Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Southern African Journal of Critical Care (Online)
On-line version ISSN 2078-676X
Print version ISSN 1562-8264
South. Afr. j. crit. care (Online) vol.36 n.1 Pretoria Jan./Jul. 2020
http://dx.doi.org/10.7196/sajcc.2020.v36i1.412
RESEARCH
An evaluation of feeding practices and determination of barriers to providing nutritional support in a multidisciplinary South African intensive care unit
E ElmezoughiI; K de VasconcellosII
IMB ChB, DA (SA), FCA (SA), MMed; OrcID 0000-0001-9140-7460; Department of Anaesthesiology and Critical Care, School of Clinical Medicine, University of KwaZulu-Natal, Durban, South Africa
IIMB ChB, FCA (SA), Cert Crit Care (SA); OrcID 0000-0002-1974-6633; Department of Anaesthesiology and Critical Care, School of Clinical Medicine, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: Adequate nutritional support is crucial to optimising intensive care unit (ICU) outcomes
OBJECTIVES: To assess adherence to current nutritional guidelines in critically ill patients in South Africa (SA). To identify risk factors for non-adherence to guidelines
METHODS: Retrospective observational chart review of nutritional practices, from 1 December 2017 to 31 May 2018, during the first week of ICU admission in adult patients admitted to a tertiary, multidisciplinary ICU in Durban, SA, for >48 hours
RESULTS: The study cohort (N=150) had a median age of 39 years and an ICU mortality of 28%. Surgical patients accounted for 50.7% of admissions. Ninety-eight percent of patients received mechanical ventilation, 75% required inotropic support, and 56% had acute kidney injury. The median time to initiation of enteral nutrition (EN) was 3 days, with EN being initiated within 48 hours in 39% of patients, and by day 7 80% of patients had received EN. Goal feeds were reached in 23% of patients by discharge, death or day 7. Parenteral nutrition was initiated in 16.7% of patients. There was an association between shock, acute kidney injury, increasing sequential organ failure assessment score and inotrope dose, and failure to initiate EN. Failure to initiate EN was predominantly due to unavoidable factors, but a number of clinical and administrative areas were identified to improve EN delivery
CONCLUSION: Adequate nutrition is associated with reduced morbidity, ICU length of stay, mortality and improved functional outcomes. More attention to avoiding barriers to adequate ICU nutrition and enhanced adherence to feeding protocols should be encouraged
Keywords: ICU, chart review, nutritional support.
Adequate nutritional support may improve morbidity and mortality, and long-term functional outcome in critically ill patients. However, there are many potential barriers to the adequate provision of nutrition to critically ill patients. It is unclear whether critically ill patients in South Africa (SA) receive adequate nutrition, and which barriers (if any) may contribute to suboptimal nutrition. Knowledge of the preceding may lead to interventions to improve the nutritional support of critically ill patients in SA.
