Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Southern African Journal of Critical Care (Online)
On-line version ISSN 2078-676X
Print version ISSN 1562-8264
South. Afr. j. crit. care (Online) vol.32 n.1 Pretoria Jan./Jul. 2016
http://dx.doi.org/10.7196/sajcc.2016.v32i1.243
ARTICLE
Reducing paediatric ventilator-associated pneumonia - a South African challenge!
H KunzmannI; K DimitriadesII, III; B MorrowIV; A ArgentV, VI
IBCur, PG Dip (Nursing Admin), PG Dip (Critical Care Child); Department of Critical Care, Red Cross War Memorial Children's Hospital, Cape Town, South Africa
IIMB ChB, FCPaed (SA), MMed (Paeds), Cert Crit Care (Paed); Department of Critical Care, Red Cross War Memorial Children's Hospital, Cape Town, South Africa
IIIMB ChB, FCPaed (SA), MMed (Paeds), Cert Crit Care (Paed); Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Cape Town, South Africa
IVBSc (Physiotherapy), PG Dip (Health Research Ethics), PhD (Paediatric Crit Care); Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Cape Town, South Africa
VMB BCh, FCPaed (SA), MMed (Paed), Cert Crit Care (Paed) Department of Critical Care, Red Cross War Memorial Children's Hospital, Cape Town, South Africa
VIMB BCh, FCPaed (SA), MMed (Paed), Cert Crit Care (Paed) Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Cape Town, South Africa
ABSTRACT
There has been a decline in ventilator-associated pneumonia (VAP) in the paediatric intensive care units of developed countries. Previous studies at the Red Cross War Memorial Children's Hospital give an incidence of VAP of >40/1 000 ventilator days, identifying VAP as a priority area for practice improvement. We outline the process and outcome of a practice improvement initiative that implemented an evidence-based bundle of care to reduce VAP. In 2011, this initiative was taken to improve healthcare-associated infections, with the support of the 'Best Care Always' project. A task team identified an evidence-based bundle of care aimed at reducing VAP. The bundle consisted of five elements that were adjusted practically to suit the unit. Standardised metrics to measure compliance with the bundle and outcomes of the intervention were instituted and collected prospectively throughout the study period. Following implementation in October 2011, VAP rates decreased from 55/1 000 to 19.1/1 000 ventilator days over the first 5-month period. During this period, compliance remained poor and metrics were poorly collected. With the introduction of a full-time VAP coordinator, compliance improved from 57% to a peak of 83%, with a decrease in VAP to an average of 4/1 000 ventilator days (January 2013 - July 2013). This practice improvement initiative resulted in a significant reduction in VAP. The success of this initiative is attributed equally to the introduction of the bundle of care and driving power of the VAP coordinator.
Ventilator-associated pneumonia (VAP) is a nosocomial pneumonia that develops in ventilated patients after 48 hours of intubation.[1] Approximately 1 400 children are admitted annually to the paediatric intensive care unit (PICU) of Red Cross War Memorial Children's Hospital (RCWMCH), Cape Town, South Africa (SA). Many of these children (~1 000) require intubation and mechanical ventilation, which places them at risk of developing VAP. Previous studies at this study site showed the incidence of VAP to be high (>40/1 000 ventilator days), with an association between VAP and both standardised mortality and morbidity.[2,3] These findings are in contrast to reports of VAP rates between 2% and 6% from PICUs in developed parts of the world such as the USA and Europe.[4]
As a result of local reports, VAP was identified as a priority focus area and a practice improvement initiative targeting VAP was commenced in the PICU in conjunction with the 'Best Care Always' (BCA) project, a campaign promoting the use of evidence-based practice to improve quality of care. The objective of this initiative was to develop and implement an evidence-based bundle of care to reduce VAP. It was considered important that the bundle of care should be relevant and practical for this specific PICU environment, to ensure global and sustainable implementation. An additional objective was to monitor and evaluate VAP incidence throughout the implementation period.
