Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Southern African Journal of Critical Care (Online)
versão On-line ISSN 2078-676X
versão impressa ISSN 1562-8264
South. Afr. j. crit. care (Online) vol.39 no.1 Pretoria Mar. 2023
http://dx.doi.org/10.7196/SAJCC.2023.v39i1.655
RESEARCH
Carbon dioxide levels of ventilated adult critically ill post-operative patients on arrival at the intensive care unit
M SlaveI; J ScribanteII; H PerrieIII; F LambatIV
IFCA(SA), MMed(Anaesth); Department of Anaesthesia, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIPhD; Surgeons for Little Lives, Department of Paediatric Surgery, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIMSc; Department of Anaesthesia, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVFCA(SA), MMed(Anaesth); Private Practice, Johannesburg, South Africa
ABSTRACT
BACKGROUND: The transportation of critically ill patients presents a precarious situation in which adverse events may occur. At Chris Hani Baragwanath Academic Hospital (CHBAH) patients were manually ventilated using a manual resuscitator bag during transportation from theatre to the intensive care unit (ICU
OBJECTIVES: To evaluate the arterial partial pressure of carbon dioxide (PaCO2) levels of ventilated adult critically ill post-operative patients on arrival at the ICU at CHBAH
METHODS: This was a cross-sectional study using convenience sampling. Pre- and post-transportation arterial blood gases were obtained from 47 patients
RESULTS: There was a statistically significant difference in the pre- and post-transport PaCO2 level (p=0.03), with a mean difference of 3.3 mmHg. The pre- and post-transport arterial partial pressure of oxygen (PaO2) level (p<0.001) and the week and weekend pre-transport (p<0.001) and post-transport (p=0.01) PaCO2 were statistically significantly different. No statistically significant difference was found in the other arterial blood gas parameters or in the post-transport PaCO2 of those patients (26 (55.3%)), who received a neuromuscular blocking drug compared with those that did not. Adverse events were noted during 12 (25.6%) of the transports, 5 (41.7%) of which were patient-related, and 7 (58.3%) of which were infrastructure-related
CONCLUSION: There was a statistically but not clinically significant difference in the pre- and post-transport PaCO2 level and between week and weekend transportations. Hypercarbia was the most common derangement in all transports. Adverse events occurred during one-quarter of transportations
Keywords: manual resuscitation bag ventilation, transportation of critically ill patients, carbon dioxide levels
The transportation of critically ill patients involves moving the patient from various destinations such as the emergency room, another hospital, or theatre to the stable environment of the intensive care unit (ICU).[1] Transportation from the ICU to a destination outside of the ICU is often required for diagnostic and interventional procedures which cannot be provided at the bedside.[2-5] The transportation of critically ill patients presents a precarious situation in which adverse events may occur.[5,6] The Harvard Medical Practice Study in 1991, defined an adverse event as 'an injury that was caused by medical management (rather than the underlying disease) and that prolonged the hospitalisation, produced a disability at the time of discharge, or both.'[7] Adverse events for transported critically ill patients may include a deterioration in the patient's haemodynamic parameters, airway and ventilator difficulties, and equipment failure.[5,6, 8-10] These adverse events may lead to an increase in morbidity and mortality in patients, arising as a consequence of the transportation challenges and not the underlying disease process.[11,12] International studies have demonstrated that portable mechanical ventilation is superior to manual ventilation using a manual resuscitator bag (MRB) during the transport of critically ill intubated patients in decreasing the risk of adverse respiratory events.[2,13-15]
MRBs do not usually have a built-in device for measuring tidal volume. The risk of hyperinflation, hyperventilation, and barotrauma with the use of MRBs has been recognised.[13,15,16] In 2005, Turki et al. [16] found peak inspiratory pressures of up to 100 cmH2O with the use of MRBs, which are well above the suggested safe limit of 30 mmHg
(40 cmH2O).[17] Some MRBs may be modified by the addition of a tidal volume meter at the exhalation valve to estimate minute volume and control ventilation.[13] Studies have indicated that the use of MRBs might result in suboptimal ventilation of patients.[5,13,14] The suboptimal ventilation includes hypoventilation with resultant hypercarbia and respiratory acidosis or the inverse, hyperventilation with hypocarbia and respiratory alkalosis. Hyperventilation with a corresponding respiratory alkalosis has been described as the most common derangement found in patients being manually ventilated.[13-15] In addition, the inability to maintain positive end-expiratory pressure (PEEP), resulting in lung de-recruitment and atelectasis when using MRBs without a PEEP valve, is a problem that can lead to hypoxaemia.[5,16]These insults to homeostasis are deleterious to critically ill patients who may not be able to compensate adequately for these maladaptations. It is, therefore, imperative that the ventilatory transportation practices of a facility be audited frequently.
