Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Southern African Journal of Critical Care (Online)
versão On-line ISSN 2078-676X
versão impressa ISSN 1562-8264
South. Afr. j. crit. care (Online) vol.38 no.3 Pretoria Nov. 2022
http://dx.doi.org/10.7196/SAJCC.2022.v38i3.546
ARTICLE
Scope and mortality of adult medical ICU patients in an Eastern Cape tertiary hospital
R FreercksI; N GigiII, III; R AylwardIV, V; S PaziVI; J EnsorVII; E van der MerweVIII, IX
IMB ChB, FCP (SA) Phys, Cert Nephrol (SA) Phys, MPhil ; Division of Nephrology and Hypertension, Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
IIMB ChB, FCP (SA); Adult Critical Care Unit, Livingstone Tertiary Hospital, Gqeberha, South Africa
IIIMB ChB, FCP (SA); Department of Medicine, Faculty of Health Sciences, Walter Sisulu University, Mthatha, South Africa
IVMB ChB ; Division of Nephrology and Hypertension, Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
VMB ChB ; Adult Critical Care Unit, Livingstone Tertiary Hospital, Gqeberha, South Africa
VIBSc, BSc (Hons), MSc; Department of Statistics, Nelson Mandela University, Gqeberha, South Africa
VIIMB ChB, FCP (SA) Phys, Cert Nephrol (SA) Phys; Division of Nephrology and Hypertension, Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
VIIIMB ChB, MMed (Int Med), Cert Crit Care (SA) Adult Critical Care Unit, Livingstone Tertiary Hospital, Gqeberha, South Africa
IXMB ChB, MMed (Int Med), Cert Crit Care (SA) Department of Medicine, Faculty of Health Sciences, Walter Sisulu University, Mthatha, South Africa
ABSTRACT
BACKGROUND. The characteristics and mortality outcomes of patients admitted to South African intensive care units (ICUs) owing to medical conditions are unknown. Available literature is derived from studies based on data from high-income countries.
OBJECTIVES. To determine ICU utilisation by medical patients and evaluate the scope of admissions and clinical associations with hospital mortality in ICU patients 12 years and older admitted to an Eastern Cape tertiary ICU, particularly in the subset with HIV disease.
METHODS. A retrospective descriptive one-year cohort study. Data were obtained from the LivAKI study database and demographic data, comorbidities, diagnosis, and mortality outcomes and associations were determined.
RESULTS. There were 261 (29.8%) medical ICU admissions. The mean age of the cohort was 40.2 years; 51.7% were female. When compared with the surgical emergencies, the medical subgroup had higher sequential organ failure assessment (SOFA) scores (median score 5 v. 4, respectively) and simplified acute physiology score III (SAPS 3) scores (median 52.7 v. 48.5), a higher incidence of acute respiratory distress syndrome (ARDS) (7.7% v. 2.9%) and required more frequent dialysis (20.3% v. 5.5%). Of the medical admissions, sepsis accounted for 32.4% of admission diagnoses. The HIV seroprevalence rate was 34.0%, of whom 57.4% were on antiretroviral therapy. ICU and hospital mortality rates were 11.1% and 21.5% respectively, while only acute kidney injury (AKI) and sepsis were independently associated with mortality. The HIV-positive subgroup had a higher burden of tuberculosis (TB), higher admission SOFA and SAPS 3 scores and required more organ support.
CONCLUSION. Among medical patients admitted to ICU, there was a high HIV seroprevalence with low uptake of antiretroviral therapy. Sepsis was the most frequently identified ICU admission diagnosis. Sepsis and AKI (not HIV) were independent predictors of mortality. Co-infection with HIV and TB was associated with increased mortality.
Keywords: ICU; HIV; mortality; Africa; medical.
Despite a growing global demand,[1,2] Intensive care unit (ICU) beds remain a limited resource[3] with rising cost implications.[4] This is especially so in low- and middle-income countries[3, 5] and across the sub-Saharan African (SSA) region where large imbalances in accessing such resources exist.[6] In 2009, the Eastern Cape Province had 252 ICU beds of which only 90 were located in the public sector. This results in a population to ICU bed ratio of 1:75 000 compared with the envisioned 1:10 000 as per the proposed National Health Insurance plan.[7] In view of the limited number of ICU beds, the process of triaging referrals to ICU is accepted practice in South Africa (SA).[8] Part of this process is deciding who is likely to benefit most from ICU admission and auditing outcomes for admitted patients.