Context
RCWMCH is a tertiary-level academic paediatric hospital situated in Cape Town, SA, with a 22-bed PICU. A large proportion of admitted children are referred via the emergency medicine department for the management of infectious diseases such as gastroenteritis and pneumonia. The remainder of the patient load consists of a mix of postoperative patients, including neurosurgical, cardiac and general surgical patients, and general paediatric patients. Particular challenges in implementing practice improvement initiatives in this environment include the high patient turnover and a relatively low nurse-to-patient ratio (with about 100 nurses in total). Additionally, the pool of about 13 doctors consists of 5 consultant intensivists and includes up to 5 registrars who rotate every 3 months, necessitating regular retraining and education.
Change implementation strategies and process
In May 2011, the chief executive officers and management of selected tertiary and secondary hospitals in the Western Cape Province of SA attended a BCA project information session addressing hospital-acquired infections (HAIs). The project aim was to reduce HAIs using care bundles developed by the Institute of Healthcare Improvement. Subsequent to this meeting, teams were launched at individual hospitals to implement the care bundles. The team tasked with implementing VAP bundles in the RCWMCH PICU consisted of the head of the clinical unit, a clinical technologist, an operational manager and a research consultant. Initial brainstorming and discussion led to the development and testing of an adapted VAP bundle. Consensus was reached that a modification of the Clinical Pulmonary Infection Score (CPIS) would be used to measure outcome, as this tool was validated and appropriate for use in the setting.[3] This tool was subsequently used throughout the study period. A CPIS score of >6 was considered diagnostic of VAP if: (i) the patient was ventilated more than 48 hours; (ii) in a patient with a high CPIS score on admission, the CPIS dropped by at least 3 points for 1 day or 2 points for 2 consecutive days before rising to >6; or (iii) in a patient previously diagnosed with VAP, the score decreased to <5 for at least 2 days before rising to >6. A registered nurse (the 'VAP Champion') and four enrolled nurses formed a five-person team to drive the practice improvement initiative in the PICU.
Following discussion and consensus, and using evidence from healthcare literature, it was decided that in addition to strict adherence to PICU infection control policies, the VAP bundle should consist of five elements:
(i) Elevating the head of the bed to 30°, on the basis of the results of an adult randomised controlled trial by Drakulovic et al.[5] An exception to this criterion was if it was contraindicated by the medical team in postoperative cardiac or neurosurgical cases. Children nursed prone or in incubators and children receiving high-frequency oscillatory ventilation would be nursed at 10° elevation, for logistical reasons. The team approach allowed for the troubleshooting of difficulties as they arose. For example, when elevating the head of the bed children tend to slip down the bed; elevating the foot of the bed and positioning a pillow under the buttocks countered this.
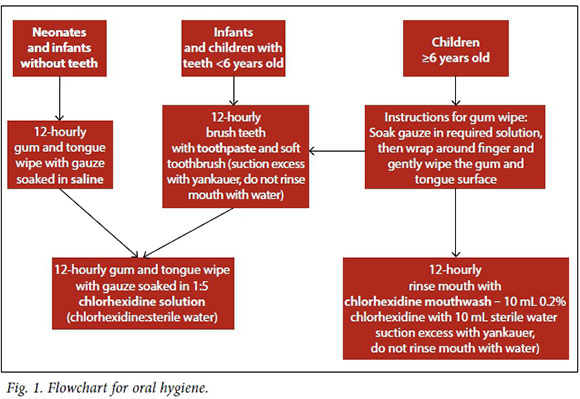
(ii) Appropriate mouth care provided to all children, based on previous recommendations.[6] Taking into consideration staffing levels, with an average of one registered nurse to two patients with the assistance of either an enrolled nurse or assistant nurse, the VAP team adjusted mouth care frequency to 6-hourly. Some adjustments in the use of chlorhexidine gluconate for different age groups were also made (Fig. 1).
(iii) Checking naso- and orogastric tubes 3 - 4-hourly to confirm position in the stomach and marking feeding tubes to allow early detection of malpositioning, to reduce the risk of aspiration, one of the known causes of VAP.[7] Marking of feeding tubes was taught to nursing staff to be performed as standard care after confirming the tube position in the stomach. The tube position was checked by either measuring a pH of between 1 and 5 of 0.5 mL gastric aspirate using a universal pH indicator strip, or by chest X-ray (including the upper abdomen).
