Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Southern African Journal of Critical Care (Online)
On-line version ISSN 2078-676X
Print version ISSN 1562-8264
South. Afr. j. crit. care (Online) vol.38 n.2 Pretoria Jul. 2022
http://dx.doi.org/10.7196/SAJCC.2022.v38i2.536
ARTICLE
Ventilator-associated pneumonia in PICU - how are we doing?
L van WykI; J T ApplegateII; S SalieIII, IV
IFCPaed (SA), MMed (Paed); Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Cape Town, South Africa
IIBCur, PG Dip (Critical Care Child); Paediatric Intensive Care Unit, Red Cross War Memorial Hospital, Cape Town, South Africa
IIICert Crit Care (Paed), MPH Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Cape Town, South Africa
IVCert Crit Care (Paed), MPH Paediatric Intensive Care Unit, Red Cross War Memorial Hospital, Cape Town, South Africa
ABSTRACT
INTRODUCTION. Ventilator-associated pneumonia (VAP) is a common hospital-acquired infection in children, leading to an increase in morbidity and mortality. A previous study in 2013 showed that VAP rates decreased dramatically after implementation of a VAP bundle and appointing a VAP coordinator. As part of a 'Plan, Do, Study, Act' cycle, it was necessary to evaluate the efficacy of these interventions.
OBJECTIVE. To evaluate the VAP rate in the paediatric intensive care unit (PICU) over 2 years (2017 - 2018), and to describe the causative organisms and antibiotic sensitivity/resistance patterns during this period.
Methods. This was a retrospective, descriptive study using the existing PICU VAP database as well as clinical folders.
RESULTS. Over the 2 years, 31 VAP cases were identified. The VAP rate for 2017 was 4.0/1 000 ventilator days and 5.4/1 000 ventilator days for 2018. Compliance with the VAP bundle was 68% in 2017 and 70% in 2018. The median (interquartile range (IQR)) duration of ventilation in 2017 was 9 (6 -12) days and 15 (11 - 28) days in 2018. The median (IQR) length of PICU stay in 2017 was 11 (8 - 22) days and 25 (17 - 37) days in 2018. The most common cultured organism was an extended-spectrum beta-lactamase (ESBL) Klebsiella pneumoniae sensitive to amikacin and carbapenems.
CONCLUSION. Our VAP rate has not decreased since 2013. It is imperative that we improve compliance with the VAP bundle, in order to reduce VAP rates. K. pneumoniae and Pseudomonas aeruginosa were the most common organisms causing VAPs and empiric use of piptazobactam and amikacin is still appropriate.
Keywords: VAP, ventilator-associated pneumonia, PICU, intensive care, paediatrics.
Ventilator-associated pneumonia (VAP) refers to a nosocomial pneumonia in patients who are ventilated for more than 48 hours and, second to bloodstream infections, is the most common hospital-acquired infection (HAI) in children.[1-3] Some studies report that up to 30% of ventilated patients develop a VAP.[1]
The consequences of a VAP may lead to an increase in both mortality and morbidity, which includes longer duration of ventilation and increased duration of paediatric intensive care unit (PICU) as well as overall hospital stay.[1,2,4]
According to the recent literature, the incidence of VAP in paediatrics is still very variable, with much higher rates in developing countries compared with developed countries. Galal et al.[1] reported a VAP rate of 21.3/1 000 ventilator days over the 12 months from September 2014 to September 2015 at Cairo University Hospital, which is among the highest in the literature. A paediatric hospital in Montreal, Canada, had a VAP rate of 7/1 000 ventilator days over a 2-year period from November 2013 to November 2015,[5] while Hatachi et al.[6] reported a VAP rate of 3.5/1 000 ventilator days in Japan during 2013. Patrick et al.[7] described rates as low as 1.9 to 0.7/1 000 ventilator days, as well as a further decrease in the incidence, among hospitals in the USA during 2007 - 2012.