Critical illness is a catabolic state associated with rapid loss of muscle mass and frequent complications including poor wound healing and infectious complications.[1,2] Critically ill patients are nutritionally 'at risk'.[3] Adequate nutritional support, in particular early enteral nutrition (EN), is associated with a reduction in morbidity, reduced intensive care unit (ICU) length of stay (LOS), improved long-term functional outcomes and, possibly, reduced mortality.[2]
Current practice guidelines agree that EN should be initiated within 48 hours after ICU admission and that early EN is preferred over parenteral nutrition (PN).[1,2] While nutritional goals and the optimal timeframe to achieve these goals are controversial, the most recent European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines recommend that full EN goal feeds are to be prescribed within 3 - 7 days, to avoid the risk of early overfeeding.[1] The timing of initiation of PN is also controversial. Initiation of PN may be delayed up to 7 days in adequately nourished patients.[1] In patients at high nutritional risk PN should be initiated as soon as possible if EN is not feasible.[2] As with EN, full PN goal rates should only be prescribed within 3 - 7 days to prevent early overfeeding. Supplemental PN may be initiated after 7 days if >60% of nutritional requirements have not been achieved.[2]
Despite an increasing awareness of the benefits of adequate nutritional support, nutritional goals are often not met.[1,3] De Jonghe et al.[4] reported that only 71% of required calories were delivered in their cohort of medical ICU patients. Delays in initiation of enteral feeding are common, with studies showing that only 33.3% of patients had feeds initiated within 48 hours of ICU admission and only 9% of patients received 80% or more of goal feeds by day 3 (D3).[5] In a multinational study, Cahill et al.[6] demonstrated that the average time to initiating EN was 46.5 hours, with only 60.8% of ICUs having a mean time to initiation of EN of <48 hours. Only 8.9% of ICUs delivered at least 80% of prescribed calories, with ~70% of prescribed calories being delivered by day seven (D7).[6]
Numerous barriers to feeding critically ill patients have been identified.[6,7] The factors and their relative importance differ according to the study site. Factors include administrative problems, such as a lack of current and user-friendly protocols, a shortage of feeding pumps, unavailability of feeds, and inadequate dietetics support.[8] Other factors are attitudinal, with other components of ICU care viewed as more important than nutritional therapy, doctors failing to initiate feeds, nursing staff failing to increase feeds as per protocol, and the reluctance of surgical colleagues to agree to EN.[8] Clinical factors include haemodynamic instability, need for surgical procedures, lack of enteral or venous access, high gastric residual volumes, feed intolerance and diarrhoea.[8]
The majority of studies investigating the above have been performed in high-income countries.[5,8] While studies have been performed in, or included data from, middle-income countries in Latin America, data from Africa are lacking.[6] Only two SA studies have been conducted to date.[9,10] Of these, one conducted in a multidisciplinary ICU reported a median time to initiation of feeds of 11 hours, with 27% of patients failing to meet at least 90% of their energy target.[9] This study presents findings that do not correlate with large international studies and did not explore barriers to nutritional therapy. The second study was conducted in a trauma ICU and reported that only 63% of patients had initiated EN within 48 hours, with only 63% achieving nutritional goals, from initiation of enteral nutrition, in less than 96 hours.[10] This study, while more in keeping with international findings, did not fully explore barriers to nutritional support and only evaluated trauma patients. There is, therefore, a need to more accurately define the current state of nutritional support in a heterogenous population of critically ill patients in SA, and to identify barriers to adequate nutritional support that may lead to interventions to improve nutritional therapy in SA critical care.
Due to controversies regarding nutritional goals and the timing of reaching these goals, this study focused on the initiation of EN (and barriers to initiating EN), as the goal of providing EN within 48 hours of ICU admission was generally accepted at the time of the study and is still the gold standard following the publication of the 2019 ESPEN guidelines.
Objectives
To provide an overview of nutritional practices in a busy ICU in a relatively resource-limited environment.
Methods
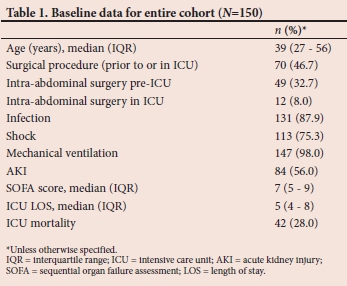
The study was a retrospective observational chart review of patients admitted to the King Edward VIII Hospital ICU, a 12-bed, multidisciplinary, intensivist-run ICU in a tertiary academic hospital in Durban, SA. The charts of all adult patients admitted to the study ICU from December 2017 to May 2018 were screened for inclusion eligibility to the study cohort. Exclusion criteria included children (>18 years old) and an admission duration less than 48 hours. A cohort of adult patients were identified and included in the study (N=150).
Approval for the study was obtained from the University of KwaZulu-Natal Biomedical Research Ethics Committee (BREC ref. no. BE671/17), the study hospital, and the Provincial Health and Research Ethics Committee of the KwaZulu-Natal Department of Health.