(iv) No saline to be instilled routinely in the endotracheal tube prior to suctioning, as this may result in dispersion of contaminated material in the lower respiratory tract, increasing the risk of nosocomial infection.[8] There are no physiological benefits to using saline with suctioning, and saline instillation is associated with hypoxia.[9] Standard practice was that saline was not routinely used during endotracheal suctioning; however, this practice had not been formalised prior to the initiative.
(v) The ventilator tubing positioned in such a manner that the condensed water could run freely away from the patient into the water trap. This required ongoing inservice training and repetitive demonstration at the bedside to improve compliance.
Prior to the practice improvement intervention, children were generally nursed flat or, on rare occasions, with the head of bed raised, but this seldom reached 30°. Mouth care was performed 4-hourly on children of all ages by wiping the inside of the mouth with gauze dipped in saline. Positioning of naso- and orogastric tubes was tested prior to commencing feeds or administering medication, but they were not marked to indicate if the tube migrated. The ventilator tubing was usually placed in a position that prevented the condensed water from draining freely into the water trap.
From October 2011, infection control improvement measures and the VAP bundle were implemented through group training and teaching of all staff categories, and VAP compliance was monitored using a standardised form. Disposable ventilator circuits were introduced into the unit and the PICU doctors were required to complete a VAP identification form (including CPIS score[3]) (Fig. 2) daily on each intubated and ventilated patient during the morning ward round.
For the first 4 months of the practice improvement initiative, data were obtained in a standardised manner, but collection was unreliable and compliance with the bundle was poor. Although the VAP rates decreased initially, they plateaued at a higher level than the target. The five-member team had little time to teach and monitor the staff owing to their own full-time patient care responsibilities. Teaching and monitoring was difficult as the shift patterns of these five members had to coordinate to ensure that each nursing shift was covered by at least one team member. Obtaining buy-in from the whole PICU team was challenging, as there was no sense of urgency to address the unacceptably high VAP rate. This was compounded by natural resistance to change, with many nurses asking, 'Why do we need to do it that way if we've been doing it this way all these years?'
It was clear that there was a need to change our implementation approach. The need for a full-time VAP coordinator, with protected time and without patient responsibilities, was identified. A coordinator would educate, monitor and observe that staff adhere to the VAP bundle and reliably report VAP incidence, developing sustainable processes. This was motivated to hospital management on the basis that preventing VAP would save costs from patient morbidity and mortality, PICU bed occupancy and staff load. In an environment where there is substantial pressure on beds, decreasing the incidence of VAP could potentially increase the number of patients the PICU could manage per annum. Management balanced this against the loss of a senior member of nursing staff from clinical duties in the context of already limited numbers of experienced critical care nurses.
The motivation for a full-time VAP coordinator was accepted and a VAP coordinator (a senior registered nurse) was appointed fulltime for an initial 4-week period in February 2011, followed by dedicated time for weekly input.
With the opportunity to focus only on implementation of the practice improvement initiative and with flexible working hours, it was possible to standardise the VAP bundle and the desired outcome of each element. The bundle elements were taught to each staff member individually, on day and night shifts, using a one-on-one teaching method at the bedside and practical assistance in executing each bundle element.
In addition to one-on-one teaching, information brochures and posters advising on the extent of VAP and the bundle elements to address the problem were made widely available throughout the PICU. Feedback was obtained from nursing staff about the practical barriers to implementing the bundle elements. The VAP coordinator used the feedback to adjust the recommendations using a 'Plan, Do, Study, Act' (PDSA) cycle, to optimise compliance.
An example of PDSA cycle use was in addressing the bundle element of raising the head of the bed to 30°. The initial planning suggested using a 40° triangle to compensate for the 10° tilt at the foot end of the bed; however, this was found to be time-consuming and there was confusion about exactly where the angle should be measured. In addition, the reference triangles were often not returned and difficult for the next user to locate. We found that 30° was almost always underestimated without use of the triangle and therefore compliance was not obtained. The VAP coordinator suggested using the triangle on admission to determine the correct 30° elevation and then applying a piece of string to the top crossbar of the bed, marking the string at the point obtained when the head of bed was elevated correctly. This was practically demonstrated to each nurse individually and the ability of the nurses to implement the procedure then was checked. This small adjustment ensured an improvement in compliance with the head of bed elevation element of the VAP bundle.