At Chris Hani Baragwanath Academic Hospital (CHBAH), the multidisciplinary ICU is located approximately 220 m from the theatre, on two separate floors, with an elevator required for access to transport the patient to either the first floor ICU (medical and surgical patients) or the second floor ICU (trauma patients). The trajectory from the theatre to the ICU emanates from outside the theatre complex into an open-air corridor exposed to the elements. This corridor is also used as a thoroughfare for other patients, visitors, staff and supplies. This pavement surface is often damaged with potholes, as a result of lack of maintenance, making it difficult to manoeuvre a stretcher. There are two elevators. Frequently, only one of the two is functional. This elevator is used by everyone needing to access the ICU. This causes delays as the elevator stops at every floor to load and unload passengers, during which time critically ill patients are waiting and being manually ventilated for a protracted period on the ground floor. When both elevators are out of service, an external cargo hoist is used to access the back entrance of the ICU. This adds a further 110 m to the journey and additionally exposes patients to the elements. Transportation may take anywhere from 5 to 25 minutes. Owing to limited resources at the time of this study, there are no portable mechanical ventilators to transport patients from the theatre back to the ICU. The only method of ventilating patients during transport is manual ventilation with MRBs without tidal volume meters. Despite the inadequacy of the maintenance of infrastructure in the public sector in South Africa (SA), clinical duties must be carried out in accordance with an acceptable standard of care. In resource-limited settings, clinicians on the ground are very rarely able to do anything about the infrastructure; however, what can be done is to attend to factors within the clinician's control in order to maintain the quality of the service delivered. It was not known how well patients were being ventilated, with specific reference to their arterial carbon dioxide levels (PaCO2) while being transported between the theatre and ICU. Therefore, this study aimed to evaluate the PaCO2 levels of ventilated adult critically ill post-operative patients on arrival at the ICU at CHBAH.
Methods
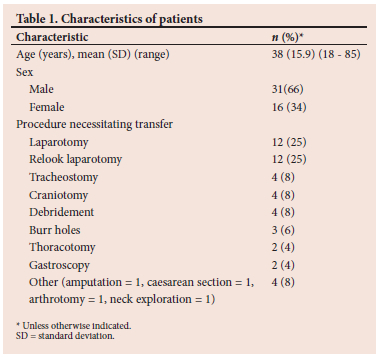
Approval to conduct the study was obtained from the Human Research Ethics Committee (Medical) (ref. no. M180779) of the University of the Witwatersrand and other relevant authorities. A cross-sectional, prospective research design was followed.
The study population consisted of adult intubated patients, who were manually ventilated with an MRB, arriving in ICU from the theatre during the study period between 7 April and 21 September 2019. Patient characteristics are described in Table 1. The patient group consisted of both trauma and surgical patients. Only those patients who were deemed by the treating physicians to be haemodynamically stable enough to be transported were transported between theatre and ICU; those too unstable had their procedures performed in the ICU or demised in theatre and were not included in the study. A convenience sampling method was used. The sample size was determined in consultation with a biostatistician using EpiInfo version 7.2.0.1 and was based on an SA study by De Vasconcellos et al.,[1] where the frequency of hypoxaemia, possibly indicating inadequate ventilation, of patients on arrival in ICU was 15.5%. Assuming a total population of 60 critically ill intubated and ventilated patients were being transported from theatre to ICU per month with a 5% margin of error, at a 95% confidence level, a sample size of 46 patients was estimated to have a minimum power of 80%. The inclusion criteria for this study were patients who had either an endotracheal tube or a tracheostomy tube in situ, an arterial line in place, and those for whom consent had been obtained. Patients with severe acute respiratory distress syndrome (ARDS)[18] were excluded from the study.
