Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Southern African Journal of Critical Care (Online)
On-line version ISSN 2078-676X
Print version ISSN 1562-8264
South. Afr. j. crit. care (Online) vol.37 n.1 Pretoria Mar. 2021
http://dx.doi.org/10.7196/SAJCC.2021.v37i1.439
RESEARCH
Stress ulcer prophylaxis use in critical care units at public hospitals in Johannesburg, South Africa
N BiyaseI; H PerrieII; J ScribanteIII; M MutebaIV; S ChettyV
IMB ChB, DA (SA), FCA (SA), MMed (Anaes); Department of Anaesthesiology, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa. OrcID 0000-0002-2120-0162
IIMSc; Department of Anaesthesiology, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa. OrcID 0000-0002-9890-7887
IIIPhD; Department of Anaesthesiology, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa. OrcID 0000-0002-2221-5024
IVMB ChB, MSc; Department of Anaesthesiology, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa. OrcID 0000-0002-8593-3592
VMB ChB, DCH (SA), DA (SA), FCA (SA), Cert Crit Care, PhD; Department of Anaesthesiology and Critical Care, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa. OrcID 0000-0002-9878-5488
ABSTRACT
BACKGROUND. Stress ulcer prophylaxis (SUP) is part of the management of critically ill patients in intensive care units (ICUs). However, inappropriate use of these drugs has important clinical implications such as ventilator-associated pneumonia and Clostridium difficile-associated gastrointestinal tract infections. The overuse of proton pump inhibitors (PPIs) as SUP is a rapidly growing problem globally.
OBJECTIVE. To describe the use of SUP in three selected ICUs in Johannesburg, South Africa (SA).
METHODS. A retrospective, descriptive, contextual study design was used. Data were collected from ICU records of adult patients admitted into these units during the study period (1 August 2013 - 31 October 2013).
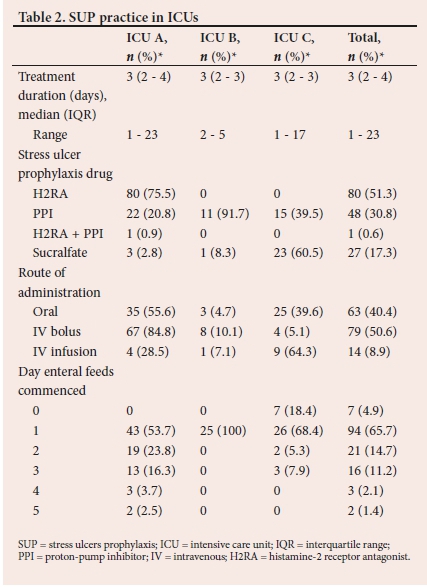
RESULTS. A total of 174 patients were included in the study. Of these, 156 were on SUP and only 38.5% (n=60/156) were appropriately treated with SUP according to the American Society of Health-System Pharmacists guidelines. There was an inappropriate use of SUP in over 50% of those who were treated. The most frequently prescribed SUP was histamine-2 receptor antagonist (H2RA) (51.3%; n=80/156), followed by PPIs (30.8%; n=48/156), sucralfate (17.3%; n=27/156), and a combination of PPI and H2RA (0.6%; n=1/156).
CONCLUSION. The study demonstrated overuse of SUP. The most commonly used drug for SUP was H2RA and not PPIs. This study demonstrates that the problem of SUP overuse internationally also exists locally. The development of local guidelines may help to improve the practice of SUP in SA.