Following the appointment of the VAP coordinator, daily compliance on every ventilated patient was assessed, using a standardised tool. This created the opportunity to identify possible obstacles to VAP compliance and made it easier to address these obstacles early. Information on VAP compliance and number of VAP cases was fed back to the unit at weekly meetings and graphic representations were posted on the notice board. For full compliance, adherence to all five bundle elements had to be maintained and a target of 90% compliance was set, similar to previous studies.[10]
VAP cases were reported, using standardised tools, at the same time each day. Initially, cases were recorded daily on one page without individual CPIS scores. However, this was not practical as it was difficult to determine the daily change in individual scores necessary to confirm the diagnosis. The form was changed to a 7-day page that was prepacked in the admission booklet and filled in on the ward round. This made it easy to compare scores for each day of the patients' admission. The forms were removed from the pack on Fridays and new ones inserted for the following week (Fig. 2).
Outcomes
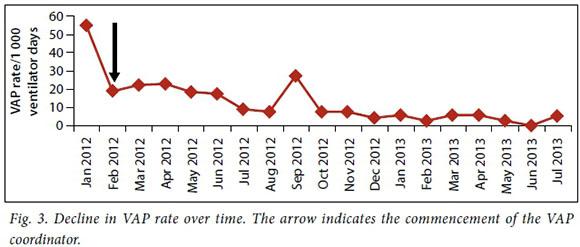
Data were collected from October 2011 to July 2013. After the introduction of the initiative in October 2011, an initial reduction in VAP rates was noted, from 55/1 000 to 19.1/1000 ventilator days (Fig. 3), despite poor compliance with the bundle. This coincided with the introduction of disposable ventilator circuits to replace the reusable circuits that were in use at the time, as well as a change in the packaging of intubation equipment in the hospital. However, this decline in VAP rates plateaued and only gradually improved following the introduction of the full-time VAP coordinator. Thereafter, VAP rates continued to decline steadily, with a noticeable reduction after June 2012 with the reappointment of the full-time VAP coordinator. In September 2012, a spike in VAP rates was noted during the unit's routine quality assurance audit. This prompted an aggressive re-education drive by the coordinator on the VAP bundle implementation Compliance to the bundle was measured on a daily basis at each bedside. This daily measurement was commenced by the VAP coordinator and continued during the period where the coordinator was no longer present (March 2012 - June 2012) (Fig. 4). The figure indicates that with an identified VAP coordinator, compliance to the VAP bundle steadily improved and this mirrored a steady decrease in VAP incidence.
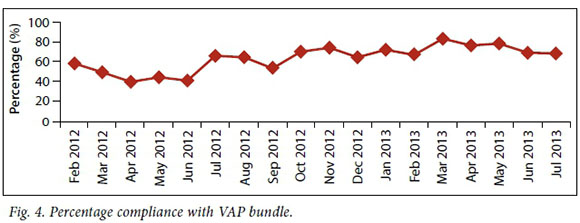
Achieving optimal compliance to the VAP bundle remains a challenge. Current challenges to achieving full compliance include shortages of consumables required for mouth care, faulty beds, and high turnover of staff resulting in an ongoing need to educate new staff about the VAP bundle. It is of concern that this process requires constant supervision and has not yet reached a self-sustaining point in the unit.
Lessons and messages
Prior to appointing a VAP coordinator, data collection was unreliable, compliance was poor and VAP rates high. Dividing attention between patient care and the VAP practice improvement process was a major obstacle to the implementation of the initiative. After a VAP coordinator with protected time was appointed, it was possible to develop processes to ensure the collection of reliable data to measure VAP bundle compliance, ventilated days and VAP identification. The proportion of beds fully compliant to all VAP bundle elements peaked at 80% (with a target of 90%) and the VAP rate dropped significantly.