As patients were incapacitated at the time of data collection, consent was obtained from the patient's family members. Deferred consent was obtained from patients when they were able to give consent and only these patients were included in the study.
A draft data collection sheet was compiled and reviewed for face and content validity by three senior anaesthesiologists, one of whom was an intensivist. The suggestions made were incorporated into the final data collection sheet. The following information was collected: date and time of transport; patient, transport, and MRB characteristics; pre- and post-transport arterial blood gas (ABG); drugs administered for transport; transport ventilation variables, and adverse events that occurred during transport.
One of the authors identified patients who were scheduled to be transported to the ICU post-operatively, from the booking information available in the theatre and ICU. The pre-transport ABG sample was collected by the patient's anaesthetist, from the patient's arterial line before the patient was disconnected from the ventilator in theatre. The post-transport ABG sample was collected by the author immediately upon arrival in the ICU before the patient was attached to the ICU ventilator. ABG samples were collected in a standardised manner. The pre- and-post-transport ABG samples were analysed using a single automated blood gas analysis machine (Radiometer ABL800 Basic; Radiometer, Denmark) within 10 minutes of collection[19] as per standard practice.
Partial ventilation occurred when the patient was spontaneously breathing and their ventilation was being supported by assisted manual breaths, while full ventilation occurred when the patient was apnoeic and received full ventilatory support using an MRB. PaCO2 is inversely proportional to alveolar ventilation[20] and was used as a marker of the adequacy of ventilation. A PaCO2 level between 35 and 45 mmHg was considered normal.[19] Normal pH was considered to be between 7.35 and 7.45. Day hours fell between 08h00 and 16h00 and weekdays were from Monday 08h00 to Friday 16h00. Adverse events in this study were reported as patient and infrastructure related.
Data were analysed in consultation with a biostatistician using STATA version 15 (StataCorp, USA). Categorical variables were described using numbers and percentages and continuous variables using means and standard deviations. Continuous variables were compared using either independent or paired t-tests. A p-value of <0.05 was considered statistically significant.
Results
Data were collected from 60 patients, 13 of whom were excluded from the study. Of these, 11 died before informed consent could be obtained from their family members, and 2 patients were transferred to other facilities before consent could be obtained. This resulted in a sample size of 47 patients. The characteristics of the patients are shown in Table 1 and the type of transport ventilation, time and duration of transport, and pre-transport drugs used are shown in Table 2. Drugs to facilitate transportation were administered to 34 (72%) patients, 20 (58%) of whom received a combination of drugs.
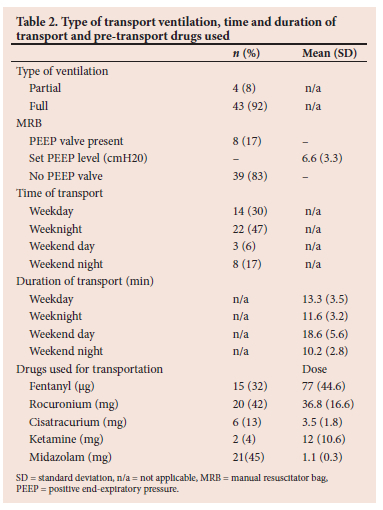
Neurosurgical procedures were performed in 7 patients (14%). In all 7 patients the carbon dioxide level increased during transportation by between 2 and 23 mmHg, resulting in post-transport PaCO2 levels ranging from 32.8 to 58.3 mmHg, which could cause a rise in intracranial pressure; however, no adverse events were noted in these patients. A neuromuscular blocking agent was administered to 26 (55%) patients before transport. No statistically significant difference was found in the post-transport PaCO2 of those patients who received a neuromuscular blocking drug (mean PaCO2 46.0) compared with those that did not (mean PaCO2 44.5) (p=0.6).