Keywords: stress ulcer prophylaxis, critically ill, proton pump inhibitors, gastrointestinal bleeding
Stress ulcer-related gastrointestinal (GI) mucosal bleeding was a common occurrence in critically ill patients in the past; however, over the years, the prevalence has decreased.[1] This has been attributed to the advancement of medical practice, a better understanding of the conditions that predispose patients to GI stress ulceration and adoption of preventive measures. Stress ulcer prophylaxis (SUP) is one of the cornerstones of the preventive measures used to treat GI ulcers in the critically ill. This has regrettably led to the international trend of SUP overuse.[2,3]
The physiological stress associated with critical illness leads to GI hypoperfusion, accumulation of acid and micro-ischaemia of the upper GI mucosal lining. These changes result in stress-related mucosal disease, which includes erosions, occult bleeding and clinically significant bleeding (CSB). The occurrence of CSB increases the length of hospital stay, medical cost and transfusion requirements.[1] Mechanical ventilation (MV) for at least 48 hours and coagulopathy are the two proven independent major risk factors for developing CSB.[4] Interventions with acid suppressive therapy (i.e. proton pump inhibitors (PPIs) and histamine-2 receptor antagonist (H2RA)) in the high-risk groups can decrease the occurrence of CSB by 50%.[5] The use of SUP in this group of patients is therefore recommended. However, there is a widespread inappropriate use of SUP in patients with low risk of CSB, with the most frequently used agent being PPIs.[3]
There is growing international concern over the overuse of SUP, especially PPIs, as they have been implicated in hospital-acquired infections such as ventilator-associated pneumonia (VAP) and Clostridium difficile-associated GI infections.[6] In a landmark study by Cook et al.,[4] MV for at least 48 hours and coagulopathy were associated with a 3.7% chance of CSB, whereas patients without these risk factors had only 0.1% risk of developing CSB. The American Society of Health-System Pharmacists (ASHP) guidelines[7] recommended that only patients with one major, or two or more minor risk factors should receive SUP. Use outside of these guidelines is therefore deemed as inappropriate in this study. In South Africa (SA), there are no national guidelines governing the prescription of SUP.
The overuse of PPIs as SUP is well described in the literature; however, the use of PPIs for SUP in SA has not been described. The objectives of this study were to describe the use of SUP in three selected intensive care units (ICUs) in Johannesburg, and to identify the risk factors that necessitated the initiation of SUP therapy. The study also sought to describe the use of SUP in accordance with the ASHP guidelines. In addition, we also aimed to determine what constitutes appropriate use of PPIs.
Methods
All complete and legible ICU charts of adult patients admitted into the ICUs of the three selected hospitals affiliated to the University of the Witwatersrand (referred to as ICU A, B and C) were included. We excluded those with existing upper GI bleeding, previous total gastrectomy, on pre-existing PPI therapy prior to ICU admission, patients who died, and those who were discharged 24 hours after admission to ICU. A consecutive and convenience sampling method was used and the number of patients admitted into the ICUs over a 3-month study period (1 August 2013 - 31 October 2013) determined the sample size. These are closed ICUs with both medical and surgical patients.
A data collection sheet was compiled following an extensive literature review ensuring content validity. The data collection sheet captured the following data: patient characteristics, risk factors for CSB, SUP received in ICU, and time to commencement of enteral feeds after starvation. In the current study, the following definitions were adopted: major risk factors were MV for more than 48 hours and coagulopathy (platelet <50 000/mm3; international normalised ratio (INR) >1.5); minor risk factors were acute renal failure, acute hepatic failure, sepsis, hypotension, history of upper GI bleeding, burns >35% of body surface area, hydrocortisone >250 mg/d or equivalent and major surgery: and CSB was overt bleeding associated with haemodynamic changes and decrease in haemoglobin in 24 hours.[4]
The data were collected by NB and entered into a Microsoft Excel spreadsheet (Microsoft, USA). Appropriate use of SUP was regarded as the initiation of SUP in the presence of one or more major risk factors, or the presence of two or more minor risk factors.[4,7] SUP was regarded as inappropriate in the presence of only one minor risk factor or the absence of risk factors. For the purpose of this study, omission of treatment in patients who qualified for SUP was classified as inappropriate SUP therapy.
Approval to conduct the study was obtained from the University of the Witwatersrand Human Research Ethics Committee (ref. no. M130104) and other relevant authorities. A retrospective, descriptive, contextual study design was used.
Stata software version 14 (Stata Corp., USA) was used to analyse data in consultation with a biostatistician. The continuous variables were not normally distributed and were described with medians and interquartile range (IQR). Pearson's χ2 test and Fisher's exact test were used for comparison of appropriate and inappropriate use of SUP. The MannWhitney U-test and Wilcoxon rank-sum test were used for independent and dependent samples, respectively. A p-value <0.05 was considered to be statistically significant.
Results
A total of 174 patients were included in the study. The patients' demographic data are presented in Table 1. A combination of major and minor risk factors were the reasons for the initiation of SUP in the majority (35.6%; n=62) of cases at the three ICUs (Fig. 1). MV was the most frequent major risk factor at the three ICUs (ICU A: n=47/56; ICU B: n=19/23; ICU C: n=24/30). Only 4 patients (ICU A: n=1/56; ICU C: n=3/30) were initiated on SUP because of coagulopathy. There were only 15 patients (ICU A: n=8/56; ICU B: n=4/23; ICU C: n=3/30) initiated on SUP for a combination of both major risk factors.