There is a paucity of studies describing the spectrum and outcomes of medical patients admitted to ICU in southern Africa. SA criteria for admission are largely influenced by studies performed in high-income countries (HIC) which have vastly different demographic and disease patterns.[8] Medical patients admitted to ICU in SA are younger, with an average age between 40 and 45 years[9,10] and HIV remains a major burden, with an estimated 8.2 million people with HIV in SA alone.[11] HIV-infected people are at higher risk for critical illness and therefore place an increased burden on ICUs.[12,13]
Improving our understanding of our cohort of medical critical care admissions and their outcomes is therefore vitally important and may inform clinicians and policymakers as to who will benefit the most from this life-saving resource. Therefore, this study was undertaken to describe the characteristics and outcomes of medical admissions to a multi-disciplinary tertiary ICU in SA, to determine poor prognostic markers and to specifically examine the cohort of known HIV-positive patients.
Ethics
The present study was approved by the research ethics and bio-safety committee at Walter Sisulu University, Mthatha (ref. no. 030/2021). The need for individual study participant consent was waived by the Ethics Unit.
Methods
Study design and setting
This was a retrospective cohort study nested in a previous study titled 'Acute Kidney Injury in critically ill patients in an Eastern Cape Tertiary Intensive Care Unit' (LivAKI)[14] which prospectively collected data on all admissions, 12 years and older, to the Livingstone Tertiary Hospital Adult ICU from 1 January 2017 to 31 December 2017. All medical and emergency surgical admissions (inclusive of all trauma-related admissions) were included in this analysis. Exclusion criteria included the following: elective surgical admissions; ICU readmission (only data from patients' first admission were included); inappropriate ICU admissions (death within 6 hours of admission); post-elective surgical procedures; obstetric patients; brain-dead patients admitted to the ICU awaiting organ procurement; and patients who were found to have end-stage kidney disease and who were not eligible for kidney replacement therapy. Patients with data sets with a missing entry regarding hospital mortality were excluded from outcome analysis.
Data collection and management
Demographic data included age, sex, race, employment history prior to hospital admission and details relating to comorbidities. Details regarding admission diagnosis, ICU stay, physiological parameters, complications and comorbidities (including HIV) were noted. Sepsis and septic shock were defined as per the third international consensus definition (Sepsis-3),[15] acute respiratory distress syndrome (ARDS) was defined by the Berlin 2012 consensus criteria[16] and acute kidney injury (AKI) was defined as per the Kidney Disease Improving Global Outcomes (KDIGO) group.[17]
Statistical analysis
Study data were collected and managed using the Research Electronic Data Capture (REDCap) tool hosted at the University of Cape Town[18] and analysed with R version 4.1.2 (R Core team, Austria). Descriptive statistics were used to summarise the medical cohort, as well as the HIV-positive subgroup.
Medical and surgical emergency admissions were compared. Continuous data were tested for normality using Pearson's chi-squared test. Normally distributed data are reported as means with standard deviations (SDs) and skewed data as medians with interquartile ranges (IQRs). Discrete data are presented as numbers (percentages). The student's f-test and Mann-Whitney U test were used to compare continuous data and the chi-squared and Fisher's exact tests were used for discrete data, as appropriate. Multivariable logistic-regression models were used to determine associations between mortality and patient characteristics of the medical group, and for those with known HIV-positive status. Variables with an alpha level <0.1 by univariable analysis, as well as other potential predictors for mortality (age, sex, hypertension, active malignancy and admission SAPS 3 score) were included in the model. The standardised mortality rate was calculated and the level of significance was 5%.
Results
During the study period, 875 patients were admitted. Medical patients accounted for 29.8% of the cohort (n=261). As depicted in Fig. 1, 163 patients were excluded for various reasons while 5 patients were lost to follow-up owing to inter-hospital transfer.
The demographic and clinical characteristics of all emergency admissions are summarised in Table 1. In the medical cohort, the prevalence of epilepsy, active tuberculosis and chronic kidney disease was more than three-fold higher than the surgical cohort (p<0.001 for all), while hypertension, diabetes mellitus and chronic kidney disease were the most frequent comorbidities. HIV status was known in 76.6% of the medical admissions and 34.0% of those patients were HIV-positive. Antiretroviral therapy (ART) uptake was low in both subgroups. The medical subgroup had a higher degree of illness compared with non-medical patients, as indicated by significantly higher SAPS 3 scores, admission SOFA scores, increased acute dialysis requirements and more frequent diagnosis of ARDS. Non-medical patients were more likely to receive vasopressor support and to be mechanically ventilated.
