Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Southern African Journal of Critical Care (Online)
versión On-line ISSN 2078-676X
versión impresa ISSN 1562-8264
South. Afr. j. crit. care (Online) vol.31 no.1 Pretoria jun. 2015
http://dx.doi.org/10.7196/SAJCC227
ARTICLE
Incidence and outcome of ventilator-associated pneumonia in Inkosi Albert Luthuli and King Edward VIII Hospital surgical intensive care units
A Awath BehariI; N KalafatisII
IMB ChB, DA (SA), FCA (SA); Department of Anaesthesia and Critical Care, University of KwaZulu-Natal, Durban, South Africa
IIMB BCh, DA (SA), FCA (SA), Cert Critical Care (SA); Department of Anaesthesia and Critical Care, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: Ventilator-associated pneumonia (VAP) is one of the most common causes of hospital morbidity and mortality, but has been poorly studied in the South African context.
OBJECTIVE: To evaluate the incidence and outcome of VAP in the intensive care units (ICUs) of two major centres in the Durban metropolitan area.
METHODS: The study was conducted over a period of 6 months with all intubated and mechanically ventilated patients who were screened on admission to ICU. A questionnaire was prepared to note patients' age, gender, date and time of intubation or reintubation. Patients were monitored from date of admission to the date of discharge from ICU or death. A diagnosis of VAP was made on a clinical pulmonary infection score (CPIS) of >6.
RESULTS: Of 32 patients evaluated, eight patients (25%) were diagnosed with VAP. Median duration of ventilation in the VAP group was 249 hours v. 65.5 hours in the non-VAP group (p=0.0002). We found no statistically significant association between age or gender with the development of VAP (p=0.28 and p=0.59, respectively). The most common organism isolated was Acinetobacter baumannii, followed by Pseudomonas aeruginosa. Three of the eight (37.5%) patients diagnosed with VAP died in the ICU.
CONCLUSION: VAP is common in critically ill patients, possibly associated with poor outcome. These results highlight the need for strict adherence to evidence-based preventive measures.
Ventilator-associated pneumonia (VAP) is one of the most common causes of hospital morbidity and mortality.[1] VAP refers to pneumonia developing in patients who have been receiving mechanical ventilation for at least 48 hours, and may be further categorised into early-onset VAP (<96 hours) and late-onset VAP (>96 hours).[2] Prevalence ranges from 10 to 25% in tertiary care hospitals, and can reach 76% in some settings.[3] VAP is associated with substantial morbidity and excess cost, and patients with VAP have been found to be twice as likely to die than those without VAP.[4] Gram-negative organisms are the most commonly associated microbial flora.[5]
The fundamental problem with the diagnosis of VAP is the lack of an internationally accepted gold standard. According to the Centers for Disease Control and Prevention,[6] VAP may be diagnosed by: (i) a new or progressive pulmonary infiltrate; (ii) fever, leukopenia or leukocytosis; (iii) purulent tracheobronchial secretions; (iv) and worsening gas exchange. However, these criteria are nonspecific and of little utility in the diagnosis of VAP. An autopsy investigation showed that only 52% of patients with pneumonia had a localised infiltrate on their chest radiograph.[7] Furthermore, fever and leukocytosis may be caused by other foci of infection in the intensive care unit (ICU) setting.
In an attempt to increase the likelihood of diagnosing VAP, Pugin et al.[3] created the Clinical Pulmonary Infection Score (CPIS, Table 1) based on sputum smear microscopy and tracheal aspirate culture, as well as on the clinical findings at the time of diagnostic suspicion. In that study, the authors concluded that there was a good correlation between clinical score and quantitative bacteriology, with a sensitivity of 72% and specificity of 80%. A CPIS threshold of 6 was found to be a fairly accurate measure of the presence or absence of pulmonary infection, as signified by bacterial culture.

VAP has been poorly studied in the South African (SA) context. A literature search showed no reported studies pertaining to VAP incidence and aetiology in adults in SA. The primary objective of this study was to evaluate the incidence and outcome of VAP in the ICUs of two major centres in the Durban metropolitan area.
Methods
A literature search was done on PubMed and Google using the search terms'ventilator-associated pneumonia, incidence, outcome, mortality.'
The study was conducted over a period of 6 months in the King Edward VIII Hospital ICU and Inkosi Albert Luthuli Central Hospital surgical ICU. Ethical approval was obtained, and consent was waived on the basis of the study being a prospective chart review with no patient contact. All intubated and ventilated patients were screened on admission to the ICUs.
A questionnaire was prepared to note each patient's age, gender, date and time of initial intubation and initiation of mechanical ventilation, and date and time of reintubation and ventilation. The parameters included in the CPIS were tabulated to enable a daily score to be calculated by the attending doctor.
