Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Southern African Journal of Critical Care (Online)
versão On-line ISSN 2078-676X
versão impressa ISSN 1562-8264
South. Afr. j. crit. care (Online) vol.38 no.1 Pretoria Mar. 2022
http://dx.doi.org/10.7196/SAJCC.2022.v38i1.522
RESEARCH
Pharmacological management of post-traumatic seizures in a South African paediatric intensive care unit
Yachad NI; Naidoo KDII
IMB ChB, DCh, Dip HIV Man; Department of Paediatrics, University of the Witwatersrand, Johannesburg, South Africa
IIMB ChB, DCh, FCPaed, MMed (Paed), Cert Crit Care (Paed); Division of Critical Care, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND. Traumatic brain injury (TBI) is a common cause of paediatric intensive care unit (PICU) admissions in South Africa. Optimal care of these patients includes the prevention and control of post-traumatic seizures (PTS) in order to minimise secondary brain injury.
OBJECTIVES. To describe the demographics of children admitted to a South African PICU, to describe the characteristics of PTS, and to describe the prophylactic and therapeutic management of PTS within the unit.
METHOD. A 3-year retrospective chart review was conducted at the PICU of the Chris Hani Baragwanath Academic Hospital (CHBAH) in Soweto, Johannesburg, from 1 July 2015 to 30 June 2018.
RESULTS. Seventy-eight patients were admitted to the PICU, all with severe TBI. A total of 66 patient files were available for analysis. The median age of admission was 6 years (interquartile range (IQR) 4 - 9) with the majority of trauma secondary to mechanical injury (89%). Prophylactic anti-epileptic drugs (AEDs) were initiated in 44 (79%) patients. Early PTS occurred in 11 (25%) patients who received prophylaxis and 4 (33%) who did not. Three (5%) patients developed late PTS, resulting in an overall incidence of PTS of 43%. The most common seizure type was generalised tonic clonic (82%). Children diagnosed with PTS were a median of 2 years younger than those without PTS, with increased prevalence of seizures (83% v. 38%) in children below 2 years of age. Maintenance therapy was initiated in all patients consistent with recommended dosages. Of the total 167 anti-epileptic levels taken during maintenance, only 56% were within target range. Of the initial 78 patients, 8 died (10%). The median length of stay was 7 (IQR 5 - 12) and 8 (IQR 8 - 24) days longer in ICU and hospital respectively, in children with PTS.
CONCLUSION. PTS is a frequent complication of severe TBI in children. There was considerable variation in the approach to both prophylaxis and maintenance therapy of PTS in terms of choice of agent, dosage, frequency of drug monitoring and approach to subtherapeutic levels. It is clear that more high-level studies are required in order to better inform these practices.
Keywords: post-traumatic seizure, paediatric traumatic brain injury, anti-epileptic drugs
Paediatric traumatic brain injury (TBI) is a major cause of morbidity and mortality globally, annually affecting ~3 million children.[1] Incidence rates are disproportionally higher in low- and middle-income countries, where road traffic accidents are more common.[2,3]
The key treatment goals in the care of TBI patients are mitigation and prevention of secondary and tertiary brain injury. Management of TBI has therefore focused on the prevention of hypoxaemia, systemic hypotension, hypercarbia, increased metabolic demands and raised intracranial pressure (ICP).[4] Thus, the prevention and effective management of post-traumatic seizure (PTS) is paramount, given that PTS results in the further release of excitatory neurotransmitters and increased metabolic demand, which further increases cerebral blood flow, intracranial pressure, cerebral oedema and tissue hypoxia.[5,6]
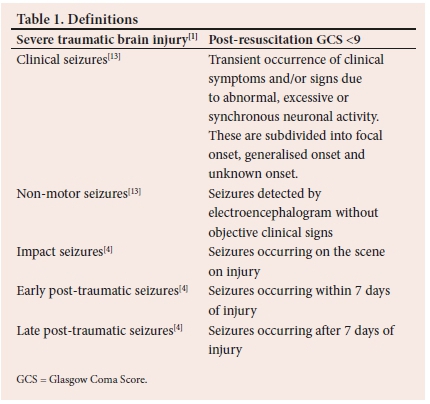
The overall incidence of PTS is at least 12%,[4] rising to around 25% with severe TBI.[7] PTS is typically classified based on onset in relation to the time of injury: impact (on scene), early (within 7 days of injury) and late (after 7 days) (Table 1).[4,5] The high incidence of PTS within 24 hours of injury has given rise to the additional classification of these seizures as immediate seizures.[5] Risk factors for the development of PTS include young age, assault, subdural haemorrhage (SDH) and a Glascow Coma Score (GCS) less than 9.[4-10]
Despite evidence of the efficacy of phenytoin prophylaxis to decrease the incidence of early post-traumatic seizure (EPTS) in severe TBI,[11] the paucity of high-level evidence to guide the approach to both the prophylaxis and treatment of PTS has resulted in considerable variability in management.[4,12] Very little is known about the incidence and management of PTS in a South African context. We sought to describe a cohort of children admitted with TBI to a South African paediatric intensive care unit (PICU), determine the incidence and characteristics of PTS and describe the patterns of prophylactic and therapeutic anti-epileptic drugs (AEDs) use within the PICU.