A local study done in the PICU at Red Cross War Memorial Children's Hospital in 2011 reported a very high VAP rate of 55/1 000 ventilator days.[8] The VAP rate decreased to 19/1 000 ventilator days in the first 5 months after the implementation of a care bundle which consisted of the following five elements:
• Elevating the head of the bed to 30° (exception made in cases where this was medically contraindicated, e.g. postoperative cardiac and neurosurgical patients, as well as children nursed prone and patients on high frequency oscillation, who were nursed at 10° elevation).
• Age-appropriate mouthcare.
• Marking of oro-/nasogastric tubes after confirmation of placement and checking their position 3 - 4 hourly, to allow early detection of displacement and thereby reducing the risk of aspiration.
• No saline to be used routinely in endotracheal tube before suctioning.
• Positioning of ventilator tubing in such a manner that condensed water runs away from the patient into the water trap.[8]
A VAP co-ordinator was appointed to improve bundle compliance, and whose responsibilities included: one-on-one training, implementation of the bundle, assessing compliance to the bundle, addressing obstacles and identifying new VAP cases. This resulted in a further decrease of VAPs to 4/1 000 ventilator days in July 2013.[8]
In addition, ventilator circuits, which were previously re-used after a decontamination process, were changed to disposable circuits. There have been no changes to the VAP care bundle in PICU over the last 5 years, but there have been several staff changes, including the appointment of three different VAP co-ordinators.
In an era of increasing antibiotic resistance, it is vital to understand and monitor the organisms causing nosocomial infections and to determine their antibiotic susceptibility patterns in order to guide empiric therapy, aid antibiotic stewardship and prevent antibiotic resistance.[2] Internationally, the most frequently isolated organism causing VAP is Pseudomonas aeruginosas[1-3,5,9,10] Other organisms include Haemophilus influenzae,[2,5] Acinetobacter baumanii,[1,9] Staphylococcus aureus [1,10] and Klebsiella pneumoniae3] The current antibiotic protocol in our PICU for treating children with a suspected VAP is piptazobactam and amikacin, or a carbapenem for children with renal failure.
As part of an ongoing health improvement initiative and 'Plan, Do, Study, Act' (PDSA) cycle, it was thought necessary to evaluate the efficacy of the previously introduced interventions. Therefore, the objectives of this study were: firstly, to re-evaluate the VAP rate in the PICU over a 2-year period from 1 January 2017 to 31 December 2018, in order to compare it with the previously published data in 2013; and secondly, to describe the organisms isolated, the bacterial resistance patterns and the appropriateness of the current empiric antibiotic therapy for VAPs.
Methods
The PICU at Red Cross War Memorial Children's Hospital (RCWMCH), Cape Town, South Africa, is a 22-bed multidisciplinary unit, admitting approximately 1 400 children annually. About two-thirds of patients require intubation and ventilation during their admission, which place them at risk of developing a VAP. This was a retrospective, descriptive study using the existing PICU VAP database to identify all patients with confirmed VAPs during 2017 and 2018.