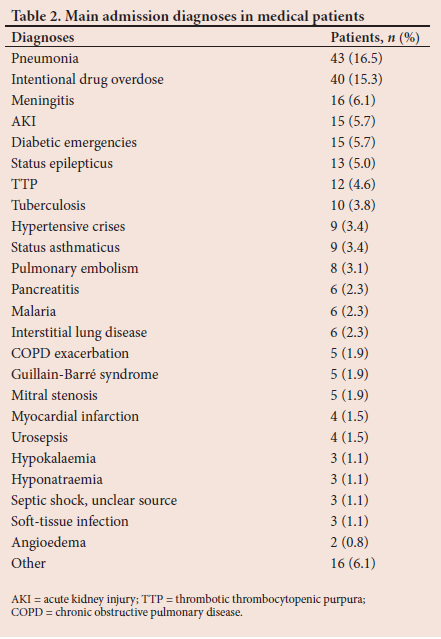
Common admission diagnoses in the medical subgroup are summarised in Table 2. Infectious diseases accounted for 32.4% of ICU admissions, with community acquired pneumonia, meningitis, tuberculosis and malaria being the main causes. Drug toxicity owing to deliberate self-harm was the second most common cause for admission (15.3%).
ICU mortality and predictors of hospital mortality in medical patients
The ICU and hospital mortality for the medical patients was 11.1% and 21.5%, respectively. By comparison, the hospital mortality for surgical emergency admissions was 24.4% (p=0.372). The standardised mortality rate for medical patients was 0.827 (95% confidence interval (CI) 0.664 - 1.030). Demographic and clinical characteristics of survivors and non-survivors are compared in Table 3. In those with known HIV status, HIV-positive patients had a significantly higher mortality compared with HIV-negative patients (29.4% v. 16.7%, respectively; p=0.036). The mortality risk was not different between those tested and not tested for HIV (21.1% v. 23.9%, respectively; p=0.817). HIV-positive non-survivors had a lower mean premorbid CD4 cell count. Other significant associations with increased mortality risk included increased age, higher admission SOFA and SAPS scores, prolonged ICU stay, sepsis, AKI, the need for mechanical ventilation, vasopressor support and dialysis.
Multivariable analysis indicated that only sepsis (odds ratio (OR) 2.91; 95% CI 1.25 - 6.87) and AKI (OR 3.14; 95% CI 1.12 - 10.30) were significantly associated with hospital mortality (Table 4). Furthermore, there was a trend of increased mortality among older patients (OR 1.02; p=0.058) and patients requiring vasopressor therapy (OR 2.34; p=0.066). HIV status was not independently associated with a higher mortality (p=0.167).
Medical subgroup comparison by HIV status
The prevalence of active tuberculosis in patients with HIV was 20.6% and 10.6% in those without HIV (p=0.087) as seen in Table 5. As indicated by higher admission SOFA and SAPS 3 illness severity scores, the HIV-positive subgroup had a higher severity of illness than those who were HIV-negative. The HIV-positive patients also had a higher incidence of sepsis and requirement for dialysis. There was no difference in the length of hospital stay, vasopressor use and ventilatory requirements between the two subgroups.
Sepsis, the presence of active tuberculosis and vasopressor requirements were associated with higher mortality in patients with HIV, as depicted in Table 6. The mortality of HIV-positive patients co-infected with TB was 57.1%.
Discussion
This study describes the case mix and outcomes of 256 adult medical patients admitted to the ICU of a tertiary hospital in a contemporary SA population. The study cohort was young (42.1 years) compared with the ICU patients in HICs[19-21] and demonstrated a high HIV seroprevalence rate. Infectious diseases were major contributors to ICU admission. Diagnosis of sepsis during any stage of the patient's admission and the presence of AKI were independent markers of mortality among medical patients, however, HIV-positive status was not.