In a resource-constrained environment, investment in dedicated staff could be cost-effective when balanced against the considerable saving in healthcare costs and the improvements in patient outcomes achieved by sustained and marked reductions in healthcare-associated infections. This was noted by Rello et al.[11] in 2002 and used as the basis for marketing the use of a VAP 'champion' to hospital management by Craven[12] in 2006. The use of a VAP champion is integral in coordinating the efforts of the team responsible for implementing the components of the VAP bundle. Other duties identified in literature include setting benchmarks and continuous use of the PDSA cycle to meet the set benchmarks.[12-14]
A large number of changes were made over the period under study, which resulted in significantly fewer cases of VAP. The implementation of the VAP prevention bundle required the introduction of a VAP champion to sustain these improvements.
Acknowledgements. We would like to thank Michele Youngleson and Gary Kantor for leading the Best Care Always initiative.
References
1. Morrow BM, Argent AC, Jeena PM, Green RJ. Guideline for the diagnosis, prevention and treatment of paediatric ventilator-associated pneumonia. S Afr Med J 2009;99(4):255-267. [ Links ]
2. Morrow BM, Argent AC. Ventilator-associated pneumonia in a paediatric intensive care unit in a developing country with high HIV prevalence. J Paediatr Child Health 2009;45(3):104-111. DOI:10.1111/j.1440-1754.2008.01437.x [ Links ]
3. Morrow BM, Mowzer R, Pitcher R, Argent AC. Investigation into the effect of closed-system suctioning on the frequency of pediatric ventilator-associated pneumonia in a developing country. Pediatr Crit Care Med 2012;13(1):e25-e32. DOI:10.1097/pcc.0b013e31820ac0a2 [ Links ]
4. Rosenthal VD, Bijie H, Maki DG, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004 - 2009. Am J Infect Control 2012;40(5):396-407. DOI:10.1016/j.ajic.2011.05.020 [ Links ]
5. Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogué S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: A randomised trial. Lancet 1999;354(9193):1851-1858. DOI:10.1016/s0140-6736(98)12251-1 [ Links ]
6. Johnstone L, Spence D, Koziol-McClain J. Oral hygiene care in the pediatric intensive care unit: Practice recommendations. Pediatr Nurs 2010;36(2):85-96. [ Links ]
7. Charles MP, Kali A, Easow JM, et al. Ventilator-associated pneumonia. Australas Med J 2014;7(8):334-344. DOI:10.4066/amj.2014.2105 [ Links ]
8. Freytag CC, Thies FL, König W, Welte T. Prolonged application of closed in-line suction catheters increases microbial colonization of the lower respiratory tract and bacterial growth on catheter surface. Infection 2003;31(1):31-37. DOI:10.1007/s15010-002-3066-1 [ Links ]
9. Morrow BM, Argent AC. A comprehensive review of pediatric endotracheal suctioning: Effects, indications, and clinical practice. Pediatr Crit Care Med 2008;9(5):465-477. DOI:10.1097/pcc.0b013e31818499cc [ Links ]
10. Weireter LJ, Collins JN, Britt RC, Reed SF, Novosel TJ, Britt LD. Impact of a monitored program of care on incidence of ventilator-associated pneumonia: Results of a long term performance-improvement project. J Am Coll Surg 2009;208(5):700-705. DOI:10.1016/j.jamcollsurg.2009.01.041 [ Links ]
11. Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 2002;122(6):2115-2121. DOI:10.1378/chest.122.6.2115 [ Links ]
12. Craven DE. Preventing ventilator-associated pneumonia in adults: Sowing seeds of change. Chest 2006;130(1):251-260. DOI:10.1378/chest.130.1.251 [ Links ]
13. O'Keefe-McCarthy S, Santiago C, Lau G. Ventilator associated pneumonia bundled strategies: An evidence based practice. Worldviews Evid Based Nurs 2008;5(4):193-204. DOI:10.1111/j.1741-6787.2008.00140.x [ Links ]
14. Sen S, Johnston C, Greenhalgh D, Palmieri T. Ventilator associated pneumonia prevention bundle significantly reduces the risk of ventilator-associated pneumonia in critically ill burn patients. J Burn Care Res 2014;37(3):166-171. DOI:10.1097/BCR.0000000000000228 [ Links ]
 Correspondence:
Correspondence:
B Morrow
brenda.morrow@uct.ac.za