Internationally, the Centers for Disease Control and Prevention (CDC)'s algorithm for VAP has been widely acceptable for surveillance and research purposes, but their diagnostic criteria are radiological findings, which are problematic because of inter-observer variability. Other factors affecting the diagnostic efficiency of chest radiographs include: high positive end expiratory pressure (PEEP) levels, which might give a false impression of resolving infiltrates; cardiac failure or excessive fluid retention can wrongly be interpreted as infiltrates; a lack of radiographic findings in immunocompromised children; and a difference in radiograph exposures. Repeated chest X-rays may also be harmful as a result of radiation exposure.[9,11] Other challenges with the CDC VAP definitions is that they rely on clinical signs and symptoms which are mostly subjective and often poorly documented in clinical notes.[12]
Proper surveillance definitions are needed in order to effectively determine prevention strategies. Therefore, the CDC recently developed an objective surveillance algorithm called 'pediatric ventilator-associated event (PedVAE)', which mainly focuses on an increase in fraction of inspired oxygen (FiO2) and mean airway pressure (MAP). It is important to note that this is strictly a surveillance tool and not a clinical definition.[12]
The definition of VAP as described by the CDC is complex and difficult to apply in our South African context, but the modified Clinical Pulmonary Infection Score (CPIS) has proved to be both sensitive and specific in diagnosing VAP in our setting.[11] The CPIS is a tool that was developed to facilitate the diagnosis of VAP and works on the basis of assigning points for various signs and symptoms of pneumonia.[13]
During the study period the modified CPIS forms were completed on a daily basis by doctors working in the PICU. Patients with high CPIS scores were flagged as possible VAPs. These suspected cases were then reviewed by the VAP co-ordinator and a PICU consultant, who checked that junior doctors had completed the modified CPIS forms correctly, and if the VAP diagnosis was appropriate. Once a patient was diagnosed as having a VAP, the VAP information was anonymised and entered onto our local PICU VAP database by the VAP co-ordinator. The VAP rate was calculated as the number of VAPs per 1 000 ventilator days. A separate list was kept with stored CPIS forms containing the names of patients, as well as VAP information for discussion at the weekly PICU morbidity and mortality meeting. The VAP co-ordinator was also responsible for monitoring compliance to the bundle by completing checklists twice a week on every ventilated patient, thereby covering both nursing shifts. Compliance with the VAP bundle was scored by the VAP co-ordinator and reported as a percentage.
All children admitted to the PICU who were diagnosed with a VAP during 2017 and 2018 were included in the study. Additional clinical information was obtained from the PICU admission database and from the patients' clinical folders.
Approval was obtained from the Departmental Research Committee, School of Child and Adolescent Health, as well as the Human Research Ethics Committee of the Faculty of Health Sciences, University of Cape Town and local hospital management before conducting the research. The study was developed and carried out in accordance with the Declaration of Helsinki, 2013.
As this was a retrospective folder review of routinely collected data and the study posed minimal risk, informed consent was not sought. Every child's parent/caregiver receives an information leaflet upon admission to the PICU explaining that routinely collected data may be used for research purposes and that data will be anonymised.
Patient confidentiality was maintained by anonymising all recorded data, as well as by storing paper records in a locked cupboard, and electronic records in a password-protected spreadsheet. No patient will be identifiable in any output arising from this study.
Statistical analysis was done using Microsoft Excel (Microsoft Corp., USA) and Stata version 11, (StataCorp., USA).
Data did not have normal distribution, and are therefore presented and summarised using median, interquartile percentiles and ranges.
Results
Over the 2-year period, 31 cases of VAP were identified. The VAP rate for 2017 was 4.0/1 000 ventilator days and for 2018 it was 5.4/1 000 ventilator days.
The characteristics of the children with VAPs are shown in Table 1.
The primary diagnoses of the children who developed VAPs in the PICU were varied and ranged across all disciplines. The top 5 primary diagnoses included traumatic brain injury due to pedestrian vehicle accidents (4 cases), severe pneumonia (2 cases), congenital diaphragmatic hernia (2 cases), total anomalous pulmonary venous drainage (TAPVD) post repair (2 cases) and ventricular septal defect (VSD) post repair (2 cases).
Of the children who developed VAPs, 8 (26%) patients were electively admitted post cardiac surgery; there were 13 (42%) emergency surgical admissions and 10 (32%) emergency medical admissions.
The most common cultured organisms are shown in Table 2.
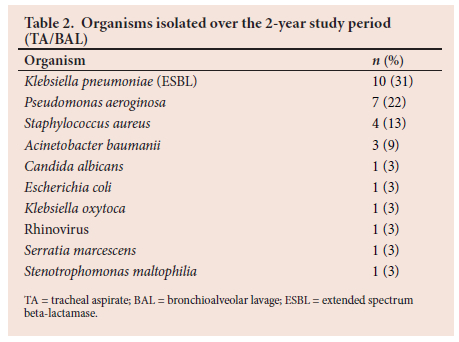
In two VAP cases no organisms were cultured and the diagnosis was made on clinical grounds, and one specimen cultured two organisms.
