):63-67. https://doi.org/10.1007/s001340050788 [ Links ]
3. Bleck TP, Smith MC, Pierre-Louis SJ, Jares JJ, Murray J, Hansen CA. Neurologic complications of critical medical illnesses. Crit Care Med 1993;21(1):98-103. https://doi.org/10.1097/00003246-199301000-00019 [ Links ]
4. Jo S, Chang JY, Jeong S, Jeong S, Jeon SB. Newly developed stroke in patients admitted to non-neurological intensive care units. J Neurol 2020;267(10):2961-2970. https://doi.org/10.1007/s00415-020-09955-5 [ Links ]
5. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013;369(14):1306-1316. https://doi.org/10.1056/NEJMoa1301372. [ Links ]
6. Behrouz R, Godoy DA, Azarpazhooh MR, Di Napoli M. Altered mental status in the neurocritical care unit. J Crit Care 2015;30(6):1272-1277. https://doi.org/10.1016/j.jcrc.2015.07.021 [ Links ]
7. Girard TD, Thompson JL, Pandharipande PP, et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: A prospective cohort study. Lancet Respir Med 2018;6(3):213-222. https://doi.org/10.1016/S2213-2600(18)30062-6 [ Links ]
8. Bergman LM, Pettersson ME, Chaboyer WP, Carlstrom ED, Ringdal ML. Safety hazards during intrahospital transport: A prospective observational study. Crit Care Med 2017;45(10):e1043-e1049. https://doi.org/10.1097/CCM.0000000000002653. [ Links ]
9. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med 1985;13(10):818-829. [ Links ]
10. Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001;286(14):1754-1758. https://doi.org/10.1001/jama.286.14.1754. [ Links ]
11. SPSS Inc. Released. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc; 2009. [ Links ]
12. Rubinos C, Ruland S. Neurologic complications in the intensive care unit. Curr Neurol Neurosci Rep 2016;16(6):57. https://doi.org/10.1007/s11910-016-0651-8 [ Links ]
13. Vera R, Lago A, Fuentes B, et al. In-hospital stroke: A multi-centre prospective registry. Eur J Neurol 2011;18(1):170-176. https://doi.org/10.1111/j.1468-1331.2010.03105.x [ Links ]
14. Park HJ, Cho HJ, Kim YD, et al. Comparison of the characteristics for in-hospital and out-of- hospital ischaemic stroke. Eur J Neurol 2009;16(5):582-588. https://doi.org/10.1111/j.1468-1331.2009.02538.x [ Links ]
15. Ambesh P, Paliwal VK, Shetty V, Kamholz S. The Babinski sign: A comprehensive review. J Neurol Sci 2017;372:477-481. https://doi.org/10.1016/j.jns.2016.10.041 [ Links ]
16. Britton M, Carlsson A, de Faire U. Blood pressure course in patients with acute stroke and matched controls. Stroke 1986;17(5):861-864. https://doi.org/10.1161/01.str.17.5.861 [ Links ]
17. Carlberg B, Asplund K, Hagg E. The prognostic value of admission blood pressure in patients with acute stroke. Stroke 1993;24(9):1372-1375. https://doi.org/10.1161/01.str.24.9.1372 [ Links ]
18. De Oliveira Manoel AL, Goffi A, Zampieri FG, et al. The critical care management of spontaneous intracranial hemorrhage: A contemporary review. Crit Care 2016;20:272. https://doi.org/10.1186/s13054-016-1432-0 [ Links ]
19. Singhal NS, Josephson SA. A practical approach to neurologic evaluation in the intensive care unit. J Crit Care 2014;29(4):627-633. https://doi.org/10.1016/j.jcrc.2014.02.014 [ Links ]
20. Posner JB, Saper CB, Schiff ND, Plum F, eds. Plum and Posner's Diagnosis of Stupor and Coma. 4th ed. New York: Oxford University Press; 2007. [ Links ]
21. Naughton MT. Pathophysiology and treatment of Cheyne-Stokes respiration. Thorax 1998;53(6):514-518. https://doi.org/10.1136/thx.53.6.514 [ Links ]
22. Hughes CG, Patel MB, Pandharipande PP. Pathophysiology of acute brain dysfunction: What's the cause of all this confusion? Curr Opin Crit Care 2012;18(5):518-526. https://doi.org/10.1097/MCC.0b013e328357effa [ Links ]
23. Smith M, Meyfroidt G. Critical illness: the brain is always in the line of fire. Intensive Care Med 2017;43(6):870-873. https://doi.org/10.1007/s00134-017-4791-3 [ Links ]
24. Tasker RC, Menon DK. Critical care and the brain. JAMA 2016;315(8):749-750. https://doi.org/10.1001/jama.2016.0701 [ Links ]
25. Nakano M, Nomura Y, Whitman G, et al. Cerebral autoregulation in the operating room and intensive care unit after cardiac surgery. Br J Anaesth 2021;126(5):967-974. https://doi.org/10.1016/j.bja.2020.12.043 [ Links ]
26. Crippa IA, Subira C, Vincent JL, et al. Impaired cerebral autoregulation is associated with brain dysfunction in patients with sepsis. Crit Care 2018;22:327. https://doi.org/10.1186/s13054-018-2258-8 [ Links ]
27. Chow DS, Lignelli A. Computed tomography. In: Louis ED, Mayer SA, Rowland LP, eds. Merritt's Neurology. 13th ed. Philadelphia: Wolters Kluwer, 2016:169-173. [ Links ]
28. Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291(14):1753-1762. https://doi.org/10.1001/jama.291.14.1753 [ Links ]
29. Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med 2009;180(11):1092-1097. https://doi.org/10.1164/rccm.200904-0537OC [ Links ]
30. Sprung CL, Peduzzi PN, Shatney CH, et al. Impact of encephalopathy on mortality in the sepsis syndrome. The Veterans Administration Systemic Sepsis Cooperative Study Group. Crit Care Med 1990;18(8):801-806. https://doi.org/10.1097/00003246-199008000-00001 [ Links ]
31. Permpikul C, Jirisan W, Srinonprasert V, Tongyoo S. Delirium in a medical intensive care unit: A report from a tertiary care university hospital in Bangkok. Siriraj Med J 2021;73(3):155-161. https://doi.org/10.33192/Smj.2021.20 [ Links ]
 Correspondence:
Correspondence:
Surat Tongyoo
surat.ton@mahidol.ac.th
Accepted 9 February 2023