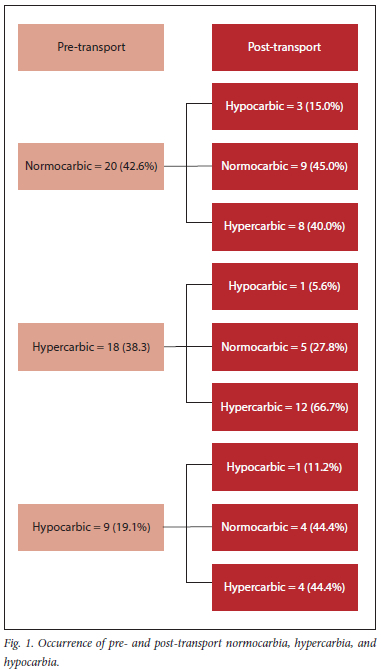
The occurrence of pre- and post-transport normocarbia, hypercarbia, and hypocarbia is shown in Fig. 1.
Comparisons of the pre- and post-transportation changes in the ABG values are shown in Table 3. The fraction of inspired oxygen concentration (FiO2) was 1.0 during post-transportation for all patients owing to the lack of an oxygen/air blender device. There was a statistically significant difference in the PaCO2 and the arterial partial pressure of oxygen (PaO2) pre- and post-transport.
Table 4 shows the comparisons in the PaCO2 pre- and post-transport during day and night and week and weekend transportations.
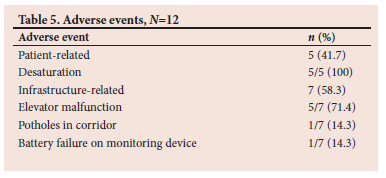
Adverse events were noted during 12 (26%) of the transports, 5 (41.7%) of which were patient-related, consisting of patient desaturation. Infrastructure problems accounted for the remaining 7 (58.3%) adverse events. The infrastructure problems included 5 (71.4%) elevator malfunctions, and 1 case each of potholes on the pavement surface and equipment failure in the form of battery failure on the monitoring device.
Discussion
Manual ventilation using MRBs without volumeters was the only mode of ventilation used to transport patients from theatre to ICU in this study. Although there was a statistically significant difference in the pre- and post-transport PaCO2 level, the mean difference was only 3.3 mmHg, with a rise in the mean PaCO2 from 42 mmHg pre-transport to 45.3 mmHg post-transport The physiological implications of this small difference are not clinically significant. This finding shows that although there was an increase in the PaCO2, it remained within acceptable levels, suggesting that manual ventilation with an MRB at CHBAH may not be injurious as described in some studies.[13,14,21] The results of this study are consistent with studies by Rajasekaram et al.[22] and O'Brien et al.,[23] who found that there was no clinical difference in the patients who were manually ventilated compared with those who were mechanically ventilated, despite there being a statistically significant change in the PaCO2 and expired end-tidal CO2, respectively.
Pre-transport, the majority of patients were normocarbic (42.6%) or hypercarbic (38.3%). Post-transport, the majority (51%) of the patients were hypercarbic. This differed from the marked respiratory alkalosis that Gervais et al.[13] and Hurst et al.[14] described. This difference could be explained by various factors. Firstly, the anaesthetists at CHBAH are probably aware of the dangers of hyperventilation, and therefore deliberately tried to avoid hyperventilating the patients. Secondly, the hyperventilation could have been unintentional. Often, patients at CHBAH are transported from theatre to the ICU accompanied by only the anaesthetist and a scrub nurse. During transportation, the healthcare practitioner manually ventilating the patient may be distracted by other tasks such as manoeuvring the bed, hailing the elevator, or attending to malfunctioning transportation equipment, and may be unable to deliver adequate minute ventilation to the patient, hence producing hypercarbia. Despite hypercarbia being so common, there was no clinically relevant effect in terms of acidosis or arrhythmias reported.