Methods
Ethical approval for this research was provided by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand (ref. no. M180834).
This 3-year retrospective chart review was carried out at the PICU of the Chris Hani Baragwanath Academic Hospital (CHBAH) in Soweto, Johannesburg, from 1 July 2015 to 30 June 2018. Patients <16 years old with admission diagnoses of TBI or polytrauma were identified using the PICU database. Data collected included baseline characteristics, injury characteristics, AED use, seizure characteristics and hospital outcomes, and were collated using Excel software (Microsoft Corp., USA). Baseline characteristics included age, sex, length of admission, mechanism of injury, past medical history, and surgical intervention before admission. Mechanism of injury was divided into mechanised and non-mechanised injury, where mechanised injury was defined as secondary to motor vehicle accidents, pedestrian-vehicle accidents and motorcycle accidents; and non-mechanised as secondary to falls, assault or blunt-force trauma.[4] Injury characteristics included post-resuscitation GCS on admission, head computed tomography (CT) findings including the presence of penetrating injury or skull fractures and severity of TBI. Severity of TBI was classified based on the post-resuscitation GCS (mild >13, moderate 9 - 12, severe <8). AED use included the choice of agent, dosing and serum levels. Outcomes data included mortality and length of PICU and hospital stays.
Seizure activity was confirmed either by clinician documentation of clinical seizures, autonomic instability or by electroencephalogram (EEG) analysis (when available). Both routine (12 lead) and bedside amplitude EEG were utilised in the unit. There was no existing EEG protocol in place during the period of investigation. Seizures were characterised by type as generalised tonic-clonic (GTC), focal, absence or subclinical when only noted by EEG or autonomic instability. The descriptor 'multiple seizure types' was used when more than one type of seizure activity was documented during admission. Follow-up of late post-traumatic seizure (LPTS) was up to hospital discharge.
There were no standard protocols in place instructing the use of AED prophylaxis or EEG monitoring. Interpretation of AED dosing was guided by the South African Medical Formulary (12th edition). Reference serum levels used were consistent with those utilised by the National Health Laboratory Service (NHLS) (2018).
Descriptive statistics were used to report the data. Categorical variables were described using median (interquartile range (IQR)) where data did not follow a normal distribution.
Results
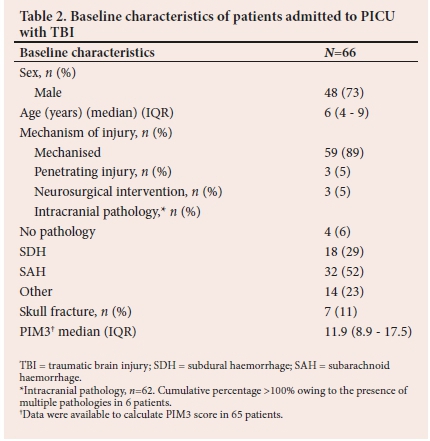
A total number of 700 patients were admitted to the Paediatric Intensive Care Unit (PICU) during the three-year study period, of whom 78 (11%) sustained TBI. The entire cohort was classified as severe TBI. Sixty-six (85%) patients' medical records were available for analysis.