Looking at the sensitivity patterns: K. pneumoniae were 100% sensitive to amikacin but had intermediate sensitivity to piptazobactam in two isolates; in these cases carbapenems showed 100% sensitivity. P. aeruginosa had 100% aminoglycocide sensitivity, and in one case showed intermediate resistance to piptazobactam. S. aureus was 75% sensitive to cloxacillin, with only 1 case methicillin resistant. A. baumanii has shown 100% sensitivity to gentamicin but 66% resistance to piptazobactam - in these cases of resistance the bacteria were sensitive to carbapenems.
Overall, 90% of the VAP organisms in our study were sensitive to the combination of piptazobactam and amikacin.
Discussion
The VAP rate of 4/1 000 ventilator days in 2017 was the same as the last published data in 2013, while the VAP rate in 2018 was slightly higher at 5.4/1 000 ventilator days. This is still higher than developed countries like Japan and the USA,[6,7] where rates are <3.5/1 000 ventilator days. Children developed a VAP after a median of 5 days' ventilation in 2017 and after a median of 8 days' ventilation in 2018. The longer median duration of ventilation in 2018 could potentially explain the increase in VAP rates during 2018, as it is well described that longer duration of ventilation leads to increased risk for developing VAP;[14] however, increased duration of ventilation could also be a consequence of VAP. Prolonged ventilation in paediatrics has been defined as mechanical ventilation for >21 days and >6 hours/day.[15] In our study, 9 of the 31 patients (29%) fulfilled that definition. In 2018, patients who required ventilation longer than 21 days were admitted with the following conditions: hypoplastic lung (39 days), encephalitis (37 days), double outlet right ventricle post op (35 days), status dystonicus (34 days), necrotising enterocolitis (NEC)/ sepsis (28 days), and tracheo-oesophageal fistula (24 days). Encephalitis is well described in the literature as requiring prolonged ventilation in the paediatric population.-16,17
Unfortunately, the patients with pulmonary hypoplasia, NEC and tracheo-oesophageal fistula demised, accounting for 3 of the 4 deaths in 2018.
In order to reduce morbidity and mortality in the PICU, the prevention of VAPs should be a priority. The low VAP bundle compliance rate of 68% in 2017 and 70% in 2018 is worrying and a reduction in our VAP rate can only be achieved by improving these compliance rates.
Infection control by means of hand washing and decontamination of surfaces remains the mainstay of prevention of any HAI.[18,19] In addition, and more specifically for VAP, a 'bundle' (a set of practices to improve patient outcomes) approach has been developed by the Institute of Healthcare Improvement (IHI) for adult patients, and a modified version has been introduced in two PICUs.[-20]
In paediatrics, not all of the adult approaches are applicable. For example, it is not recommended to stop sedation on a daily basis to review the child's extubation readiness. Although one study in a meta-analysis by de Neef et al.[20]found no safety issues with daily sedation interruption. Sedation breaks pose a high risk for accidental extubation, with the process of re-intubation potentially increasing the risk of developing a VAP.[18,20,21]
Furthermore, the use of H2 antagonists and antacids is not recommended in children. The natural acidity of stomach contents plays a role in decreasing colonisation with harmful bacteria. Increasing the pH of stomach content poses the risk of possible colonisation with pathogenic bacteria and increasing the risk of VAP.-19,22-24] In adults, however, sucralfate, which does not alter stomach pH, showed a significant decrease in VAP rates.[20,25] Deep-vein thrombosis (DVT) prophylaxis is not routinely recommended in children.[21]- It is advisable to assess every child individually and not routinely make DVT prophylaxis part of the VAP-prevention bundle.[18]