All data were obtained from existing patient ICU records, and included demographic data, disease severity data, outcome data and data on nutritional support for the first 7 days of ICU admission or until ICU discharge, whichever occurred first. Reasons for failure to initiate EN were obtained by review of the doctors', nurses' and dieticians' notes and were classified according to specified categories. Shock was defined by the need for inotropic support. Acute kidney injury (AKI) was defined and staged according to the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline.[11] Only the creatinine and renal replacement criteria were used, as patient's weight is generally not accurately known in the ICU and thus urine output data are inaccurate. Baseline creatinine was taken as a lowest known creatinine. ICU disease severity was described using the admission sequential organ failure assessment (SOFA) score.
The study ICU had nutritional guidelines in place at the time of the study. These were devised by the ICU dietician and had been revised in November 2016. While current ESPEN guidelines differ in some respects, they provide the framework within which nutritional support was provided in the study ICU. Briefly, the guidelines were as follows:
• Initiate enteral feeding as soon as the patient is haemodynamically stable, and within 24 to 48 hours post admission in ICU
• Increase feeding rate every 6 to 12 hours
• Aim for goal feeding rate by D3
• Do not monitor gastric residual volumes
• If patient vomits, reduce to previously tolerated rate and start prokinetic agent
• By ICU day 4 (D4), if patient only tolerates <45% of the maximum rate, consider supplementing with parenteral or changing to parenteral feeding.
Decisions on nutritional support were made by the treating intensivist, in consultation with the dietician. The ICU is a closed unit and decisions on surgical contraindications were made by the treating intensivist, although input from the treating surgeon (in the case of surgical patients) was considered. Haemodynamic stability was not specifically defined and was at the discretion of the treating intensivist. Although not specified in the guidelines, EN was not initiated until nasogastric tube position was confirmed radiologically. Unit practice was also to hold EN prior to abdominal or airway surgery.
Failure to provide EN was defined as either failure to initiate EN or failure to deliver EN for an ICU day. Reasons for not providing EN were grouped into the following categories: haemodynamic instability, surgical contraindication, pending extubation or intubation, pending surgical procedure, failure to prescribe EN, failure to follow EN prescription, awaiting confirmation of feeding tube position, and feed intolerance. Each patient could have more than one reason for not being fed. Feed intolerance was defined as vomiting/regurgitation of feed or abdominal distension following EN. The combination of failure to prescribe EN or failure to follow an EN prescription, in the absence of any other reasons for not providing EN, was classified as 'avoidable' failure to provide EN.
Data were captured on an Excel spreadsheet and analysed using SPSS versions 24 and 25 (IBM Corp., USA). Categorical data were analysed using descriptive statistics and presented as numbers and percentages where appropriate. Categorical data were analysed using the Fisher's Exact Test or Pearson's χ1 test as appropriate. Due to predominantly non-Gaussian distribution, continuous data were analysed using descriptive statistics and presented as the median and interquartile range (IQR) and compared using the Mann-Whitney (7-test.
Results
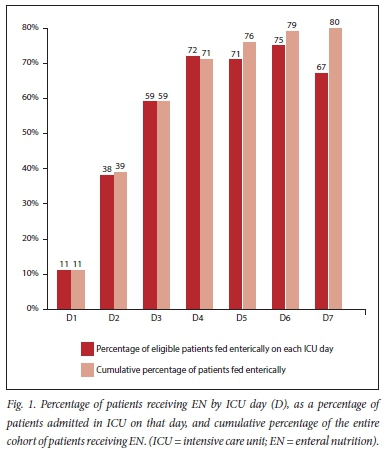
The demographic and clinical data for the entire cohort were captured from the patient charts (Table 1). A total of 80.0% (n=120) of patients received EN during the first week of their ICU stay (Fig. 1). When patients for whom feeding was not initiated due to limitation of life-sustaining therapy were excluded (n=18), the cumulative percentage of patients receiving EN rose to 87.1% (n=115). The median (IQR) time to initiation of feeds was 3 (2 - 4) days, with the mode being 2 days. Patients were not fed for a median period of 2 (1 - 3) days and were fed for 3 (1 - 4.25) days during their ICU admission. In total patients were fed for 375 out of 744 patient days (50.4%). Up to and including day 2 (D2), patients were fed for 72 out of 296 (24.3%) patient days, while after D2 patients were fed for 303 out of 448 (67.6%) patient days. Full enteral goal feeds were reached in 23.4% of patients (n=18) by D7 or ICU discharge (whichever came first). The median (IQR) time to EN goal was 59 (42 - 92) hours.