Patient characteristics
Baseline patient characteristics are presented in Table 2. None of the patients had a history of epilepsy or TBI prior to admission. The median age of admission was 6 years (IQR 4 - 9), with an overall male preponderance (73%). Mechanised injury was the most common cause of injury (n=59; 89%). The remaining causes of injury were: falls from height (n=4), assault (n=2), and 1 child sustained injury associated with a collapsed wall. Sixty-two patients (94%) underwent a head CT scan prior to admission.
Prophylactic anti-epileptic drug use
Prophylactic AEDs were initiated in 44 patients (79%) either by Emergency Department (ED) or PICU staff, including 2 patients who had sustained impact seizures. Prophylaxis was deferred in the remaining 12 patients (21%) as per physician preference.
Phenytoin (n=26 (59%)) and sodium valproate (n=18) were the only two AEDs used as prophylaxis. Phenytoin and sodium valproate were administered prophylactically for a median of 6 (IQR 4 - 7) and 8 days (IQR 6 - 10), respectively. Dosing of AEDs for this purpose was consistent with formulary ranges in the majority of patients (77% on phenytoin and 94% on sodium valproate). Of the remaining patients on phenytoin, 3 (12%) were given AEDs dosed below, and 3 above, the formulary range. Only 1 patient on sodium valproate was dosed below the formulary range.
A total of 25 patients (38%) developed early post-traumatic seizure (EPTS), consisting of the 10 patients initiated on treatment in the ED, 11 who were on prophylaxis and 4 who did not receive prophylaxis. The overrall incidence of EPTS in the prophylaxis group was 25%, compared with 33% in the deferred group. The incidence of EPTS among the phenytoin and valproate groups was 39% and 13%, respectively. The relationships between the administration (or omission) of seizure prophylaxis, adequacy of serum drug levels and the development of EPTS are illustrated in Fig. 1.
All serum levels were reviewed within 24 hours of seizure occurrence.
Seizures
Thirty-five patients completed a 7-day follow-up without demonstrating EPTS. Of these, 3 patients (9%) developed LPTS, resulting in a combined total of 28 children (43%) with PTS at the time of ICU discharge, of whom 89% were EPTS. Immediate seizures accounted for 96% of EPTS. Patients experienced multiple seizures more commonly (64%) than isolated seizures. Twenty-one patients (75%) sustained a single seizure type, with generalised tonic clonic (GTC) being the most common (n=16), followed by subclinical (n=4) and a single focal seizure. The other 7 patients had multiple seizure types including at least 1 GTC in addition to focal seizures (n=5), subclinical (n=1) and absence seizure (n=1). Three of the subclinical seizures were detected by amplitude EEG monitoring and 2 on the basis of autonomic instability. EEG monitoring was utilised in 7 patients, 4 with amplitude EEG and 3 with routine EEG monitoring. All patients sustaining LPTS completed a 7-day course of prophylactic AEDs and were recognised by day 9 of admission. Two of the 3 patients were not on an AED at the time and the third patient sustained seizures on day 8, while still on prophylactic phenytoin. Status epilepticus was reported in 9 patients (32%), 7 of whom belonged to the group of 10 patients who sustained seizures prior to prophylaxis initiation.
Children diagnosed with PTS were a median of 2 years younger than those without PTS. Post-traumatic seizures were noted in 5 of the 6 (83%) children under 24 months of age, in contrast to 38% of those above 24 months old. Post-traumatic seizures were observed in 53% (n=17) of patients with subarachnoid haemorrhage (SAH) as compared with 22% (n=4) patients with subdural haemorrhage (SDH).
Maintenance anti-epileptic drug use
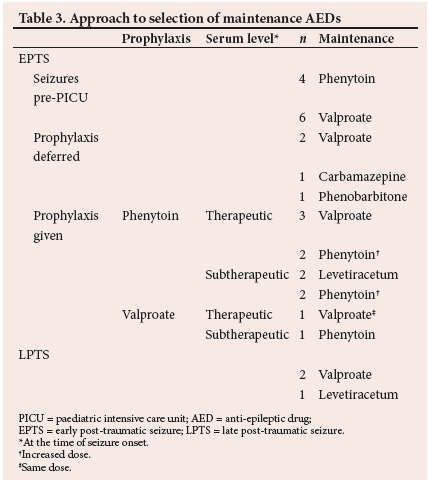
All 28 patients who developed PTS were initiated onto maintenance anti-epileptic therapy. The initial maintenance agents utilised were sodium valproate in 14 (50%) patients, phenytoin in 9 (33%), levetiracetum in 3 (11%), with carbamazepine and phenobarbital in 1 (4%) patient each. The approach to selection of maintenance agents is detailed in Table 3.