In 2019 a recently published meta-analysis by de Neef et al.[20] and a systematic review by Niedzwiecka et al.-[26] reviewed the effectiveness of VAP bundles in ventilated children. They concluded that the implementation of a ventilator care bundle can help reduce the incidence of VAP in ventilated children.[20,26]
Other strategies to prevent VAPs in paediatrics include: using cuffed endotracheal tubes, and checking cuff pressures regularly;-19,21,27-minimising aspiration; changing ventilator circuits when visibly soiled or malfunctioning;[21,27] allowing condensate to drain away from patients;[21] and selective oropharyngeal decontamination, which entails the application of topical antibiotics to the oropharynx, but at the risk of causing increased antimicrobial resistance.[28,29]
There is insufficient literature regarding the effect of oral v. nasal intubations on the incidence of VAPs in the paediatric population. However, when staff shortages in developing countries such as our own are taken into account, there is a higher potential for accidental extubations with oral endotracheal tubes.[18]
Successful and effective ventilator care bundles are dependent on good compliance, but this remains a challenge. Accountability forms a crucial component of VAP prevention as it bridges the gap between science and outcome and includes leadership, education, execution, evaluation and feedback.[20,21]
By far the most commonly cultured organism in our study was an extended-spectrum beta-lactamase-producing K. pneumoniae (30%), which is in contrast to international data, where P. aeruginosa is described as the most common causative agent.[1-3,5,9,10]
Culture yields in our audit were high (94%), but included Gram-positive and Gram-negative cultures, as well as viruses and yeasts. A study by Chomton et al.[5] in Canada documented a 60% Gram-negative culture yield.
Empirical use of amikacin and piptazobactam in children with suspected VAPs, who do not have renal impairment, is still appropriate as 90% of the causative organisms showed sensitivity to either the one or the other. This is crucial in view of the increasing concerns about carbapenem-resistant enterobacteriaceae (CRE) colonisation and infection. Of major concern is the developing intermediate resistance pattern seen with carbapenems, as these are the alternative drugs of choice for children with renal impairment.
The study unfortunately has several limitations. The data were extracted from existing databases. The CPIS forms were completed by varying junior doctors, and these forms were often filled in retrospectively. Multiple VAP co-ordinators between 2013 and 2018 could have affected monitoring, as well as teaching and training. Leucocyte counts and chest X-rays were not performed routinely each day on ventilated patients. Inter-observer variability in interpretation of X-rays and bacterial culture results from tracheal aspirates instead of bronchoalveolar lavage could play a role in missing or overdiagnosing VAPs.
Recommendations
Maintaining and improving VAP compliance is of utmost importance and could include more regular checks, and keeping staff motivated, informed and educated,[20] as well as ongoing audits. It would be valuable to look at the components of VAP compliance scores to determine which areas require additional attention, and research could be aimed at assessing barriers to implementation of the VAP bundle by nursing staff in developing countries.
Conclusion
Despite seeing an initial decrease in VAPs in our unit after implementing the VAP bundle in 2013, our VAP rate has not decreased further. The VAP rate was slightly higher in 2018, and needs to be evaluated for subsequent years. It is also imperative that we improve compliance with the VAP bundle, in order to improve VAP rates.
K. pneumoniae and P. aeruginosa were the commonest organisms causing VAPs and empirical use of piptazobactam and amikacin in combination is still appropriate.
Declaration. None.
Acknowledgements. PICU team and previous VAP co-ordinatiors.
Author contributions. JA maintained VAP database; LvW and SS contributed to protocol development, data analysis and interpretation, and development of the final manuscript.
Funding. None.
Conflicts of interest. None.
