Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Southern African Journal of Critical Care (Online)
On-line version ISSN 2078-676X
Print version ISSN 1562-8264
South. Afr. j. crit. care (Online) vol.31 n.2 Pretoria Nov. 2015
http://dx.doi.org/10.7196/SAJCC.2015.V31I2.233
CASE REPORT
Atrial myxoma-related embolism resulting in acute limb ischaemia in a critical care patient
M KnightI; R D WiseII, III
IMA, MB ChB (Hons), MRCEM, MRCP;Department of Anaesthetics, Critical Care, and Pain Management, Edendale Hospital, Pietermaritzburg, South Africa
IIMB ChB, FCA (SA), Cert Crit Care (SA), MMed (Anaes), Dip Obst (SA), Dip PEC (SA); Perioperative Research Group, Discipline of Anaesthesiology and Critical Care, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIIMB ChB, FCA (SA), Cert Crit Care (SA), MMed (Anaes), Dip Obst (SA), Dip PEC (SA); Department of Anaesthetics, Critical Care, and Pain Management, Edendale Hospital, Pietermaritzburg, South Africa
ABSTRACT
This report presents an unusual case of limb ischaemia in the critical care setting, the cause of which was elucidated on echocardiography. Evaluation of the case highlights the importance of appropriate and timely investigation, in particular the role of bedside echocardiography. Although atrial myxomas are uncommon, a thorough investigation of patients presenting with acute peripheral ischaemic events should be undertaken to facilitate the diagnosis of this treatable condition.
We report an interesting case of a patient presenting with a constellation of symptoms and signs, all of which may be attributable to an underlying left atrial myxoma. The availability of bedside echocardio-graphy in the intensive care unit (ICU) permitted the timely evaluation and diagnosis of this rare cardiac lesion.
Case report
A 41-year-old male suffered a sudden loss of consciousness at home while eating dinner. He had no complaints of preceding symptoms and had been well earlier that day. He remained unconscious and was transferred to a regional hospital where he was found to have signs of left ventricular failure. His initial management, including tracheal intubation, occurred in the Emergency Department, after which he was transferred to the ICU for ventilatory support.
It was discovered in his history and in discussion with his family that he had recently been admitted to hospital following a non-ST elevation myocardial infarction (NSTEMI) and was awaiting outpatient cardiology follow-up. A provisional diagnosis of dilated cardiomyopathy secondary to excess alcohol intake had been made based on clinical and radiographic assessment; however, echocardiographic evaluation had not yet been performed owing to resource limitations. Over the preceding month he had also been complaining of progressive dyspnoea.
Clinical examination on this admission revealed signs of chronic alcohol consumption including parotid enlargement and hepatomegaly. He was noted to have a laterally displaced, thrusting apex beat and a grade 3/6 early diastolic murmur at the lower left sternal edge, with no other added sounds. His electrocardiogram showed lateral T-wave inversion and cardiac enzymes were markedly raised, in keeping with a further NSTEMI. Throughout this, however, he remained haemodynamically stable. Despite a low Glasgow Coma Scale score, he did not have any localising neurological signs, or signs or history of recent toxin ingestion. On investigation, he did not display any metabolic or electrolyte derangements at the time of his admission; however, thrombocytopenia and a prolonged PT were noted in keeping with chronic alcohol use.
He was treated for an NSTEMI but could not be transferred to the local tertiary hospital's coronary care unit owing to a bed shortage. Two days after ICU admission acute ischaemia of his right leg developed; an emergency above-knee amputation took place without complication.
A bedside transthoracic echocardiogram (TTE) performed in ICU following the operation identified a 4 cm x 3 cm highly mobile polypoid mass in the left atrium that could be seen prolapsing into the mitral valve orifice (Fig. 1), in keeping with an atrial myxoma. Two smaller, immobile masses attached to the atrial septum were also seen, which most likely represented atrial thrombi. These were absent on subsequent studies. Further echocardiographic evaluation revealed dilated and poorly contractile ventricles and severe aortic regurgitation.
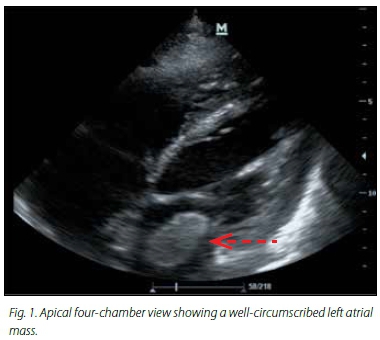
The patient's condition continued to deteriorate. He developed an acute kidney injury and his level of consciousness failed to improve despite the discontinuation of sedative agents. A computed tomography scan of his head identified several infarcts in the territories of the left middle cerebral artery, the left and right posterior cerebral artery, and lacunar infarcts in the left basal ganglia and right thalamus. He was considered unsuitable for surgical resection of the tumour and unfortunately he died with multiorgan failure 6 days after his ICU admission.