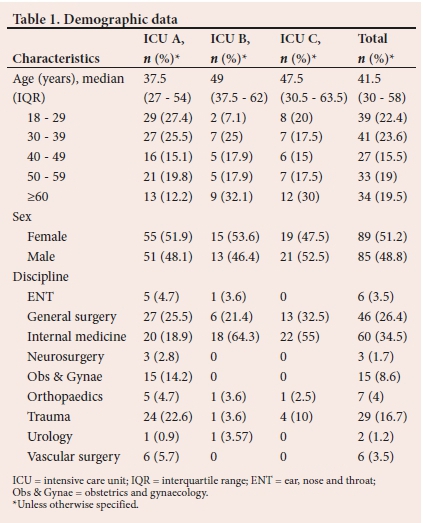
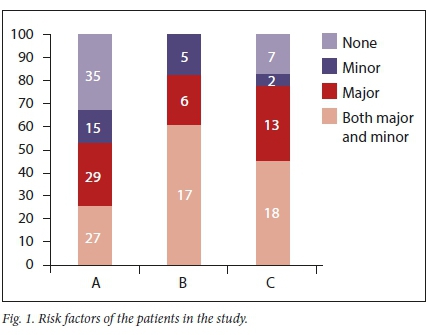
The practice of SUP in the three ICUs is presented in Table 2. A total of 156 patients were on SUP. The characteristics of the patients on SUP and the relationship between the appropriateness of SUP and drugs used are shown in Table 3. PPI use is higher in the inappropriate category (Table 3).
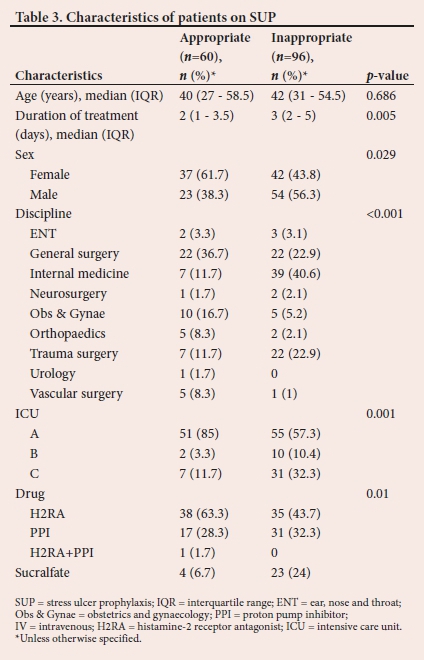
The comparison of prescription practices among the three ICUs shows that the facility influenced the drug prescribed. Inappropriate PPI use was associated with patients from internal medicine, general surgery and orthopaedics/trauma (p<0.001). Of the 174 patients included in the study, 10.3% (n=18) had risk factors for developing CSB and did not receive any prophylaxis.
Discussion
The routine use of SUP in patients who are not at risk of developing CSB is reported to be on the rise in ICUs across the world, with PPIs being the commonly prescribed agents.[8] Factors such as costs, availability of stock and local guidelines influence the prescription practices of SUP agents in the ICU.[9]
PPIs have been proven to be highly effective at suppressing gastric acid secretion by elevating gastric pH.[10] They have also been shown to be the most effective drugs for the prevention of CSB; hence, their widespread use as the agents of choice.[11] There are however other studies stating the contrary, and the ability of PPIs to increase gastric pH may lead to GI bacterial proliferation, which is associated with increased risk of developing infectious complications.[6,12-14] Similarly, we showed in the present study that the most commonly prescribed SUP agent was H2RA and not PPIs. It is evident from the results that locality influenced practice, with ICU A using predominantly H2RA, ICU B using PPIs and ICU C using sucralfate.
A comparison of inappropriate prescription practices among the three ICUs followed similar trends, whereby the facility significantly influenced the drug prescribed inappropriately. ICU A had the highest proportion of these incidents, followed by ICU C. In general, H2RA accounted for a higher proportion of inappropriately used drugs, followed by PPIs and then sucralfate. Internationally, it is reported that the most common inappropriately used agents are PPIs followed by H2RA.[2,3,8]
These locality/facility-related practices are likely to be influenced by drug costs, as our study was conducted in public sector ICUs, where formulations are predominantly driven by price. In addition to the higher costs of PPIs, there is no substantial evidence demonstrating that these agents are more effective at preventing CSB than other groups of SUP drugs,[16] which may influence therapeutic choices in public ICUs.