Inclusion criteria were as follows: all patients >18 years of age admitted to the ICU who were intubated and ventilated for a minimum of 48 hours. Exclusion criteria were suspected or confirmed community-acquired or nosocomial pneumonia on admission, patient age <18 years, and patients who were managed on non-invasive ventilation. Patients were monitored from the date of admission into the ICU to the date of discharge or date of death. A diagnosis of VAP was made on a CPIS of >6. Due to the cost and ethical issues surrounding daily radiographic and microbiological testing, a score of 0 was allocated to these parameters (chest X-ray infiltrates and microbiology) if none was available on the day of evaluation. Semiquantitative and qualitative tracheal aspirations and blood cultures were obtained.
At the time of study, patients were managed by the attending clinician and all interventions were decided upon based on the clinician's individual assessment of the patient, including the initiation of antimicrobial therapy according to the unit's antimicrobial stewardship programmes.
The association between the diagnosis of VAP and duration of ventilation was statistically analysed using the Mann-Whitney test, the association between age and development of VAP was studied using the two-sample f-test and the association between gender and VAP was studied using the one-sided Fisher's exact test. A p-value of <0.05 was considered significant.
Results
The study cohort comprised 32 patients (male n=19, 59.4%) admitted for various surgical and medical pathologies. Eight of the 32 patients (male n=5, 25.0%) were diagnosed with VAP, corresponding to a rate of 9.9 per 1 000 ventilator days. There were no statistically significant associations between age or gender and the development of VAP (Table 2). Patients with VAP had a significantly longer duration of mechanical ventilation than those without VAP (Table 2). Ofthe patients diagnosed with VAP, three out of eight (37.5%) died in the ICU. Mortality in the non-VAP group was not recorded.

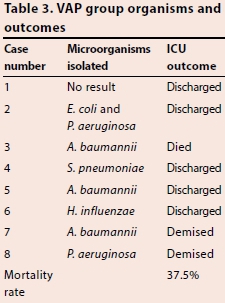
All of the VAP cases except Case 1 fell under the definition of late-onset VAP. The most common organisms associated with VAP in were Acinefobacfer baumannii (37.5%), Pseudomonas aeruginosa (25.0%), followed by Haemophilus influenzae, Sfrepfococcus pneumoniae, and Escherichia coli (12.5% each) (Table 3), with no organsim isolated in one of the patients diagnosed with VAP by CPIS.
Discussion
The primary objective of this study was to assess the incidence, aetiology and outcome of VAP in a heterogenous patient population admitted to our ICUs with both medical and surgical pathologies. The incidence of VAP in our setting was 25%, which is in keeping with rates of 15.5 - 27.5% quoted in other studies using similar methodology.[8] However, a recent surveillance study by Kollef ef al.[9] on the epidemiology of VAP due to P. aeruginosa found the global incidence of VAP to be lower, at 15.6%, with a regional incidence of 13.5% in the USA, 19.4% in Europe, 13.8% in Latin America and 16.0% in Asia Pacific.
Despite the clinical popularity of the CPIS, debate continues regarding its diagnostic validity. Its apparent straightforward calculation is beneficial; however, the inter-observer variability in CPIS calculation remains substantial, jeopardising its routine use in clinical trials.[101 The lack of an international gold standard for the diagnosis of VAP makes comparison of different studies difficult and inaccurate. Unfortunately, we as clinicians are nowhere near achieving this goal due to the overlapping of clinical presentations with other causes of sepsis or ARDS, resulting in a high sensitivity but low specificity using clinical diagnostic criteria. It is hoped that new developments in the isolation of specific biomarkers would provide a solution to this diagnostic conundrum.
Age and gender were not found to be statistically significant contributors to the development of VAP, but duration of mechanical ventilation was found to be highly significantly associated with the development of VAP. However, we are unable to determine cause and effect on the basis of this study. The association between duration of mechanical ventilation and the development of VAP has been reported previously, for example Gadani et a/.,[8] in a study of 100 patients, concluded that the incidence of VAP was directly proportional to the duration of mechanical ventilation. This highlights the need to avoid intubation and mechanical ventilation if at all possible. The rate of VAP in non-invasive positive pressure-ventilated patients is lower and should be the ventilation modality utilised if proven to be equal if not superior to invasive means of ventilation for the disease process.[11] Both units in our study had VAP bundles in place, including head-up position, hand-washing protocols and early weaning protocols incorporating sedation holds.
We found that Gram-negative organisms were the most common associated pathogens, with A. baumannii and P. aeruginosa being the most common organisms isolated in the patients with VAP. Although these organisms are commonly associated with VAP in different settings, patient profile and nature of the ICU can contribute to a higher prevalence of other organisms.[5] The majority of the VAP cases were of the late-onset subtype in which multidrug-resistant bacteria are known to be the most prevalent.[11] Aetiological data collection and interpretation provides vital information on most likely causative organisms and resistance patterns. This assists clinicians in directing their choice of empirical treatment if VAP is suspected. Therefore, it is suggested that surveillance studies be adopted in all ICU settings.