Thirteen (46%) patients were prescribed more than one AED during the follow-up period and 4 (31%) patients required more than 1 AED simultaneously for seizure control. Dosing of all patients' maintenance drugs was consistent with formulary dosing regimens. Of the 18 patients in whom maintenance AEDs were initiated in the PICU, 12 (67%) received a loading dose before commencing maintenance dosing.
At least one serum anti-epileptic level (AEL) was measured in 23 (82%) patients during their hospital admission. A total of 167 AELs were drawn, following the initiation of maintenance therapy during the study period. Of these, 93 (56%) were within the target range, 67 (40%) were subtherapeutic and 7 (4%) were above the recommended therapeutic range. Fig. 2 illustrates the range of AELs for both phenytoin (Fig. 2A) and sodium valproate (Fig. 2B).
The responses to subtherapeutic levels were: administration of a loading dose of the current AED (n=16), increased dosing (n=10), both loading and increasing the dose (n=9), addition of a second AED (n=2), change to another AED (n=7) or no change (n=23). The proportion of AELs within therapeutic ranges declined with time from 62% during the first 7 days to 45% thereafter.
Nineteen patients experienced further seizures (total of 52) while on maintenance therapy. An AEL, taken within 24 hours of seizure onset, was available for 30 (58%) of these seizures, with 19 (63%) within therapeutic limits and 11 subtherapeutic. Subsequent management included attempts at optimising the serum level of the current drug (n=7) or a change to an alternative AED. A total of 13 therapeutic changes were made solely in response to AELs. Of the 13 changes, 8 levels (38%) were taken in the absence of a loading dose and before 4.5 half-lives had elapsed.
Outcomes
Of the 78 patients who sustained TBI, a total of 8 (10%) died. The median ICU and hospital lengths of stay were 12 and 19 days, respectively in those children with PTS, compared with 5 and 11 days, respectively in those who did not develop PTS.
Discussion
TBI represents a common reason for admission to PICUs (11% of all admissions in this cohort).[14] The extreme pressure on ICU beds in public healthcare in South Africa dictates that patients admitted are usually in need of organ support, hence limiting the admission of most patients with moderate TBI. Consequently, the present study adds to the subset of evidence pertaining to severe paediatric TBI. Predominantly our cohort displayed characteristics in keeping with similar studies, viz. a male predominance (ratio 2.7), a median age of 6 years,[15,16] an overwhelming majority of injuries being non-penetrating and the presence of numerous intracranial haemorrhages.[7,8,15] Notable differences included the relative lack of cases thought to have resulted from assault or non-accidental injury,[4,8,15] the finding of a higher number of subarachnoid rather than subdural haemorrhages[7,8] and the higher proportion of mechanised injury (consistent with only one other South African study).[3]
The overall incidence of PTS was 47%, with the vast majority being EPTS (91%). The incidence of PTS in our cohort, perhaps unsurprisingly, exceeded rates previously reported (19 - 39%),[8] given the correlation between PTS incidence and severity of TBI.[6,9] Children younger than 2 years were once again more prone to the development of PTS (83 v. 38% among older children)[4,6-8] while, unexpectedly, PTS occurred more often in patients with SAH (53%) rather than SDH (22%).[5,6,9] Consistent with existing literature, almost all the EPTS were immediate seizures (96%),[4-6] highlighting the time-sensitive need for appropriate PTS prophylaxis in high-risk groups, particularly the value of a loading dose in order to rapidly achieve therapeutic levels.