Parenteral nutrition was initiated in 16.7% of patients (n=25), with the median (IQR) time to initiation of PN being 3 (2 - 4) days. A total of 86.0% of patients (n=129) received EN and/or PN. When patients (n=18) with limitation of life-sustaining therapy were excluded, 92.4% of patients (n=122) received EN and/or PN.
A number of reasons were identified for the failure to initiate EN for each ICU day, where data on the reason/s were available (Fig. 2). On ICU day 1 (D1), 45.8% of patients (n=60) had an 'avoidable' cause for failure to provide EN. On D2, this had reduced to 23.6% of patients (n=21), and on day 5 (D5) only 12.0% patients (n=3) had an 'avoidable' cause for failure to provide EN. With respect to other causes of failure to provide EN, haemodynamic instability was prominent early in the ICU stay, but was superseded by delays while awaiting extubation or theatre later in the ICU stay.
Univariate associations with the early and late initiation of EN by D2 and D7, respectively, were investigated (Table 2). ICU mortality was significantly higher in patients for whom EN had not been initiated by D2 and D7. The presence of AKI or shock was associated with both early and late failure to initiate EN, as was increasing SOFA score and inotropic dose. Surgical procedures were associated with early failure to start EN, but not late failure. The association between reasons for failure to provide EN during the first 24 hours of ICU admission and failure to provide EN on D2 or D7 were also explored as possible predictors of inadequate EN. While haemodynamic instability was associated with failure to provide EN by D2, this was no longer a significant factor on D7. However patients with a surgical contraindication on D1, and those where there was a failure to prescribe EN on D1, were significantly more likely not to have received EN by D2 and D7. Similarly, patients who received EN on D2 were significantly more likely to reach goal feeds than those who did not receive EN on D2.
Discussion
The cohort of patients represented in this study was young, with a high incidence of surgical procedures, sepsis and multiple organ failure. Almost all patients (98%) received mechanical ventilation, with a large proportion having circulatory shock (75%) and AKI (56%). The severity of illness of the cohort is evidenced by a mortality rate of 28% and a median SOFA score of 7. There are geographic differences in critically ill patient populations in terms of both patient and disease profile.[12-14] It is reasonable to hypothesise that these differences may extend to differences in nutritional support practices, which may be due to clinical or administrative differences. This study adds to the limited information on nutritional support practices in critically ill patients in sub-Saharan Africa.
The median time to initiation of EN in this study was 3 days, with EN being initiated within 48 hours in only 39.3% of patients. This contrasts with the study ICU protocol and current guideline recommendations that EN should be initiated within 48 hours of ICU admission. Veldsman et al.[9] reported a median time to EN initiation of 11 hours, while Lofgren et al.[10] reported that 63.3% of patients were fed within 48 hours of ICU admission. In this study, 80% of patients received EN by D7, which is in contrast with the 94% of patients reported by Lofgren et al.[10]There are key differences in the patient cohorts represented in these studies, as Veldsman et al.[9]reported on a predominantly medical population, while Lofgren et al.[10] evaluated trauma patients. These differences were associated with patient demographic and outcome differences, including differences in median age and, lower rates of sepsis, organ support and ICU mortality. Although all three of these studies are from SA, the large variation in nutritional outcomes highlights the need to further explore barriers to EN, and to the best of our knowledge this is the first study to explore these barriers in the region.
