Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Southern African Journal of Critical Care (Online)
versão On-line ISSN 2078-676X
versão impressa ISSN 1562-8264
South. Afr. j. crit. care (Online) vol.30 no.1 Pretoria Ago. 2014
http://dx.doi.org/10.7196/SAJCC.197
ARTICLE
A prospective comparison of the efficacy and safety of fully closed-loop control ventilation (Intellivent-ASV) with conventional ASV and SIMV modes
A AbutbulI; S SviriI; W ZbedatII; D M LintonIII; P V van HeerdenIV
IMD; Medical Intensive Care Unit, Hadassah-Hebrew University Medical Center, Ein Kerem, Jerusalem, Israel
IIRN, MPA; Medical Intensive Care Unit, Hadassah-Hebrew University Medical Center, Ein Kerem, Jerusalem, Israel
IIIMB ChB; Medical Intensive Care Unit, Hadassah-Hebrew University Medical Center, Ein Kerem, Jerusalem, Israel
IVMB BCh, PhD; Medical Intensive Care Unit, Hadassah-Hebrew University Medical Center, Ein Kerem, Jerusalem, Israel
ABSTRACT
BACKGROUND. Intellivent-adaptive support ventilation (ASV) is a closed-loop, fully automatic method of mechanical ventilation. This advanced mode of ventilation adjusts ventilation and oxygenation parameters according to patient weight, lung function (as assessed by the ventilator) and continuous input of end-tidal carbon dioxide and oxygen saturation. Our study compares the efficacy of this new mode with ASV and synchronised intermittent mandatory ventilation (SIMV) modes.
METHODS. We conducted a within-group comparison of three modes of ventilation, ASV, Intellivent-ASV and SIMV, using a Hamilton S1 ventilator (Hamilton Medical, Switzerland). Subjects were ventilated for 2 hours on each mode, and at the end of each 2-hour period, parameters of ventilation and haemodynamics were measured.
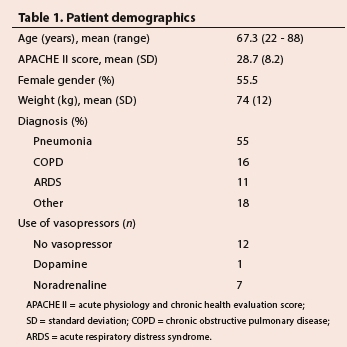
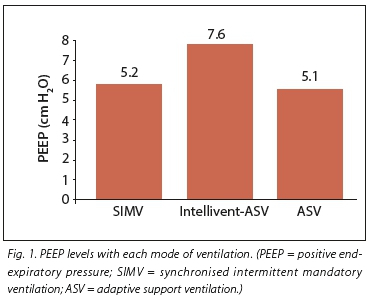
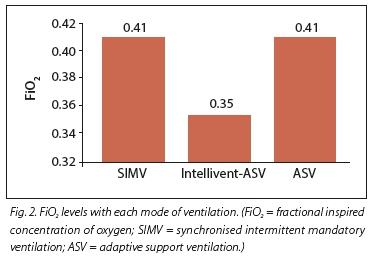
RESULTS. Twenty subjects participated in this study. Their mean age was 67.3 years (range 22 - 82 years). The most common diagnosis at presentation was pneumonia (55%), followed by chronic obstructive pulmonary disease (16%) and acute respiratory distress syndrome (11%). Mean (standard deviation) levels of positive end-expiratory pressure (PEEP) were significantly higher in the Intellivent-ASV group (7.6 (5) v. 5.1 (2) and 5.2 (2) cm H2O in the ASV and SIMV groups, respectively (p<0.005). Fractional inspired concentration of oxygen (FiO2) was significantly lower in the Intellivent-ASV group (0.35 (0.7)) v. 0.41 (0.6) and 0.41 (0.6) for the ASV and SIMV groups, respectively (p<0.005). The mean spontaneous breathing rate in the Intellivent-ASV group was 8.6 (7.5) breaths per minute (b/min), significantly higher than in the ASV group (2.9 (5.7) b/min) and the SIMV group (2.4 (4.5) b/min) (p=0.002), while there was no difference in the total respiratory rate between the groups. There was no significant difference in haemodynamic parameters between the different ventilation modes. ASV tended to produce lower partial pressure of carbon dioxide (PCO2) levels than SIMV and Intellivent-ASV (p<0.05).