Inappropriate PPI use by discipline was more common in patients from internal medicine, general and trauma surgery. Krag et al.[17] concluded that since co-existing disease is associated with GI bleeding, patients with co-existing disease have a higher chance of being prescribed SUP on ICU admission. The observational nature of our study limited us from exploring other reasons for initiation of SUP in the absence of risk factors, which would have explained this association between different disciplines.
The ASHP guidelines recommend that only patients with one major, or two or more minor risk factors should receive SUP.[7] The Eastern Association for the Surgery of Trauma also has similar guidelines.[18] Acute renal failure and acute or chronic liver disease have also emerged as potential independent risk factors for GI ulceration[17,19]
Our data revealed that MV was the most common risk factor associated with the initiation of SUP therapy. Coagulopathy was an indication for therapy in only 4.7% of the patients and multiple minor risk factors were the least frequent indication for SUP initiation. These findings are consistent with reports in the literature, where most critically ill patients admitted into ICU require MV at some point in their ICU stay.[19]
Despite global acceptance of guidelines on appropriate prescription practices for SUP therapy in the ICU,[7,18,19] we revealed that 61.5% of patients in the cohort were on SUP without any risk factors for CSB. This is a common finding in international literature and highlights the extent of the problem of overuse globally.[2,3,8] This practice is associated with worse morbidity in patients.[6]
The choices on route of administration mirrored those reported in the literature.[7,18] We had one patient that was on a combination of PPIs and H2RA, which is an unusual combination. The administration of PPIs as a bolus has been demonstrated to have the same efficacy as infusions, with an added advantage of reduced cost.[20,21] The impact of early enteral feeds is visible in ICU B, where all patients commenced these on day 1, resulting in less than 50% of their patients needing SUP.
A concerning factor in this study was that 10.3% of patients who were eligible for SUP according to the guidelines[7] did not receive them. The omission of treatment has a potential of increasing morbidity, mortality and the cost of care. The simultaneous presence of overuse and underuse of SUP reinforces the importance of setting up guidelines or following internationally described guidelines. It is also important that audits of practice be performed regularly to inform these guidelines. Influencing practice in this way will further reduce risk and improve patient care.
In the group that was on SUP, 78% of patients were receiving enteral nutrition from day two of ICU admission. The early introduction of enteral nutrition is an important protective mechanism to maintain gut integrity and reduce the risk of CSB. SUP is not indicated in patients who are on full enteral nutrition.[19-21] Adherence to recommendations to stop pharmacological SUP in patients on full enteral nutrition can therefore further reduce the negative consequences of inappropriate SUP in the critically ill.
Study limitations
We did not look at the rate of occurrence of CSB, which might have provided a more comprehensive view into the use of SUP in our setting. This study was conducted in three selected public sector ICUs affiliated to the University of the Witwatersrand and the findings may not be applicable in the private sector, as drug costs and availability may have a greater influence on the choice of SUP therapy in the public sector. In addition, the contextual nature of this study prohibits its use in describing generalised practices in SA. A national study of practices in the public and private sector ICUs would achieve this endpoint. The implied trend of inappropriately prescribed SUP therapy from our data is however of concern and should encourage discussion and further national research in this area.
This study would also have been strengthened by auditing rates of VAP and Clostridium difficile infection, and their association with SUP use. The literature highlights growing concerns in the association of PPIs and H2RA use with VAP and Clostridium difficile infection in critically ill patients.[22-26] In contrast, a study by Krag et al.[27] found that the number of clinically important events such as CSB, pneumonia and Clostridium difficile infection were similar between those who received 40 mg of pantoprazole and those given placebo as SUP, while the effects of PPI and H2RA on acquiring these infections were inconclusive in another study.[28]
The estimated per case cost of VAP and Clostridium difficile infections in adult in-patient population was USD40 144 and USD11 285 in the USA, respectively. [29] We did not find published data in SA evaluating the cost of hospital-acquired infections. There was also paucity of data reporting SUP and PPI practice in the intensive care environment in SA. In addition, no national practice guidelines for SUP in ICU have been published.