The finding of a relatively young medical cohort was similar to ICU cohorts described in reports from other low- and middle-income countries, where the average age ranged between 31 and 47 years.[22-25]The most common reason for ICU admission among medical patients was infection, including community acquired pneumonia, meningitis, malaria and tuberculosis, followed by drug toxicity. The number of malaria cases over the course of the study period was notable as the centre is not situated in a malaria area. A high burden of intentional overdose was also evident.
The ICU and in-hospital mortality rates in our cohort study were 11.1% and 21.5%, respectively. The hospital mortality rate was not significantly different from that predicted by the SAPS 3 score. The mortality rate was comparable to a study conducted in KwaZulu-Natal that reported a hospital mortality rate of 17.5%.[26] Numerous other studies in Africa have reported higher mortality rates among critically ill medical patients: 30.5% in Egypt;[27] 40% in Malawi;[28] 41.4% in Tanzania; [29] and 43.7% in Uganda.[24] This variation may be a result of differences in the availability of resources across the various SSA countries.[30] As expected, the non-survivors in our study had higher illness severity scores and required more interventions such as vasopressor infusions, mechanical ventilation and dialysis. Furthermore, non-survivors had a high prevalence of sepsis (69%) as the likely underlying driver of morbidity and mortality as seen in local[31,32] and international studies.[33-35] The present study did not distinguish between sepsis on admission and nosocomial sepsis. However, the finding that the presence of sepsis was independently associated with mortality, emphasises the importance of preventing ICU-acquired infections by implementing prevention bundles and infection-control measures. Similar to international studies,[36,37] AKI was an independent marker of mortality and guidelines for the prevention for AKI in ICU patients[38] should be promoted in order to improve outcomes.
The HIV seropositivity rate in the study population was 34%. This was considerably higher than the 21% prevalence reported in a recent Eastern Cape study[39] and the 22.6% prevalence reported in a recent ICU study in Johannesburg.[32] Of the medical patients, 76.6% were tested for HIV, which was higher than the 39.0% in a recent report from Cape Town.[40] We found the uptake of ART to be low in our cohort, with only 57.4% of those with HIV on ART at admission. Other African studies have also demonstrated low rates of ART utilisation (range 48% - 54%) in patients admitted to the respective ICUs.[23,40] Notably, this falls well behind the ambitious 90-90-90 treatment target by the UNAIDS which aimed to have 90% of people diagnosed with HIV on ART by 2020.[41]
While HIV was associated with a higher mortality rate, it was not independently associated with mortality on multivariable analysis. This can be explained by the fact that the HIV-positive patients had a higher severity of disease (as indicated by higher SAPS 3 and SOFA scores), more frequent sepsis and higher requirements for dialysis. While earlier studies associated HIV infection with increased mortality in the ICU,[9,42] equivalent ICU outcomes for those with and without HIV have been demonstrated in the ART era after adjustment for disease severity.[23,43]
The prevalence of active tuberculosis among the medical cohort was 11.7%, which was somewhat lower than the 15.8% reported in a recent SA study.[32] Active tuberculosis was associated with mortality in patients with HIV. The high mortality rate in co-infected patients (57.1%) may partly be explained by the high number of patients who were ART-naïve. Prior studies of HIV and TB co-infected patients in ICUs have also demonstrated high mortality rates, ranging between 32.5% and 78.3%, respectively, with mechanical ventilation,[44,45] a high admission SOFA score,[46] a higher SAPS score[47] and AKI[44,48] as predictors of mortality.
Study strengths and limitations
The present study had a number of strengths. It was based on one of the largest complete ICU data sets in SSA, with a cohort of 875 admissions (including 261 medical admissions) over a 1-year period and encompassing a wide referral base. Secondly, owing to the use of a prospective data collection process, the results are unlikely to be biased. In addition to ICU mortality, hospital mortality was also reported. Limitations of the study include the fact that this was a single-centre study and was therefore subject to local ICU admission criteria and ICU practices. In view of this, the findings may not necessarily be generalisable. Secondly, 23.4% of our patients did not have an HIV test and this may result in the over- or underestimation of the HIV incidence in our cohort. However, the mortality rate was not different between the groups, suggesting a low risk of selection bias in initiating testing.
Conclusion
This study contributes to the body of literature by describing the epidemiology and mortality of critically ill medical adults in an SA hospital. Infectious diseases remain a major cause of ICU admission and the presence of sepsis and AKI were independent markers of mortality. A high HIV seroprevalence with a low uptake of ART were found in this cohort. However, despite low ART uptake, HIV-positive status was not independently associated with mortality.