The difference between week and weekend PaCO2 pre- and posttransport was statistically significant. During the weekend, at CHBAH, there is an increase in trauma-related surgery and only emergency surgeries are conducted. Therefore, patients presenting for surgery during the weekend may be more seriously ill compared with those operated on during the week.
In this study, there was no statistically significant difference in PaCO2 levels in those patients who were administered a neuromuscular blocking drug compared with those patients who were not. This is an unexpected finding, considering neuromuscular blockade facilitates artificial ventilation and eliminates patient-ventilator dyssynchrony. Of the 92% of patients who were fully ventilated during transportation, 55% had received neuromuscular blocking agents for transportation; however, the residual effects of drugs given during the anaesthetic may have prevented the other patients from breathing spontaneously. Additionally, sedatives and opioids have altered pharmacokinetics in critically ill patients and may produce apnoea in the presence of organ dysfunction, particularly if hepatic and renal dysfunction were present.
The pre- and post-transport PaO2 was statistically significantly different. This difference was due to the FiO2 being titrated to the patient's requirements in theatre, to maintain a saturated oxygen (SaO2) between 88 and 96%, whereas all patients were transported with an FiO2 of 1.0 owing to the absence of an oxygen/air blender. Although the difference in pre- and post-transport SaO2 was not statistically significant, 4.3% of patients were hypoxaemic (SaO2 <88%) on arrival in the ICU. This is lower than the 9.3% observed by De Vasconcellos et al.[1] in patients on arrival in a multidisciplinary ICU who were transported by anaesthetists. Overall De Vasconcellos et al.[1] found an incidence of hypoxaemia of 15.5% among all patients in their study; however, not all patients were transported with a pulse oximeter. At CHBAH, the use of pulse oximetry during the transportation of patients to the ICU is mandatory. De Vasconcellos et al.[1] described pulse oximetry as a negative predictive factor for hypoxaemia.
Adverse events occurred during 26% of the transportations; this is within the range of 3 - 75% reported by Droogh et al.[24] Two SA studies reported adverse event incidences of 23.4%[25] and 56.1%,[26] respectively, of transported critically ill patients, the former being similar to the reported incidence in this study. Five patients were reported to have desaturated during transportation. Of these, only two patients were hypoxaemic on arrival in the ICU, as shown by the ABG. In the three patients who arrived without hypoxaemia, the possible reasons may have included saturation probe displacement with loss of contact or the patient being vasoconstricted, causing unreliable readings. Alternatively, suspected desaturation during transportation may have been recognised and managed by hyperventilation using the MRB. Regarding the two hypoxaemic patients, one was documented as having desaturated during transportation, possibly as a result of lung de-recruitment. In the other patient, the battery of the transport monitor failed during transport; however, no respiratory complication was reported. Owing to the infrastructure problems faced during the transportation of patients at CHBAH, such as the long distance from the theatre to the ICU and the poor maintenance of the corridor and elevators, more adverse events could have been expected.
Owing to the contextual design of this study, the results may not be generalisable to other contexts. However, many of the infrastructural difficulties described in this facility are applicable to other healthcare centres in the SA context, as well as other low-to-middle income countries, and therefore these results are relevant to any centre involved in the management of critically ill patients in an under-resourced setting. Additionally, the reassurance that even with a lack of dedicated transport ventilators, appropriate ventilatory care can be delivered to most critically ill patients during transportation with MRBs is assuaging for many clinicians who find themselves practising medicine in under-resourced settings. Convenience sampling was used and therefore the sample may not have been representative of the population. Furthermore, the diverse pathologies of the patients may have influenced the ventilatory parameters. Although the neurosurgical patients who formed part of the sample all had increases in their PaCO2 levels, no adverse events were noted. This may be because they were post-operative patients who had already been treated for potentially raised intracranial pressure and may not have been as sensitive to fluctuations in their PaCO2 as pre-operative neurosurgical patients might be. Patients with severe ARDS were excluded from the study, and pulmonary hypertension was not described in any of the patients. With regard to pulmonary hypertension, it is this institution's practice to avoid prolonged positive pressure ventilation where possible in patients with moderate to severe pulmonary hypertension. Pre-operative neurosurgical patients, patients with severe ARDS and those with pulmonary hypertension are the populations of patients who are most sensitive to inadequate ventilation and may decompensate as a result of inadequate ventilation. Therefore the results of this study are not generalisable to these populations. Additionally, all transportations were performed by trained anaesthetists who are experienced in manual ventilation during transportation. The results of this study may have been different if junior staff or staff from other disciplines who are less experienced in manual ventilation had been conducting the transportation. Based on the findings of this study, the authors recommend that in the absence of transport ventilators, MRBs equipped with PEEP valves and volumeters and oxygen cylinders fitted with oxygen/air blenders should be made available to allow better control of ventilation.