Conclusion
SUP therapy is a well-established intervention for at-risk patients in the ICU. The inappropriate use of these therapies is associated with increased healthcare costs and morbidity in the ICU. Therefore, judicious use of these agents, in line with internationally accepted guidelines, is very important to reduce adverse events.
We identified inappropriate SUP prescription in three ICUs in Johannesburg, SA. Not only should the prescription practices of these university-affiliated ICUs be reviewed, but a larger national study on the use of SUP therapy also needs to be conducted to determine if the identified trend is a national problem. Although the incidence of CSB,
VAP and Clostridium difficile infections in relation to SUP were not in the scope of this study, an investigation into this would be invaluable. The development of a local set of guidelines more suitable to the SA healthcare sector would also be beneficial.
Declaration. This research was done in partial fulfilment of a MMed degree.
Acknowledgements. We would like to thank Dr Palesa Motshabi, academic Head of the Department of Anaesthesia at the University of the Witwatersrand for assisting with the editing of the manuscript.
Author contributions. SC, HP, JS, NB conceptualised the study. NB collected data and MM analysed the data. NB wrote the manuscript and SC, HP, JS and MM revised the manuscript. All the authors approved the manuscript for publication.
Funding. None.
Conflicts of interest. None.
References
1. Krag M, Perner A, Wetterslev J, et al. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med 2015;41(5):833-845. https://doi.org/10.1007/s00134-015-3725-1 [ Links ]
2. Farrell CP, Mercogliano G, Kuntz CL. Overuse of stress ulcer prophylaxis in the critical care setting and beyond. J Crit Care 2010;25(2):214-220. https://doi.org/10.1016/j.jcrc.2009.05.014 [ Links ]
3. Frandah W, Colmer-Hamood J, Nugent K, Raj R. Patterns of use of prophylaxis for stress-related mucosal disease in patients admitted to the intensive care unit. J Intensive Care Med 2014;29(2):96-103. https://doi.org/10.1177/0885066612453542 [ Links ]
4. Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. N Engl J Med 1994;330(6):377-381. https://doi.org/10.1056/NEJM199402103300601' [ Links ]
5. Cook DJ, Witt LG, Cook RJ, Guyatt GH. Stress ulcer prophylaxis in the critically ill: A meta-analysis. Am J Med. 1991;91(5):519-527. https://doi.org/10.1016/0002-9343(91)90189-5 [ Links ]
6. MacLaren R, Reynolds PM, Allen RR. Histamine-2 receptor antagonists v. proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Intern Med 2014;174(4):564-574. https://doi.org/10.1001/jamainternmed.2013.14673 [ Links ]
7. ASHP therapeutic guidelines on stress ulcer prophylaxis. ASHP Commission on Therapeutics and approved by the ASHP Board of Directors on November 14, 1998. Am J Health Syst Pharm 1999;56(4):347-379. https://doi.org/10.1093/ajhp/56.4.347 [ Links ]
8. Barletta JF, Kanji S, MacLaren R, Lat I, Erstad BL. Pharmacoepidemiology of stress ulcer prophylaxis in the United States and Canada. J Crit Care 2014;29(6):955-960. https://doi.org/10.1016/j.jcrc.2014.06.025 [ Links ]
9. Benatar SR. The challenges of health disparities in South Africa. S Afr Med J 2013;103(3):154-155. https://doi.org/10.7196/samj.6622 [ Links ]
10. Hung WK, Li VK, Chung CK, et al. Randomised trial comparing pantoprazole infusion, bolus and no treatment on gastric pH and recurrent bleeding in peptic ulcers. ANZ J Surg 2007;77(8):677-681. https://doi.org/10.1111/j.1445-2197.2007.04185.x [ Links ]
11. Barkun AN, Bardou M, Pham CQ, Martel M. Proton pump inhibitors v. histamine-2 receptor antagonists for stress-related mucosal bleeding prophylaxis in critically ill patients: A meta- analysis. Am J Gastroenterol 2012;107(4):507-520. https://doi.org/10.1038/ajg.2011.474 [ Links ]