Prophylaxis to prevent EPTS was administered to 79% of patients admitted to the PICU, with 21% receiving none as per physician preference. The Brain Trauma Foundation presently recommends phenytoin as first-line therapy for seizure prophylaxis based on two single-centre class 3 studies which showed decreased seizure incidence in the group receiving phenytoin prophylaxis; notably, both these studies included TBI with various levels of severity. Interestingly, the use of levetiracetam is still not recommended based on the lack of studies showing either increased efficacy or reduced toxicity compared with phenytoin prophylaxis.[11] Given the weak recommendations provided in contemporary TBI guidelines,'171 inconsistent use of prophylaxis was perhaps to be expected. However, the rate of prophylaxis prescription in this cohort was similar to a recent study investigating the variation of seizure prophylaxis use in paediatric trauma centres'121 and higher than rates reported in earlier studies.'41
Although phenytoin was the more commonly prescribed agent, the rate of sodium valproate use (41%) as a prophylactic agent was surprising. Since the landmark (adult) RCT comparing phenytoin and sodium valproate for PTS prophylaxis found no benefit and hinted at a potential increase in the mortality rate among the valproate group,[18] sodium valproate has not featured as an option for prophylaxis in any of the relevant subsequent TBI management guidelines.[17,19] Although the study was not powered to investigate the efficacy of the agents, the lower incidence rate of EPTS in the valproate prophylaxis group (13 v. 39%) possibly begs further consideration. Despite the recent emergence of levetiracetum as a potential prophylactic agent in the adult population,'51 the absence of utilisation was probably due to limited availability, given the increased cost relative to phenytoin and valproate. Another consideration for the lack of use of levetiracetam is that therapeutic drug monitoring is not available in public hospitals, making it difficult to assess whether patients' drug levels are within the recommended therapeutic serum levels.
The Brain Trauma Foundation recently defined post-traumatic epilepsy (PTE) as recurrent seizures after 7 days post injury; however, this definition remains inconsistent in the literature,[11] with some focussing on the development of at least one[6,19,21] or more[17] LPTS and others including the development of recurrent seizures irrespective of timing.[22] In this cohort, maintenance anti-epileptic therapy was uniformly initiated following the occurrence of a single seizure, irrespective of timing. An aggressive approach to management is perhaps influenced by the report that early AED use has been associated with decreased mortality in the PICU setting.[14] However, early initiation of maintenance therapy has not been shown to affect overall quality of life or result in sustained seizure remission.[21] Additionally, the use of phenytoin and carbamazepine has been shown to negatively affect cognition, with potentially deleterious long-term neurobehavioural effects in children.[22]
The approach to the selection of maintenance AEDs appears to have been based on the type of seizures noted and local availability, consistent with previous reports.[5,22] Sodium valproate and phenytoin were the most commonly used agents in this setting (83%), both of which being suitable agents for GTC seizures, which was the most common seizure type encountered (57%).
Phenytoin is a drug characterised by nonlinear pharmacokinetics, significant protein binding, a narrow therapeutic range and saturable metabolism.[23] Critical illness itself is often associated with altered drug pharmacokinetics and pharmacodynamics. Consequently, appropriate dosing of maintenance AEDs is challenging. To this end, therapeutic drug monitoring (TDM) provides clinicians with a target against which to titrate dosing.
Despite the frequent use of TDM during the study period, there appeared to be notable inconsistencies in physician practice. First was the issue of the timing of AEL sampling. Of the 13 management changes solely in response to subtherapeutic AELs, more than a third were of questionable accuracy, given that they were taken in the absence of a loading dose and before 4.5 half-lives had elapsed. This practice raised the possibility of management decisions being taken based on potentially inaccurate AELs. Second was the subsequent approach to the management of AEDs in response to subtherapeutic serum levels. Changes to management occurred more often (66%) but 5 different approaches were noted during the study period. Perhaps of greater interest was the lack of a response in one-third of cases.
Given that nearly two-thirds of seizures while on maintenance therapy occurred in the presence of AEL within the therapeutic range, coupled with a recent report of the absence of LPTS only in adult TBI patients achieving serum phenytoin levels of at least 52 mmol/L,[24] it brings into question the appropriateness of established AED reference ranges for TBI patients.