Conclusion
The purpose of this study was to evaluate the PaCO2 levels of critically ill patients at CHBAH during transportation from theatre to the ICU. There was a statistically but not clinically significant difference in the PaCO2 level pre- and post-transport and between week and weekend transportations. Hypercarbia was the most common derangement in all transports. Adverse events occurred during one-quarter of transportations. These findings indicate that manual ventilation of critically ill patients during transport between theatre and the ICU during the study period was not injurious. Recommendations for future research include reproducing the study in patients with severe ARDS and pulmonary hypertension to ascertain if manual ventilation is safe in this population and reproducing the study with healthcare practitioners other than anaesthesiologists, who may not be as experienced in manual ventilation.
Declaration. This research was done in partial fulfilment of a Master of Medicine degree.
Acknowledgements. None.
Author contributions. Concept and design: MS, JS, HP, FL; data collection: MS; data analysis: MS, JS, HP, FL; drafting article: MS, JS, HP, FL.
Funding. None.
Conflicts of interest. None.
References
1. De Vasconcellos K, Skinner DL, Singh D. Hypoxaemia on arrival in a multidisciplinary intensive care unit. S Afr Med J 2016;106(5):510-513. https://doi.org/10.7196/SAMJ.2016.v106i5.10251. [ Links ]
2. Fromm RE Jr, Dellinger RP. Transport of critically ill patients. J Intensive Care Med 1992;7(5):223-233. https://doi.org/10.1177/088506669200700503. [ Links ]
3. Low M, Jaschinski U. Intra-hospital transport of critically ill patients. Anaesthetist 2009;58(1):95-105. https://doi.org/10.1007/s00101-008-1499-3. [ Links ]
4. Indeck M, Peterson S, Smith J, Brotman S. Risk, cost, and benefit of transporting ICU patients for special studies. J Trauma 1988;28(7):1020-1025. https://doi.org/10.1097/00005373-198807000-00018. [ Links ]
5. Blakeman TC, Branson RD. Inter- and intra-hospital transport of the critically ill. Respir Care 2013;58(6):1008-1023. https://doi.org/10.4187/respcare.02404. [ Links ]
6. Gimenez FMP, de Camargo WHB, Gomes ACB, et al. Analysis of adverse events during intrahospital transportation of critically ill patients. Crit Care Res Pract 2017;2017(8):1-7. https://doi.org/10.1155/2017/6847124. [ Links ]
7. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalised patients: Results of the Harvard Medical Practice Study I. N Engl J Med 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604. [ Links ]
8. Beckmann U, Gillies DM, Berenholtz SM, Wu AW, Pronovost P. Incidents relating to the intrahospital transfer of critically ill patients: An analysis of the reports submitted to the Australian Incident Monitoring Study in Intensive Care. Intensive Care Med 2004;30(8):1579-1585. https://doi.org/10.1007/s00134-004-2177-9. [ Links ]
9. Shirley PJ, Bion JF. Intra-hospital transport of critically ill patients: Minimising risk. Intensive Care Med 2004;30(8):1508-1510. https://doi.org/10.1007/s00134-004-2293-6. [ Links ]
10. Schwebel C, Clec'h C, Magne S, et al. Safety of intra-hospital transport in ventilated critically ill patients: A multi-centre cohort study. Crit Care Med 2013;41(8):1919-1928. https://doi.org/10.1097/CCM.0b013e31828a3bbd. [ Links ]