12. Lin PC, Chang CH, Hsu PI, Tseng PL, Huang YB. The efficacy and safety of proton pump inhibitors v. histamine-2 receptor antagonists for stress ulcer bleeding prophylaxis among critical care patients: A meta-analysis. Crit Care Med 2010;38(4):1197-1205. https://doi.org/10.1097/CCM.0b013e3181d69ccf [ Links ]
13. Lilly CM, Aljawadi M, Badawi O, et al. Comparative effectiveness of proton pump inhibitors v. histamine type 2 receptor blockers for preventing clinically important gastrointestinal bleeding during intensive care: A population-based study. Chest 2018;154(3):557-566. https://doi.org/10.1016/j.chest.2018.05.015 [ Links ]
14. Krag M, Perner A, Wetterslev J, Moller MH. Stress ulcer prophylaxis in the intensive care unit: Is it indicated? A topical systematic review. Acta Anaesthesiol Scand 2013;57(7):835-847. https://doi.org/10.1111/aas.12099 [ Links ]
15. Toews I, George AT, Peter JV, et al. Interventions for preventing upper gastrointestinal bleeding in people admitted to intensive care units. Cochrane Database Syst Rev 2018;6(6):CD008687. https://doi.org/10.1002/14651858.CD008687.pub2 [ Links ]
16. Australian PIft, New Zealand Intensive Care Society Clinical Trials Group AHSCCSCN, the Irish Critical Care Trials Group, et al. Effect of stress ulcer prophylaxis with proton pump inhibitors vs histamine-2 receptor blockers on in-hospital mortality among ICU patients receiving invasive mechanical ventilation: The PEPTIC randomised clinical trial. JAMA 2020;323(7):616-626. https://doi.org/10.1001/jama.2019.22190 [ Links ]
17. Krag M, Perner A, Wetterslev J, et al. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med 2015;41(5):833-845. https://doi.org/10.1007/s00134-015-3725-1 [ Links ]
18. Guillamondegui OD, Gunter O, Bonadies JA, et al. Practice management guidelines for stress ulcer prophylaxis. Chicago: Eastern Association for the Surgery of Trauma, 2008:1-24. [ Links ]
19. Madsen KR, Lorentzen K, Clausen N, et al. Guideline for stress ulcer prophylaxis in the intensive care unit. Dan Med J 2014;61(3):C4811. [ Links ]
20. El-Kersh K, Jalil B, McClave SA, et al. Enteral nutrition as stress ulcer prophylaxis in critically ill patients: A randomised controlled exploratory study. J Crit Care 2018;43:108-113. https://doi.org/10.1016/j.jcrc.2017.08.036 [ Links ]
21. Huang HB, Jiang W, Wang CY, Qin HY, Du B. Stress ulcer prophylaxis in intensive care unit patients receiving enteral nutrition: A systematic review and meta-analysis. Crit Care 2018;22(1):20. https://doi.org/10.1186/s13054-017-1937-1 [ Links ]
22. Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta-analyses. JAMA 1996;275(4):308-314. https://doi:10.1001/jama.1996.03530280060038 [ Links ]
23. Bateman BT, Bykov K, Choudhry NK, et al. Type of stress ulcer prophylaxis and risk of nosocomial pneumonia in cardiac surgical patients: Cohort study. BMJ 2013;347:f5416. https://doi.org/10.1136/bmj.f5416 [ Links ]
24. Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med 2010;170(9):772-778. https://doi.org/10.1001/archinternmed.2010.73 [ Links ]
25. Tariq R, Singh S, Gupta A, Pardi DS, Khanna S. Association of gastric acid suppression with recurrent Clostridium difficile infection: A systematic review and meta-analysis. JAMA Intern Med 2017;177(6):784-791. https://doi.org/10.1001/jamainternmed.2017.0212 [ Links ]
26. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: Meta-analysis. Am J Gastroenterol 2012;107(7):1011-1019 [ Links ]
27. Krag M, Marker S, Perner A, et al. Pantoprazole in patients at risk for gastrointestinal bleeding in the ICU. N Engl J Med 2018;379(23):2199-2208. https://doi.org/10.1056/NEJMoa1714919 [ Links ]
28. Barbateskovic M, Marker S, Granholm A, et al. Stress ulcer prophylaxis with proton pump inhibitors or histamine-2 receptor antagonists in adult intensive care patients: A systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 2019;45(2):143-158. https://doi.org/10.1007/s00134-019-05526-z [ Links ]
29. Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763 [ Links ]
 Correspondence:
Correspondence:
N Biyase
nana.biyase@yahoo.com
Accepted 6 January 2021.