Study limitations
This was a single-centre study, which limits generalisability and provides a limited sample size that has prevented meaningful associations between practice patterns and outcomes. Selection bias may have been introduced by the fact that 16% of files were unavailable for analysis. The retrospective nature of the study design has resulted in some incomplete data, which raises the possibility of reporting bias, specifically with respect to outcomes. Outcomes reported at day 28 would have been preferable; however, these data were not available for two-thirds of the patients; hence outcomes were reported to ICU or hospital discharge, which may have underestimated both late seizure incidence and mortality. The absence of a formal EEG protocol within the unit and the resultant intermittent use of EEG monitoring may have underestimated the incidence of nonconvulsive seizures, which is notable, given the high incidence of subclinical seizures in children with TBI.
Future directions
Despite the presence of TBI guidelines, there remain several open questions with respect to the management of PTS and PTE. The high frequency of seizure onset within the first 24 hours of injury suggests the possible value of prophylaxis in the prehospital setting. The indication for prophylaxis and most suitable agent remains unclear, given the emergence of levetiracetam. The wide variation in practice evident from this study highlights the potential value of both investigation (TDM and EEG) and treatment protocols to homogenise the management of TBI - specifically with regards to standardising AED dosage, the administration of loading doses, the timing of bloods taken for drug levels and response to subtherapeutic levels to improve the quality of care. In addition, motivating for access to continuous EEG monitoring would improve seizure detection and early management. The lack of a consensus definition for PTE probably underlies much of the inconsistencies regarding management. Thus, there is an urgent need for additional trials in both paediatric and adult TBI in order to inform management of this common condition.
Conclusion
Severe TBI is a frequent cause for PICU admissions in our setting. Furthermore, post-traumatic seizures are a common complication of severe TBI and therefore represents a key treatment area to optimise the prognoses for these children. To the best of our knowledge, this is the first description of the pharmacological management of post-traumatic seizures in critically ill children. Unsurprisingly, given the lack of data informing relevant guidelines, there was considerable variation in physician practices in terms of approach to the use of both AEDs and TDM. This hiatus should serve as an urgent call for additional research in both paediatric and adult TBI in order to inform the optimal management of this complication.
Author contributions. Equal contributions.
Acknowledgements. Nadir Yehya for his assistance and valuable insights.
Funding. None.
Conflict of interest. None.
References
1. Dewan M, Mummareddy N, Wellons J, Bonfield C. Epidemiology of global pediatric traumatic brain injury: Qualitative review. World Neurosurgery 2016;91:497-509.e1. https://doi.org/10.1016/j.wneu.2016.03.045 [ Links ]
2. Naidoo D. Traumatic brain injury: The South African landscape. S AfT Med J 2013;103(9):613. http://dx.doi.org/10.7196%2FSAMJ.7325 [ Links ]
3. Schrieff L, Thomas K, Dollman A, Rohlwink U, Figaji A. Demographic profile of severe traumatic brain injury admissions to Red Cross War Memorial Children's Hospital, 2006 - 2011. S Afr Med J 2013;103(9):616. https://doi.org/10.7196/samj.7137 [ Links ]
4. Liesemer K, Bratton S, Zebrack C, Brockmeyer D, Statler K. Early post-traumatic seizures in moderate to severe pediatric traumatic brain injury: Rates, risk factors, and clinical features. J Neurotrauma 2011;28(5):755-762. https://doi.org/10.1089/neu.2010.1518 [ Links ]
5. Zimmermann L, Martin R, Girgis F. Treatment options for posttraumatic epilepsy. Curr Opin Neurol 2017;30(6):580-586. https://doi.org/10.1097/wco.0000000000000505 [ Links ]