Failure to initiate or provide EN for a 24-hour period was predominantly due to reasons that may be considered 'unavoidable' during the first week in ICU. 'Avoidable' reasons for failure to provide EN declined over the course of the first 5 days in ICU. The most common 'unavoidable' reason on D1 and D2 was haemodynamic instability, which became progressively less frequent during the ICU stay. From D3 the most common 'unavoidable' reasons were pending extubation or surgical contraindication and/or awaiting theatre. By D5 and D6 the reasons for these delays were largely due to pending procedures or extubations/intubations. O'Leary-Kelley et al.[3] reported that more than 50% of the feeding interruptions in their cohort were due to planned procedures or extubations. The incidence of feed intolerance was low, peaking at 11.8% on D6. The low rate of feed intolerance maybe due to a nutritional support protocol that does not utilise gastric residual volumes.
While the above reasons for failure to initiate EN are described as 'unavoidable', they are still potential areas for intervention when it comes to improving EN delivery. Haemodynamic instability, while a common contraindication to EN, is poorly defined, and EN is likely to be initiated earlier if a clear definition is used in EN guidelines. With respect to surgical contraindications, ongoing educational efforts, at undergraduate, postgraduate and continuing medical education levels, are required to dispel commonly held surgical myths regarding surgical contraindications. Withholding EN pending theatre or extubation is well-suited to interventions that aim to limit unnecessary delays in this regard. These include prioritising patients from ICU that are booked for theatre and applying appropriate fasting times for appropriate surgical procedures, including not holding EN for non-airway or non-gastrointestinal procedures. It also includes a reassessment of the appropriateness of holding EN (for non-evidence based and variables times) prior to extubation. O'Meara et al.[15]reported that technical issues and waiting to confirm the position of enteral tubes accounted for 25.5%, while in this study the highest rate for confirming tube position was only 7.9% on D2 of admission. EN not being initiated, or held for prolonged periods pending theatre, extubation or radiological confirmation of feeding tube position may be due to organisational delays (e.g. busy emergency theatres and limited radiological support services). Addressing these organisational challenges represents a potential target to reduce unnecessary delays in providing EN. These delays may also be attitudinal and reflect clinical practice where decisions on whether to provide EN are made during ward rounds or at other prespecified times, and are not frequently re-evaluated. These practices would potentially be amenable to ongoing educational processes aimed at prioritising initiation of EN as an important therapeutic goal, and to interventions such as modifying nutritional protocols, especially with respect to implementing liberal, nurse-driven nutritional protocols. This also highlights the need to have dynamic protocols that allow for catch-up on no-feed periods.
The 'avoidable' barriers to EN are important to highlight, as these are potentially readily amenable to intervention. Failure to follow EN prescriptions was relatively uncommon in this study, but should be able to be completely addressed by improved communication within the multidisciplinary team. Most notable, however, was the failure to prescribe EN, which was the predominant single reason for failure to initiate EN on D1 (45.8%). Although the incidence dropped off rapidly from D2, by D5 it still accounted for the failure to initiate EN in 12.0% of patients. The reasons for failing to prescribe EN are speculative but likely include clinicians prioritising other more immediate aspects of clinical care. This is may be addressed by improving educational activities and amending protocols to allow nursing staff to initiate EN as a default. The wording of protocols that allow for EN to be introduced at any point up to 48 hours (or similar wording), should also be reconsidered, with EN protocols being crafted to emphasise initiation of EN at the earliest appropriate opportunity. As an example, guidelines may instead recommend starting EN within 24 hours as opposed to 48 hours. Failure to prescribe EN may also have been due to undocumented contraindications to EN, as it is noted that failure to prescribe EN on D1 is significantly associated with failure to receive EN by D7. This may highlight the complexity of nutritional decision-making in a severely ill cohort of patients but is still a potential action point, as clinicians should be encouraged to document their reasons for not initiating EN.