11. Voigt LP, Pastores SM, Raoof ND, Thaler HT, Halpern NA. Review of a large clinical series: Intrahospital transport of critically ill patients: Outcomes, timing, and patterns. J Intensive Care Med 2009;24(2):108-115. https://doi.org/10.1177/0885066608329946. [ Links ]
12. Waddell G. Movement of critically ill patients within hospital. Br Med J 1975;2(5968):417-419. https://doi.org/10.1136/bmj.2.5968.417. [ Links ]
13. Gervais HW, Eberle B, Konietzke D, Hennes HJ, Dick W. Comparison of blood gases of ventilated patients during transport. Crit Care Med 1987;15(8):761-763. https://doi.org/10.1097/00003246-198708000-00010. [ Links ]
14. Hurst JM, Davis K Jr, Branson RD, Johannigman JA. Comparison of blood gases during transport using two methods of ventilatory support. J Trauma 1989;29(12):1637-1640. https://doi.org/10.1097/00005373-198912000-00008. [ Links ]
15. Dockery WK, Futterman C, Keller SR, Sheridan MJ, Akl BF. A comparison of manual and mechanical ventilation during paediatric transport. Crit Care Med 1999;27(4):802-806. https://doi.org/10.1097/00003246-199904000-00040. [ Links ]
16. Turki M, Young MP, Wagers SS, Bates JH. Peak pressures during manual ventilation. Respir Care 2005;50(3):340-344. [ Links ]
17. Butterworth JF, Mackey DC, Wasnick JD, Morgan GE, Mikhail MS. Morgan and Mikhail's Clinical Anesthesiology. 6th ed. New York: McGraw Hill, 2013. [ Links ]
18. Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012;307(23):2526-2533. https://doi.org/10.1001/jama.2012.5669. [ Links ]
19. Walker HK. The origins of the history and physical examination. In: Walker HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths, 1990. [ Links ]
20. West J, Luks A. West's Respiratory Physiology: The Essentials. 10th ed. Philadelphia, PA: Wolters Kluwer, 2016. [ Links ]
21. Holets SR, Davies JD. Should a portable ventilator be used in all in-hospital transports? Respir Care 2016;61(6):839-853. https://doi.org/10.4187/respcare.04745. [ Links ]
22. Rajasekaram R, Reade MC, Shortal B, Hart GK, Shaw M, Bellomo R. Variability in adequacy of ventilation during transport of cardiac surgery patients: A cohort study. Anaesth Intensive Care 2011;39(3):465-471. https://doi.org/10.1177/0310057X1103900319. [ Links ]
23. O'Brien EO, Newhouse BJ, Cronin B, et al. Hemodynamic consequence of hand ventilation versus machine ventilation during transport after cardiac surgery. J Cardiothorac Vasc Anesth 2017;31(4):1246-1249. https://doi.org/10.1053/j.jvca.2016.11.006. [ Links ]
24. Droogh JM, Smit M, Absalom AR, Ligtenberg JJ, Zijlstra JG. Transferring the critically ill patient: Are we there yet? Crit Care 2015;19(1): 62. https://doi.org/10.1186/s13054-015-0749-4. [ Links ]
25. Seilbea LY, deVasconcellos K. Adverse events during the intra-hospital transfer of critically ill perioperative patients in a South African tertiary hospital. South Afr J Anaesth Analg 2020;26(3):131-138. https://doi.org/10.36303/SAJAA.2020.26.3.2307. [ Links ]
26. Geldenhuys, L, Wise R, Rodseth R. The impact of a bundled intra-hospital transfer protocol on the safety of critically ill patients in a South African Metropolitan Hospital System. South Afr J Anaesth Analg 2020;26(3):139-148. https://doi.org/10.36303/SAJAA.2020.26.3.23 [ Links ]
 Correspondence:
Correspondence:
M Slave
mulai@hotmail.co.uk
Accepted 26 January 2023