6. Statler K. Pediatric posttraumatic seizures: Epidemiology, putative mechanisms of epileptogenesis and promising investigational progress. Dev Neurosci 2006;28(4-5):354-363. https://doi.org/10.1159/000094162 [ Links ]
7. Bennett K, DeWitt P, Harlaar N, Bennett T. Seizures in children with severe traumatic brain injury. Ped Crit Care Med 2017;18(1):54-63. https://doi.org/10.1097/pcc.0000000000000948 [ Links ]
8. Arango J, Deibert C, Brown D, Bell M, Dvorchik I, Adelson P. Posttraumatic seizures in children with severe traumatic brain injury. Child Nerv Sys 2012;28(11):1925-1929. https://doi.org/10.1007/s00381-012-1863-0 [ Links ]
9. Annegers J, Coan S. The risks of epilepsy after traumatic brain injury. Seizure 2000;9(7):453-457. https://doi.org/10.1053/seiz.2000.0458 [ Links ]
10. Young K, Okada P, Sokolove P, et al. A randomized, double-blinded, placebo-controlled trial of phenytoin for the prevention of early posttraumatic seizures in children with moderate to severe blunt head injury. Ann Emerg Med 2004;43(4):435-446. https://doi.org/10.1016/j.annemergmed.2003.09.016 [ Links ]
11. Kochanek P, Tasker R, Carney N, et al. Guidelines for the management of pediatric severe traumatic brain injury, third ed. Ped Crit Care Med 2019;20(4):404. [ Links ]
12. Ostahowski P, Kannan N, Wainwright M, et al. Variation in seizure prophylaxis in severe pediatric traumatic brain injury. Neurosurg Pediatr 2016;18(4):499-506. https://doi.org/10.3171/2016.4.peds1698 [ Links ]
13. Fisher R, Cross J, French J, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58(4):522-530. https://doi.org/10.1111/epi.13670 [ Links ]
14. Tilford J, Simpson P, Yeh T, et al. Variation in therapy and outcome for pediatric head trauma patients. Crit Care Med 2001;29(5):1056-1061. https://doi.org/10.1097/00003246-200105000-00037 [ Links ]
15. Buitendag J, Kong V, Bruce J, Laing G, Clarke D, Brysiewicz P. The spectrum and outcome of paediatric traumatic brain injury in KwaZulu-Natal Province, South Africa has not changed over the last two decades. S Afr Med J 2017;107(9):777-780. https://doi.org/10.7196%2FSAMJ.2017.v107i9.12394 [ Links ]
16. Dewan M, Mummareddy N, Wellons J, Bonfield C. Epidemiology of global pediatric traumatic brain injury: Qualitative review. World Neurosurg 2016;91:497-509.e1. https://doi.org/10.1016/j.wneu.2016.03.045 [ Links ]
17. Kochanek P, Carney N, Adelson P, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents - second edition. Pediatr Crit Care Med 2012;13:S1-S2. https://doi.org/10.1097/pcc.0b013e31823f435c [ Links ]
18. Temkin N, Dikmen S, Anderson G, et al. Valproate therapy for prevention of posttraumatic seizures: A randomized trial. J Neurosurg 1999;91(4):593-600. https://doi.org/10.3171/jns.1999.9L4.0593 [ Links ]
19. Chang B, Lowenstein D. Practice parameter: Antiepileptic drug prophylaxis in severe traumatic brain injury: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2003;60(1):10-16. https://doi.org/10.1212/01.wnl.0000031432.05543.14 ' [ Links ]
20. Iudice A, Murri L. Pharmacological prophylaxis of post-traumatic epilepsy. Drugs 2000;59(5):1091-1099. https://doi.org/10.2165/00003495-200059050-00005 [ Links ]
21. Szaflarski J, Nazzal Y, Dreer L. Post-traumatic epilepsy: current and emerging treatment options. Neuropsychiatr Dis Treat 2014;10:1469-1477. https://doi.org/10.2147/ndt.s50421 [ Links ]
22. Agrawal A, Timothy J, Pandit L, Manju M. Post-traumatic epilepsy: An overview. Clin Neurol Neurosurg 2006;108(5):433-439. https://doi.org/10.1016/j.clineuro.2005.09.001 [ Links ]
23. Patsalos P, Spencer E, Berry D. Therapeutic drug monitoring of antiepileptic drugs in epilepsy: A 2018 update. Ther Drug Monitor 2018;40(5):526-548. https://doi.org/10.1097/ftd.0000000000000546 [ Links ]
24. Young B, Rapp R, Norton J, Haack D, Tibbs P, Bean J. Failure of prophylactically administered phenytoin to prevent early posttraumatic seizures. J Neurosurg 1983;58(2):231-235. https://doi.org/10.3171/jns.1983.58.2.0231 [ Links ]
 Correspondence:
Correspondence:
N Yachad
nyachad@gmail.com
Accepted 24 February 2022.
Contribution of the study
To the best of our knowledge, this article represents the first description of incidence and management practices of paediatric post traumatic seizures.