References
1. Galal YS, Youssef MRL, Ibrahiem SK. Ventilator-associated pneumonia: Incidence, risk factors and outcome in paediatric intensive care units at Cairo University Hospital. J Clin Diagn Res 2016;10(6):SC06. https://doi.org/10.7860/jcdr/2016/18570.7920 [ Links ]
2. Foglia E, Meier M, Elward A. Ventilator-associated pneumonia in neonatal and pediatric intensive care unit patients. Clin Microbiol Rev 2007;20(3):409-425. https://doi.org/10.1128/cmr.00041-06 [ Links ]
3. Becerra M, Tantaleán J, Suárez V, Alvarado M, Candela J, Urcia F. Epidemiologic surveillance of nosocomial infections in a pediatric intensive care unit of a developing country. BMC Pediatr 2010;10(1):66. https://doi.org/10.1186/1471-2431-10-66 [ Links ]
4. Awasthi S, Tahazzul M, Ambast A, Govil YC, Jain A. Longer duration of mechanical ventilation was found to be associated with ventilator-associated pneumonia in children aged 1 month to 12 years in India. J Clin Epidemiol 2013;66(1):62-66. https://doi.org/10.1016/j.jclinepi.2012.06.006 [ Links ]
5. Chomton M, Brossier D, Sauthier M, et al. Ventilator-associated pneumonia and events in pediatric intensive care: A single center study. Pediatr Crit Care Med 2018;19(12):1106. https://doi.org/10.1097/pcc.0000000000001720 [ Links ]
6. Hatachi T, Tachibana K, Takeuchi M. Incidences and influences of device-associated healthcare-associated infections in a pediatric intensive care unit in Japan: A retrospective surveillance study. J Intensive Care 2015;3(1):44. https://doi.org/10.1186/s40560-015-0111-6 [ Links ]
7. Patrick SW, Kawai AT, Kleinman K, et al. Health care-associated infections among critically ill children in the US, 2007-2012. Pediatrics 2014:peds.2014-0613. https://doi.org/10.1542/peds.2014-0613 [ Links ]
8. Kunzmann H, Dimitriades K, Morrow B, Argent A. Reducing paediatric ventilator- associated pneumonia - a South African challenge! South Afr J Crit Care 2016;32(1):17-20. https://doi.org/10.7196%2FSAJCC.2016.v32i1.243 [ Links ]
9. Iosifidis E, Stabouli S, Tsolaki A, et al. Diagnosing ventilator-associated pneumonia in pediatric intensive care. Am J Infect Control 2015;43(4):390-393. https://doi.org/10.1016/j.ajic.2015.01.004 [ Links ]
10. Almuneef M, Memish ZA, Balkhy HH, Alalem H, Abutaleb A. Ventilator-associated pneumonia in a pediatric intensive care unit in Saudi Arabia: A 30-month prospective surveillance. Infect Control Hosp Epidemiol 2004;25(9):753-758. https://doi.org/10.1086/502472 [ Links ]
11. Morrow BM, Mowzer R, Pitcher R, Argent AC. Investigation into the effect of closed-system suctioning on the frequency of pediatric ventilator-associated pneumonia in a developing country. Pediatr Crit Care Med 2012;13(1):e25-e32. https://doi.org/10.1097/pcc.0b013e31820ac0a2CDC [ Links ]
12. Pediatric Ventilator-Associated Event 2020. https://www.cdc.gov/nhsn/pdfs/pscmanual/pedvae-current-508.pdf [ Links ]
13. Zilberberg MD, Shorr AF. Ventilator-associated pneumonia: The clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin Infect Dis 2010;51(Supplement_1):S131-S5. https://doi.org/10.1086/653062 [ Links ]
14. Wu D, Wu C, Zhang S, Zhong Y. Risk factors of ventilator-associated pneumonia in critically ill patients. Front Pharmacol 2019;10:482. https://doi.org/10.3389%2Ffphar.2019.00482 [ Links ]
15. Sauthier M, Rose L, Jouvet P. Pediatric prolonged mechanical ventilation: Considerations for definitional criteria. Respir Care 2017;62(1):49-53. https://doi.org/10.4187/respcare.04881 [ Links ]
16. Schubert J, Bramer D, Huttner HB, et al. Management and prognostic markers in patients with autoimmune encephalitis requiring ICU treatment. Neurol Neuroimmunol Neuroinflamm 2019;6(1):e514. https://doi.org/10.1212/NXI.0000000000000514 [ Links ]