Patients with AKI and shock were significantly less likely to be receive EN on D2 or D7, and similarly patients not fed on D2 or D7, had significantly higher SOFA score and inotropic requirements. This suggests a strong association with increasing severity of illness and failure to initiate EN. There is also a significant association between failure to initiate EN at D2 or D7 and increased ICU mortality. While this may represent evidence of failure to provide EN leading to increased mortality, it is most likely a reflection of the above finding of increased disease severity resulting in failure to initiate EN. While some of this likely to be due to haemodynamic instability, it may be due to other reasons, including physician perceptions of futility. This is evidenced by the finding that limitation of life-sustaining therapy is associated with significantly lower rates of initiation of EN on both D2 and D7. However, a word of caution is worthwhile here as failure to provide nutrition to the most severely ill patients out of concern for a poor prognosis, may become a self-fulfilling act. Undergoing any surgical procedure was associated with an increased risk of early failure to initiate EN but not with late failure to initiate EN. However, patients referred from surgical disciplines were more likely not to have EN initiated by D7, suggesting an association between failure to initiate EN and the patient's underlying pathology.
Finally, it is worth noting that patients who received EN on D2 were significantly more likely to reach their EN goal. While this may be simply due to the absence of unavoidable factors, it may indicate that early EN, and adhering to EN guidelines, leads to an increased chance of meeting EN goals.
Study limitations
This was a retrospective analysis and potentially subject to the inherent biases associated with retrospective studies. The study was based upon a single centre, which may limit external validity. However, the study ICU is a busy referral centre for the second most populous province in SA as well as being a multidisciplinary ICU with a relatively even mix of patients. Therefore, the results are likely to be reflective of critical care in many multidisciplinary ICUs in the region. Issues of severely ill patients, limited numbers of dieticians and administrative delays in theatre and radiology are not well described academically but are familiar to most practising clinicians. It may be argued that given the nature of the severely ill patient cohort, EN would have been inappropriate in many patients de novo, and the data are thus skewed and overly negative. The aim of the study was not to find fault in the nutritional practices of the study ICU, but to describe the real-world application of nutritional support in an underexplored context. It has achieved this, and further interventional studies are required to further explore whether the barriers to nutritional support that have been identified are amenable to interventions or are unmodifiable factors in the clinical context. It may also be argued that the cohort is too heterogenous and that it is best to evaluate specific subsets of patients (e.g. medical versus surgical patients). Current international nutritional targets such as the recommendation to provide EN within 48 hours, are however generally interpreted as a standard target for critically ill patients, and as such it is reasonable to explore whether a multidisciplinary cohort of critically ill patients can achieve these targets, and if not, why not.
Recommendations
This study has highlighted potential areas for intervention that are applicable to both the study ICU and similar ICUs in SA, namely attempts to reduce administrative delays, the need for user-friendly, dynamic nutritional protocols, and ongoing in-service training to emphasise the importance of nutritional support. Further studies (preferably multicentre) need to be conducted in a variety of critical care settings in SA to document nutritional practices, identify barriers to achieving nutritional goals, and evaluate the effect of interventions aimed at improving nutritional support in the critically ill.
Conclusion
Most critical care nutritional research is conducted in higher-income countries, and as a consequence most guidelines are based on these data. This study significantly adds to the limited data available from sub-Saharan Africa on nutritional practices in critical care, and in particular barriers to provision of EN.
Adequate nutrition in critically ill patients has been associated with reduced morbidity, ICU length of stay, and mortality and improved functional outcomes. Early EN is important in meeting patients' nutritional goals. This study highlighted frequent delays in providing EN, and a low rate of attainment of EN goals, in a multidisciplinary ICU with a high severity of illness. Many barriers to providing EN were identified, which allowed for several potential areas of intervention to be identified. These include improving EN protocols, reducing systems delays in confirming feeding tube position, performing surgical procedures and extubating patients, and revisiting preconceived notions of contraindications to feeding and reasons for holding EN.
Declaration. This manuscript was submitted as partial fulfilment of the requirements for a Master of Medicine (Anaesthesia), at the University of KwaZulu-Natal.
Acknowledgements. None.
Author contributions. EE contributed to study design, data collection, drafting of the manuscript and editing the manuscript. KdV contributed to study design, data analysis, drafting of the manuscript and editing of the manuscript.