CONCLUSIONS. Intellivent-ASV provided a significant reduction in the FiO2 with higher PEEP levels, but without haemodynamic detriment. Intellivent-ASV encouraged significantly more spontaneous breathing, which may translate to faster weaning. Further studies to examine this effect are warranted.
Synchronised intermittent mandatory ventilation (SIMV) has been the conventional mode of ventilation in many intensive care units (ICUs) around the world for decades. In SIMV, the physician sets the respiratory rate, tidal volume and levels of pressure support (PS), positive end-expiratory pressure (PEEP) and fractional inspired concentration of oxygen (FiO2). Once set, the settings are static, until changed again by the operator on the basis of changing monitored parameters such as respiratory rate, pulse oximetry (SpO2), end tidal CO2 (PetCO2) or intermittent arterial blood gas (ABG) measurements.
More advanced modes of ventilation have appeared in the last 20 years. One such mode is adaptive support ventilation (ASV), first described in 1994 by Laubscher and colleagues.[1,2] This is a microprocessor-controlled, closed-loop method of automatic mechanical ventilation. The patient's target minute ventilation is determined and delivered to the patient, based on ideal body weight and percent minute volume settings. ASV automatically adapts the respiratory rate and level of inspiratory pressure to the patient's lung mechanics and offers a breath-by-breath adjustment of mechanical ventilation. In this mode, the physician sets the patient's ideal body weight, percent minute volume, PEEP and FiO2. The rest of the parameters, such as inspiratory pressure (aimed at reaching the target tidal volume) and respiratory rate, are determined and changed automatically by the ventilator. Previous studies have tested the efficiency, safety and adaptability of ASV and have demonstrated improvement in patient-ventilator interaction and reduction in signs of asynchrony with ASV, compared with SIMV.[3-7]
Intellivent-ASV (Hamilton Medical, Switzerland) is a recently released development of ASV that automatically adjusts both ventilation and oxygenation parameters. The only parameter that the physician sets when using this modality is the patient's ideal body weight, based on the gender and height of the patient. Minute volume is then adjusted according to PetCO2, and the spontaneous breathing rate. If the measured PetCO2 or the spontaneous respiratory rate is higher than the set upper limit threshold, the ventilator will automatically increase the target minute ventilation, which is met by automatically increasing the respiratory rate or the inspiratory pressure. Oxygenation is adjusted according to arterial oxygen saturation measured by SpO2. The ventilator responds to hypoxia by increasing the PEEP and/or the FiO2 according to the acute respiratory distress syndrome (ARDS) network tables.[8-10] The PEEP levels set by the Intellivent-ASV controller are within the range of 5 - 15 cm H2O.
In light of these advances in ventilator technology, our objective was to compare the safety and efficacy of the Intellivent-ASV mode with the currently practised ventilation mode in our medical ICU, namely ASV, as well as to the standard ventilation mode used in most other ICUs (SIMV). Our hypothesis was that Intellivent-ASV is safe and provides ventilation that results in arterial blood gas (ABG) results that are at least equivalent to ASV or SIMV.
Methods
The study took place in the nine-bed medical ICU (MICU) at the Hadassah-Hebrew University Medical Center, a 750-bed academic tertiary referral centre. The MICU admits critically ill, non-surgical cases with acute respiratory, infectious, neurological, haematological-oncological, renal, metabolic and other medical problems (Table 1).
Patients were recruited into the study between February 2013 and July 2013. All patients admitted to our MICU and who were ventilated for at least 24 hours prior to recruitment were eligible for inclusion. Patients included were also required to have had stable haemodynamic and respiratory parameters over the previous 12 hours. We excluded patients due for extubation on the same day, patients with unstable haemodynamic parameters (labile BP or pulse rate or escalating vasopressor or inotrope requirements), those requiring mandatory ventilation (such as continuous mandatory ventilation) or those receiving inhaled nitric oxide. Pregnant women and minors (<18 years old) were also excluded.