Discussion
Although rare, with an annual incidence of 0.5 per million,[1] atrial myxomas are the most common of the benign cardiac tumours, accounting for approximately half of identified cases. Seventy-five percent of myxomas occur in women and the majority of patients are aged between 30 and 60 years at diagnosis.[2] Most cases are sporadic; however, familial cardiac myxomas may occur, in association with conditions such as the Carney Complex. This autosomal dominant condition results from a germline mutation in the PRKAR1A gene involved in regulating cell proliferation, and is characterised by skin pigmentation, endocrinopathies, and endocrine and non-endocrine tumour formation, including cardiac tumours.[3]
Myxomas are thought to derive from multipotential mesenchymal cells, and have a variable macroscopic appearance ranging from soft and friable to smooth and bosselated.[4] Most tumours are found in the atria, with 75% originating from the fossa ovalis region of the left atrium. Fifteen to twenty percent of cases are detected in the right atrium and only 3 - 4% are found in either the left or right ventricle.
While between 2 and 12% of patients remain asymptomatic,[1,5] most present with one or more of the triad of constitutional symptoms, intracardiac obstruction and embolic phenomena.[6]
Embolism occurs in ~30 - 40% of cases and may involve any internal organ; however, cerebral arteries are most commonly affected.[6] The diagnosis of myxoma is occasionally made after histological assessment of embolic material reveals myxomatous tissue, with subsequent investigation leading to identification of the primary cardiac tumour.[7] Constitutional symptoms are common, reported in approximately one-third of patients, and include weight loss, fever, fatigue and symptoms mimicking connective tissue disorders.[4] These have been attributed to the expression of interleukin-6 by the myxoma and typically resolve following tumour resection.[8]
Syncope and sudden death caused by cardiac myxomas have been reported.[9] Large left atrial tumours impairing intracardiac blood flow can produce variable clinical features, from slowly progressive orthopnoea and paroxysmal nocturnal dyspnoea, to acute pulmonary oedema, syncope or sudden death.[10,11] Sudden death resulting from very small tumours is more likely to be related to embolism to the coronary or cerebral arteries.
In the case described here, obstruction of the mitral valve by the myxoma may have provoked syncope and features of left ventricular failure. The history of presumed dilated cardiomyopathy secondary to chronic alcohol use may have distracted from the possibility of other aetiologies, and led to a delay in diagnosis, although dual pathologies may well have existed in this case. Tumour embolism, or dislodgement of the associated left atrial thrombi, may also have contributed to the initial deterioration of the patient, and are most likely to be the cause of the acute lower limb ischaemia and multiterritorial cerebral infarcts. Unfortunately, the embolic material was not removed during the operation, preventing histological confirmation. Several case reports have implicated atrial myxoma as the cause of acute myocardial infarction, and this may also have been a contributing factor in this case.[12,13] Aortic regurgitation has been found in association with atrial myxomas where the tumour pedicle is attached to the atrial septum close to the aortic root.[14] Tractional forces applied to the aortic root as the tumour moves towards the left ventricle during diastole have been implicated in the aortic incompetence.
TTE is a valuable investigation, allowing identification, localisation and measurement of cardiac tumours, and should be considered in all cases of suspected cardiogenic embolism. Where myxoma is identified without preceding embolism, the subsequent risk can be evaluated based on echocardiographic morphology. Polypoid and polylobulated tumours have been associated with increased risk of embolism and therefore may merit more urgent surgery.[15,16] Transoesophageal echocardiography is more sensitive than TTE (95.2% and 100%, respectively);[17] however, the limited availability, particularly in the developing world, restricts its use as a diagnostic tool. Indeed, since most district-level hospitals in South Africa lack echocardiography services and the sensitivity of clinical examination and plain chest radiography for the diagnosis of cardiac tumours is low, many cases are likely to go undiagnosed.
Treatment of cardiac myxoma is by surgical resection via median sternotomy and is generally curative. Rates of tumour recurrence among sporadic cases are between 1 and 3%, but up to 20% in familial cases. Operative mortality is low in most studies. In a single centre French study, Pinede et al.[5]followed 112 patients postoperatively for a median of 3 years and reported only four deaths, which is consistent with mortality rates in other series.[18]
Conclusion
Atrial myxoma is rare and can present with a wide range of symptoms. A low threshold for TTE in suspected cases may facilitate diagnosis and expedite definitive surgical management that carries with it an excellent prognosis. In the case of cardiomyopathy with embolic phenomena, urgent TTE should be sought to identify potential causes such as thrombi, endocarditis and, as in this case, atrial myxoma.