Active tuberculosis, sepsis and vasopressor support were associated with mortality in HIV-positive patients. While HIV status did not predict ICU outcome per se, the premorbid CD4 cell count, frailty and presence of comorbidities such as tuberculosis, should be considered when assessing HIV-positive patients for ICU in a resource-limited setting. In SA, multicentre studies of medical ICU patients are needed to shed light on ways to improve outcomes and the establishment of critical care registries may be an important step to address this concern.
Declaration. This research study was conducted in partial fulfilment of NG's MMed degree at Walter Sisulu University, South Africa.
Acknowledgements. The authors gratefully acknowledge the medical officers working at the Livingstone Hospital ICU for their assistance with data collection.
Author contributions. NG wrote the protocol, interpreted the data and wrote the first draft, supervised by EvdM and RF. RA collected the data and designed the original study. SP performed statistical analysis. RF wrote the final draft and all authors assisted with and approved the final manuscript.
Funding. The authors gratefully acknowledge Roche South Africa for an unrestricted research grant that was used to fund data capturing (ref. no. SA/ NONP/1812/0050). The funder did not contribute to the design, conduct of the study, analysis or interpretation of results. We also gratefully acknowledge the Kidneys, Infectious Diseases and Critical Care (KICC) public benefit organisation for assistance with publication funding.
Conflicts of interest. None.
References
1. Needham DM, Bronskill SE, Calinawan JR, Sibbald WJ, Pronovost PJ, Laupacis A. Projected incidence of mechanical ventilation in Ontario to 2026: Preparing for the aging baby boomers. Crit Care Med 2005;33(3):574-579. https://doi.org/10.1097/01.CCM.0000155992.21174.31 [ Links ]
2. Schumaker G, Hill NS. Utilisation of Critical Care Resources Is Increasing - are we ready? Sage Publications: Thousand Oaks, 2006. https://doi.org/10.1177/0885066605282775 [ Links ]
3. Adhikari NKJ, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet 2010;376(9749):1339-1346. https://doi.org/10.1016/S0140-6736(10)60446-1 [ Links ]
4. Mahomed S, Mahomed O. Cost of intensive care services at a central hospital in South Africa. S Afr Med J 2019;109(1):35-39. https://doi.org/10.7196/SAMJ.2018.v109i1.13268 [ Links ]
5. Bhagwanjee S, Scribante J. National audit of critical care resources in South Africa: Unit and bed distribution. S Afr Med J 2007;97(12):1311-1314. [ Links ]
6. Okafor UV. Challenges in critical care services in Sub-Saharan Africa: Perspectives from Nigeria. Indian J Crit Care Med 2009;13(1):25-27. https://doi.org/10.4103/0972-5229.53112 [ Links ]
7. Naidoo K, Singh J, Lalloo U. Critical analysis of ICU/HC beds in South Africa: 2008 - 2009. S Afr Med J 2013;103(10):751-753. https://doi.org/10.7196%2FSAMJ.6415 [ Links ]
8. Joynt GM, Gopalan DP, Argent AA, et al. The Critical Care Society of Southern Africa Consensus Statement on ICU Triage and Rationing (ConICTri). S Afr Med J 2019;109(8b):613-629. [ Links ]
9. Van der Merwe E, Kidd M, Meltzer S, Bolliger C, Irusen E. Validating the use of the APACHE II score in a tertiary South African ICU. South Afr J Crit Care 2005;21(1):46-54. [ Links ]
10. Van Zyl-Smit R, Burch V, Willcox P. The need for appropriate critical care service provision at non-tertiary hospitals in South Africa. S Afr Med J 2007;97(4):268-272. [ Links ]
11. Statistics South Africa (StatsSA). Mid-year population estimates 2021. Pretoria: StatsSA, 2021. [ Links ]
12. Azoulay É, de Castro N, Barbier F. Critically ill patients with HIV: 40 years later. Chest 2020;157(2):293-309. https://doi.org/10.1016/j.chest.2019.08.002. [ Links ]
13. Akgün KM, Gordon K, Pisani M, et al. Risk factors for hospitalisation and medical intensive care unit (MICU) admission among HIV-infected veterans. JAIDS 2013;62(1):52-59. https://doi.org/10.1097/QAI.0b013e318278f3fa [ Links ]