The question of the effect of VAP on mortality of critically ill patients is certainly a pertinent one to the clinician. Of the patients who developed VAP, 37.5% died in the ICU. A recent meta-analysis on the attributable mortality of VAP conducted by Melsen et a/.[12] showed an overall attributable mortality of VAP to be 13%. Due to the heterogenous nature of our study population, with differing comorbidities and admission diagnoses as well as limited outcome data collection in those who did not develop VAP, we are unable to comment on VAP being an independent risk factor for mortality. However, the mortality rate in our study does warrant further investigation.
Study limitations
The major limitation is the small sample and the lack of patient stratification prior to investigation, preventing multivariate analysis. Our sample was based on other similarly designed studies, with populations of between 51 and 100 patients.[3,8] Furthermore, we did not look at medical and surgical admissions separately but instead looked at the patients in both ICUs as a single population. Patients' baseline function, comorbidities, injury severity score and management confounders such as dialysis and patient transfer complications need to be documented and evaluated as possible contributing factors for the development of VAP. Possible areas of study for this unit include adherence to VAP bundle elements and the utilisation of other prevention studies such as feeding protocols, monitoring of endotracheal tube cuff pressures, minimising transfers out of ICU, etc., with subsequent similar surveillance studies to ascertain if these interventions affect the development of VAP. Larger, multicentred studies are recommended to address and help minimise the effect of this disease process on a national level.
Conclusion
VAP is a common pathology in critically ill patients, possibly associated with poor outcome. We found a significant association between duration of ventilation and development of VAP, which highlights the essential need for implementation of VAP preventive bundles, weaning protocols and strict adherence to infection control policies.
References
1. Rotstein C, Evans G, Born A, et al. Clinical practical guidelines for hospital-acquired pneumonia and ventilator-associated pneumonia in adults. Can J Infect Dis Med Microbiol 2008;19(1):19-53. [ Links ]
2. Vanhems P, Bénet T, Voirin N, et al. Early-onset ventilator-associated pneumonia incidence in intensive care units: A surveillance-based study. BMC Infect Dis 2011;11:236. [http://dx.doi.org/10.1186/1471-2334-11-236] [ Links ]
3. Rakshit P, Nagar VS, Deshpande VAK. Incidence, clinical outcome, and risk stratification of ventilator-associated pneumonia: A prospective cohort study. Indian J Crit Care Med 2005;9(4):211-216. [http://dx.doi.org/10.4103/0972-5229.19761] [ Links ]
4. Nseir S, Di Pompeo C, Jozefowicz E, et al. Relationship between tracheotomy and ventilator- associated pneumonia: A case-study. Euro Resp Journal 2007;30(2):314-320. [http://dx.doi.org/10.1183/09031936.06.00024906] [ Links ]
5. Rello J, Quintana E, Austina V, et al. Incidence, etiology, and outcome of nosocomial pneumonia in mechanically ventilated patients. Chest 1991;100(2):439-444. [http://dx.doi.org/10.1378/chest.100.1.439] [ Links ]
6. Centres for Disease Control. Ventilator associated pneumonia guideline. http://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent (accessed 9 September 2014). [ Links ]
7. Prescott HC, O'Brien JM. Prevention of ventilator-associated pneumonia in adults. Med Rep 2010;2(15):15. [http://dx.doi.org/10.3410/M2-15] [ Links ]
8. Gadani H, Vyas A, Kar A. A study of ventilator associated pneumonia: Incidence, outcome, risk factors and measures to be taken for prevention. Indian J Anaesth 2010;54(6):535-540. [http://dx.doi.org/10.4103/0019-5049.72643] [ Links ]
9. Kollef MH, Chastre J, Fagon JY, et al. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit Care Med 2014;42(10):2178-2187. [http://dx.doi.org/10.1097/CCM.0000000000000510] [ Links ]
10. Kalanuria AA, Zai W, Mirsk M. Ventilator-associated pneumonia in the ICU. Crit Care 2014;18(2):208 [http://dx.doi.org/10.1186/cc13775] [ Links ]
11. Hess DR. Non-invasive positive-pressure ventilation and ventilation-associated pneumonia. Respir Care 2005;50(7):924-931. [ Links ]
12. Melsen WG, Rovers MM, Groenwald RH, et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient date from randomised prevention studies. Lancet Infect Dis 2013;13(8):665-671. [http://dx.doi.org/10.1016/S1473-3099(13)70081-1] [ Links ]
 Correspondence:
Correspondence:
A Awath Behari
a.behari1@gmail.com