Learning points
- Atrial myxomas have a variable clinical presentation and should be included in the differential diagnosis of confirmed or suspected cardiogenic embolism.
- Transthoracic echocardiography is a highly sensitive investigation in cases of suspected cardiac myxoma.
- Surgical resection of atrial myxomas is generally curative and carries with it an excellent prognosis, highlighting the importance of early diagnosis.
References
1. MacGowan S, Sidhu P, Aherne T, et al. Atrial myxoma: National incidence, diagnosis and surgical management. Ir J Med Sci 1993;162(6):223-226. [ Links ]
2. Sarjeant J, Butany J, Cusimano R. Cancer and the heart: Epidemiology and management of primary tumors and metastases. Am J Cardiovasc Drugs 2003;3(6):407-421. [ Links ]
3. Mabuchi T, Shimizu M, Ino H, et al. PRKAR1A mutation in patients with cardiac myxoma. Int J Cardiol 2005;102(2):273-277. [http://dx.doi.org/10.1016/j.ijcard.2004.05.053] [ Links ]
4. Butany J, Nair V, Ather N, et al. Cardiac tumours: Diagnosis and management. Lancet Oncol 2005;6(4):219-228. [http://dx.doi.org/10.1016/S1470-2045(05)70093-0] [ Links ]
5. Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial myxoma: A series of112 consecutive cases. Medicine (Baltimore) 2001;80(3):159-172. [http://dx.doi.org/10.1097/00005792-200105000-00002] [ Links ]
6. Reynen K. Cardiac myxomas. N Engl J Med 1995;333(24):1610-1617. [ Links ]
7. Silverman J, Olwin J, Graettinger J. Cardiac myxomas with systemic embolization: Review of the literature and report of a case. Circulation 1962;26:99-103. [http://dx.doi.org/10.1161/01.CIR.26.1.99] [ Links ]
8. Mendoza C, Rosado M, Bernal L. The role of interleukin-6 in cases of cardiac myxoma: Clinical features, immunologic abdnormalities, and a possible role in recurrence. Tex Heart Inst J 2001;28(1):3-7. [ Links ]
9. Modi K, Venkatesh P, Agnani S, et al. Sudden death in a patient with left atrial myxoma: Report of two cases and review of literature. BJMP 2010;3(2):318. [ Links ]
10. Fisicaro A, Slavich M, Agricola E, et al. Acute pulmonary oedema caused by a giant atrial myxoma. Case Rep Med 2013;2013:904952. [http://dx.doi.org/10.1155/2013/904952] [ Links ]
11. Nogueira D, Bontemp D, Menardi A, et al. Left atrial myxoma as the cause of syncope in an adolescent. Arq Bras Cardiol 2003;81(2):206-209. [ Links ]
12. Sankar N, Vaidyanathan R, Prasad G, et al. Left atrial myxoma presenting as acute inferior wall infarction: A case report. J Card Surg 2006;21(5):478-479. [http://dx.doi.org/10.1111/j.1540-8191.2006.00282.x] [ Links ]
13. Panos A, Kalangos A, Sztajzel J. Left atrial myxoma presenting with myocardial infarction: Case report and review of the literature. Int J Cardiol 1997;62(1):73-75.[http://dx.doi.org/10.1016/s0167-5273(97)00178-2] [ Links ]
14. Bourdillon P, Monro J, Johnson A. Left atrial myxoma with aortic regurgitation. Br Heart J 1978;40(5):575-578. [http://dx.doi.org/10.1136/hrt.40.5.575] [ Links ]
15. Scott N, Veinot J, Kwan-Leung C. Symptoms in cardiac myxoma. Chest 2003;124(6):2408. [ Links ]
16. Acebo E, Val-Bernal F, Gómez-Román J, et al. Clinicopathologic study and DNA analysis of 37 cardiac myxomas: A 28-year experience. Chest 2003;123(5):1379-1385. [ Links ]
17. Engberding R, Daniel W, Erbel R, et al. Diagnosis of heart tumours by transoesophageal echocardiography: A multicentre study in 154 patients. Eur Heart J 1993;14(9):1223-1228. [ Links ]
18. Centofanti P, Di Rosa E, Deorsola L, et al. Primary cardiac tumours: Early and late results of surgical treatment in 91 patients. Ann Thorac Surg 1999;68(4):1236-1241. [ Links ]
 Correspondence:
Correspondence:
R D Wise
robertwise@webafrica.org.za