14. Aylward RE, van der Merwe E, Pazi S, et al. Risk factors and outcomes of acute kidney injury in South African critically ill adults: A prospective cohort study. BMC Nephrol 2019;20(1):460. https://doi.org/10.1186/s12882-019-1620-7 [ Links ]
15. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315(8):801-810. https://doi.org/10.1001/jama.2016.0287 [ Links ]
16. Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med 2012;38(10):1573-1582. https://doi.org/10.1007/s00134-012-2682-1 [ Links ]
17. Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2(1):1-138. https://doi.org/10.1038/kisup.2012.1 [ Links ]
18. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010 [ Links ]
19. Zhou J, Qian C, Zhao M, et al. Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China. PLoS ONE 2014;9(9):e107181. https://doi.org/10.1371/journal.pone.0107181 [ Links ]
20. Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: The contribution of mechanical ventilation. Crit Care Med 2005;33(6):1266-1271. https://doi.org/10.1097/01.CCM.0000164543.14619.00 [ Links ]
21. McLaughlin AM, Hardt J, Canavan JB, Donnelly MB. Determining the economic cost of ICU treatment: A prospective 'micro-costing' study. Intensive Care Med 2009;35(12):2135-2140. https://doi.org/10.1007/s00134-009-1622-1 [ Links ]
22. Obsa MS, Adem AO, Gete GB. Clinical outcomes of patients admitted in intensive care units of Nigist Eleni Mohammed Memorial Hospital of Hosanna, Southern Ethiopia. Int J Med Medic Sci 2017;9(6):79-85. https://doi.org/10.5897/IJMMS2017.1297 [ Links ]
23. Kwizera A, Nabukenya M, Peter A, et al. Clinical characteristics and short-term outcomes of HIV patients admitted to an African intensive care unit. Crit Care Res Pract 2016;2016. https://doiorg/10.1155/2016/2610873 [ Links ]
24. Michel MM. Sociodemographic characteristics and outcomes of critically ill patients in a Lubumbashi ICU, DR Congo: A 3 years review. EC Anaesthesia 2018;4(8):318-328. [ Links ]
25. Sulieman H, El-Mahdi W, Awadelkareem M, Nazer L. Characteristics of critically-ill patients at two tertiary care hospitals in Sudan. Sultan Qaboos University Med J 2018;18(2):e190. https://doi.org/10.18295/squmj.2018.18.02.011 [ Links ]
26. Anesi GL, Gabler NB, Allorto NL, et al. Intensive care unit capacity strain and outcomes of critical illness in a resource-limited setting: A 2-hospital study in South Africa. J Intensive Care Med 2020;35(10):1104-1111. https://doi.org/10.1177/0885066618815804 [ Links ]
27. Ghoneim AHA, Hussein RM, El-Ghamry R, Mahmoud LY. Patterns of admitted cases to respiratory intensive care unit at Zagazig University Hospital, Egypt. Egyptian J Chest Dis Tuber 2013;62(4):661-668. https://doi.org/10.1016/j.ejcdt.2013.09.003 [ Links ]
28. Prin M, Itaye T, Clark S, et al. Critical care in a tertiary hospital in Malawi. World J Surg 2016;40(11):2635-2642. https://doi.org/10.1007/s00268-016-3578-y [ Links ]
29. Sawe HR, Mfinanga JA, Lidenge SJ, et al. Disease patterns and clinical outcomes of patients admitted in intensive care units of tertiary referral hospitals of Tanzania. BMC Int Health Human Rights 2014;14(1):26. https://doi.org/10.1186/1472-698X-14-26 [ Links ]
30. Rudd KE, Kissoon N, Limmathurotsakul D, et al. The global burden of sepsis: Barriers and potential solutions. Crit Care 2018;22(1):1-11. https://doi.org/10.1186/s13054-018-2157-z [ Links ]
31. Prin M, Onofrey L, Purcell L, Kadyaudzu C, Charles A. Prevalence, etiology, and outcome of sepsis among critically ill patients in Malawi. Am J Trop Med Hygiene 2020;103(1):472-479. https://doiorg/10.4269/ajtmh.19-0605 [ Links ]
32. Maphula R, Laher A, Richards G. Patterns of presentation and survival of HIV-infected patients admitted to a tertiary-level intensive care unit. HIV Med 2020;21(5):334-341. https://doi.org/10.1111/hiv.12834 [ Links ]
33. Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA 2017;317(3):290-300. https://doi.org/10.1001/jama.2016.20328 [ Links ]
34. Wang M, Jiang L, Zhu B, et al. The prevalence, risk factors, and outcomes of sepsis in critically ill patients in China: A multicenter prospective cohort study. Front Med 2020;7:Article 593808. https://doi.org/10.3389/fmed.2020.593808 [ Links ]
35. Chiang H-H, Hung C-C, Lee C-M, et al. Admissions to intensive care unit of HIV-infected patients in the era of highly active antiretroviral therapy: Etiology and prognostic factors. Crit Care 2011;15(4):1-10. https://doi.org/10.1186/cc10419 [ Links ]
36. Linder A, Fjell C, Levin A, Walley KR, Russell JA, Boyd JH. Small acute increases in serum creatinine are associated with decreased long-term survival in the critically ill. Am J Resp Crit Care Med 2014;189(9):1075-1081. https://doi.org/10.1164/rccm.201311-2097OC [ Links ]
37. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalised individuals. Clin J Am Soc Nephrol 2014;9(1):12-20. https://doi.org/10.2215/CJN.02730313 [ Links ]
38. Joannidis M, Druml W, Forni LG, et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit: Update 2017. Expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med 2017;43(6):730-749. https://doi.org/10.1007/s00134-017-4832-y [ Links ]
39. Hansoti B, Stead D, Parrish A, et al. HIV testing in a South African emergency department: A missed opportunity. PLoS ONE 2018;13(3):e0193858. https://doi.org/10.1371/journal.pone.0193858 [ Links ]
40. Mkoko P, Raine R. HIV-positive patients in the intensive care unit: A retrospective audit. S Afr Med J 2017;107(10):877-881. [ Links ]
41. Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS, 2014. [ Links ]
42. Stoneburner R, Laroche E, Prevots R, et al. Survival in a cohort of human immunodeficiency virus-infected tuberculosis patients in New York City: Implications for the expansion of the aids case definition Arch Intern Med 1992;152(10):2033-2037. https://doi.org/10.1001/archinte.152.10.2033 [ Links ]
43. Turvey SL, Bagshaw SM, Eurich DT, Sligl WI. Epidemiology and outcomes in critically ill patients with human immunodeficiency virus infection in the era of combination antiretroviral therapy. Can J Infect Dis Med Microb 2017;2017:7868954 . https://doi.org/10.1155/2017/7868954 [ Links ]
44. Amâncio F, Lambertucci J, Cota G, Antunes C. Predictors of the short-and long-term survival of HIV-infected patients admitted to a Brazilian intensive care unit. Int J STD AIDS 2012;23(10):692-697. https://doi.org/10.1258/ijsa.2012.011389 [ Links ]
45. Ferreira MD, Neves CPd, Souza ABd, Beraldi-Magalhães F, et al. Predictors of mortality among intensive care unit patients coinfected with tuberculosis and HIV. J Brasileiro de Pneumologia 2018;44:118-124. https://doi.org/10.1590/s1806-37562017000000316 [ Links ]
46. Pecego AC, Amancio RT, Ribeiro C, et al. Six-month survival of critically ill patients with HIV-related disease and tuberculosis: A retrospective study. BMC Infect Dis 2016;16(1):1-9. https://doiorg/10.1186/s12879-016-1644-6 [ Links ]
47. Lanoix J, Gaudry S, Flicoteaux R, Ruimy R, Wolff M. Tuberculosis in the intensive care unit: A descriptive analysis in a low-burden country. Int J Tuberc Lung Dis 2014;18(5):581-587. https://doi.org/10.5588/ijtld.13.0901 [ Links ]
48. Balkema C, Irusen E, Taljaard J, Koegelenberg C. Tuberculosis in the intensive care unit: A prospective observational study. Int J Tuberc Lung Dis 2014;18(7):824-830. https://doi.org/10.5588/ijtld.13.0044 [ Links ]
 Correspondence:
Correspondence:
R Freercks
robert.freercks@uct.ac.za
Accepted 11 August 2022
Contribution of the study.
The epidemiology and outcomes of adults who are critically ill from medical conditions in South African intensive care units was previously unknown but has been described in this study. The association of sepsis, TB, HIV and acute kidney injury with mortality is discussed.