Funding. None.
Conflicts of interest. None.
References
1. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019;38(1):48-79. https://doi.org/10.1016/j.clnu.2018.08.037 [ Links ]
2. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). J Parenter Enteral Nutr 2016;40(2):159-211. https://doi.org/10.1177/0148607115621863 [ Links ]
3. O'Leary-Kelley CM, Puntillo KA, Barr J, Stotts N, Douglas MK. Nutritional adequacy in patients receiving mechanical ventilation who are fed enterally. Am J Crit Care 2005;14(3):222-231. [ Links ]
4. De Jonghe B, Appere-De-Vechi C, Fournier M, et al. A prospective survey of nutritional support practices in intensive care unit patients: What is prescribed? What is delivered? Crit Care Med 2001;29(1):8-12. [ Links ]
5. Stewart ML, Biddle M, Thomas T. Evaluation of current feeding practices in the critically ill: A retrospective chart review. Intensive Crit Care Nurs 2017;38:24-30. https://doi.org/10.1016/j.iccn.2016.05.004 [ Links ]
6. Cahill NE, Dhaliwal R, Day AG, Jiang X, Heyland DK. Nutrition therapy in the critical care setting: What is "best achievable" practice? An international multicenter observational study. Crit Care Med 2010;38(2):395-401. https://doi.org/10.1097/ccm.0b013e3181c0263d [ Links ]
7. Kim H, Stotts NA, Froelicher ES, Engler MM, Porter C. Why patients in critical care do not receive adequate enteral nutrition? A review of the literature. J Crit Care 2012;27(6):702-713. https://doi.org/10.1016/j.jcrc.2012.07.019 [ Links ]
8. Cahill NE, Murch L, Cook D, Heyland DK, Group CCCT. Barriers to feeding critically ill patients: A multicenter survey of critical care nurses. J Crit Care 2012;27(6):727-734. https://doi.org/10.1016/j.jcrc.2012.07.006 [ Links ]
9. Veldsman L, Richards GA, Blaauw R. The dilemma of protein delivery in the intensive care unit. Nutrition 2016;32(9):985-988. https://doi.org/10.1016/j.nut.2016.02.010 [ Links ]
10. Löfgren E, Mabesa T, Hammarqvist F, Hardcastle T. Early enteral nutrition compared to outcome in critically ill trauma patients at a level one trauma centre. S Afr J Clin Nutrition 2015;28(2):70-76. https://doi.org/10.1080/16070658.2015.11734534 [ Links ]
11. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120(4):c179-c184. https://doi.org/10.1159/000339789 [ Links ]
12. Vincent JL, Marshall JC, Namendys-Silva SA, et al. Assessment of the worldwide burden of critical illness: The intensive care over nations (ICON) audit. Lancet Respir Med 2014;2(5):380-386. https://doi.org/10.1016/s2213-2600(14)70061-x [ Links ]
13. Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet 2010;376(9749):1339-1346. https://doi.org/10.1016/s0140-6736(10)60446-1 [ Links ]
14. Elhouni AA, de Vasconcellos K. The utility of hyperlactataemia in the definition of septic shock: Evaluating the Sepsis-3 definitions in a sub-Saharan African intensive care unit. S Afr Med J 2019;109(11):880-884. http://doi.org/10.7196%2FSAMJ.2019.v109i11.13968 [ Links ]
15. O'Meara D, Mireles-Cabodevila E, Frame F, et al. Evaluation of delivery of enteral nutrition in critically ill patients receiving mechanical ventilation Am J Crit Care 2008;17(1):53-61. [ Links ]
 Correspondence:
Correspondence:
E Elmezoughi
beladalandalus@yahoo.com
Accepted 14 January 2020
Contribution of study
This study significantly adds to the limited data available from sub- Saharan Africa on nutritional practices in critical care, and in particular barriers to provision of EN. It is further anticipated that the findings of the study will contribute in making recommendations in an attempt to improve the outcomes