17. Harutyunyan G, Hauer L, Dunser MW, et al. Autoimmune encephalitis at the neurological intensive care unit: Etiologies, reasons for admission and survival. Neurocrit Care 2017;27(1):82-89. https://doi.org/10.1007%2Fs12028-016-0370-7 [ Links ]
18. Morrow B, Argent A, Jeena PM, Green RJ. Guideline for the diagnosis, prevention and treatment of paediatric ventilator-associated pneumonia. S Afr Med J 2009;99(4):255-267. [ Links ]
19. Chang I, Schibler A. Ventilator associated pneumonia in children. Paediatr Respir Rev 2016;20:10-16. https://doi.org/10.1016/j.prrv.2015.09.005 [ Links ]
20. De Neef M, Bakker L, Dijkstra S, Raymakers-Janssen P, Vileito A, Ista E. Effectiveness of a ventilator care bundle to prevent ventilator-associated pneumonia at the PICU: A systematic review and meta-analysis. Pediatr Crit Care Med 2019;20(5):474-480. https://doi.org/10.1097/pcc.0000000000001862 [ Links ]
21. Klompas M, Branson R, Eichenwald EC, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35(8):915-936. https://doi.org/10.1086/677144 [ Links ]
22. Mourani PM, Sontag MK. Ventilator-associated pneumonia in critically ill children: A new paradigm. Pediatr Clin North Am 2017;64(5):1039-1056. https://doi.org/10.1016/j.pcl.2017.06.005 [ Links ]
23. Manonita M, Suman D, Moukoli P, Indraneel S, Srinath Reddy A. Incidence, risk factors, clinico-microbiological profile, change in ventilator settings needed and outcome of 135 ventilator associated pneumonia cases in pediatric intensive care unit (PICU) of a tertiary care centre in Eastern India. J Pediatr Neon Individual Med 2018;7(1):e070122-e. https://doi.org/10.7363/070122 [ Links ]
24. Albert BD, Zurakowski D, Bechard LJ, et al. Enteral nutrition and acid-suppressive therapy in the pediatric intensive care unit: Impact on the risk of ventilator-associated pneumonia. Pediatr Crit Care Med 2016;17(10):924. https://doi.org/10.1097/pcc.0000000000000915 [ Links ]
25. Alquraini M, Alshamsi F, M0ller MH, et al. Sucralfate versus histamine 2 receptor antagonists for stress ulcer prophylaxis in adult critically ill patients: A meta-analysis and trial sequential analysis of randomised trials. J Crit Care 2017;40:21-30. https://doi.org/10.1016/j.jcrc.2017.03.005 [ Links ]
26. Niedzwiecka T, Patton D, Walsh S, Moore Z, O'Connor T, Nugent L. What are the effects of care bundles on the incidence of ventilator-associated pneumonia in paediatric and neonatal intensive care units? A systematic review. J Spec Pediatr Nurs 2019;24(4):e12264. https://doi.org/10.1111/jspn.12264 [ Links ]
27. Haut C. Preventing pediatric ventilator-associated pneumonia. Nursing Critical Care 2015;10(6):42-47. https://journals.www.com/nursingcriticalcare/Fulltext/2015/11000/Preventing_pediatric_ventilator_associated.8.aspx [ Links ]
28. Landelle C, Boyer VN, Abbas M, et al. Impact of a multifaceted prevention program on ventilator-associated pneumonia including selective oropharyngeal decontamination. Intensive Care Med 2018;44(11):1777-1786. https://doi.org/10.1007%2Fs00134-018-5227-4 [ Links ]
29. Petros A, Silvestri L, Booth R, Taylor N, van Saene H. Selective decontamination of the digestive tract in critically ill children: Systematic review and meta-analysis. Pediatr Crit Care Med 2013;14(1):89-97. https://doi.org/10.1097/pcc.0b013e3182417871 [ Links ]
 Correspondence:
Correspondence:
L van Wyk
lianivw@gmail.com
Accepted 19 May 2022
Contribution of the study
This study highlights the need for ongoing evaluation of quality improvement initiatives in PICU, considering that VAP rates remained largely unchanged from 2013 to 2018..