The study was reviewed and approved by the Hadassah-Hebrew University Hospital Institutional Review Board (IRB) (0041-13-HMO). The IRB allowed 'delayed' consent (consent signed after the fact, when the patient regained consciousness), provided consent was obtained at the time of enrollment from either the subject's legal guardian or from a physician independent of the study.
Study design and parameters recorded
Participants were all initially ventilated for at least 2 hours on ASV, the standard ventilation mode in our MICU. The usual initial settings were 100% minute ventilation using the patient's ideal body weight, PEEP within the range of 2 - 12 cm H2O and the lowest FiO2 allowing adequate oxygenation (arterial oxygen saturation as measured by a blood gas analyser (SaO2) >93% or an arterial partial pressure of oxygen (PaO2) >70 mmHg) in the range of FiO2 0.35 - 0.5 (Table 2). At the end of this 2-hour period, baseline parameters and arterial blood gases were obtained. These included blood pressure (BP) and heart rate (HR) by continuous electrocardiogram and BP monitoring, FiO2, PEEP, compliance, resistance, peak inspiratory pressure, respiratory rate (total breaths per minute (b/min) and spontaneous b/min), spontaneous minute volume, expiratory tidal volume and actual minute volume.

The ventilation mode was then changed to Intellivent-ASV for a period of 2 hours, after which the same parameters were recorded.
The patient was subsequently returned to the standard (baseline) mode of ventilation, ASV, for 2 hours, followed by a change to SIMV mode for a period of 2 hours, after which the same, previously described parameters were obtained. SIMV settings were determined by the treating intensivist according to individual preference and patient condition, based on tidal volume of 6 - 10 mL/kg, respiratory rate of 10 - 18 b/min, PEEP of 2 - 12 cm H2O and pressure support of 10 - 20 cm H2O. These settings are the practice in our unit and we wished to simulate real-life conditions. Patients with ARDS were ventilated according to the ARDSNet guidelines.[8-10]
We used a consistent order of ventilation modes, rather than randomised, because the investigators were not blinded to the mode of ventilation and the duration of the study was short, so temporal changes were unlikely to affect the results.
The doses of sedatives administered to the patients were not altered throughout the study period. All the patients were lightly sedated (eyelid movement in response to glabellar tap) with either a combination of morphine and midazolam, or propofol by infusion.
Safety was defined as follows: that ABG results were not worse than the baseline after each intervention; that high inspiratory pressures (greater than 35 cm H2O) were not encountered; and that haemodynamic parameters (BP and HR) were not compromised, compared with baseline.
Data analysis
The results are presented as mean (standard deviation (SD) or (range). To analyse the differences between the three groups, we used the repeated measures analysis test for within-subject effect. This test was appropriate as the data were normally distributed.
The study was designed based on arbitrary data collection from 20 subjects, as it was not known what differences we could expect in measured parameters. A post hoc power calculation found that for a 2.4 cm H2O difference in PEEP between modes, with 1.7 SD and α=0.05, the statistical power of a study with 20 subjects is 100%. To reach a statistical power of 90%, we therefore needed only seven subjects. A p-value of <0.05 was considered significant.
Results
Data were collected from 20 patients. Their mean age was 67.3 (22 - 88) years, and 55% were female. The most common diagnosis at presentation was pneumonia (55%), followed by chronic obstructive pulmonary disease (COPD) (16%) and ARDS (11%) (Table 1).
The ventilator settings for the three groups are shown in Table 2, and the repiratory parameters and ABG results are shown in Table 3.
Respiratory parameters
The mean PEEP level was significantly higher in the Intellivent-ASV group of results at 7.6 (5) cm H2O v. 5.1 (2) and 5.2 (2) cm H2O in the ASV and SIMV groups, respectively (p<0.005) (Fig. 1). FiO2 was significantly lower in the Intellivent-ASV group (0.35 (0.7)) v. 0.41 (0.6) and 0.41 (0.6) for ASV and SIMV, respectively (p<0.005) (Fig. 2). The mean spontaneous breathing rate in the Intellivent-ASV group was 8.6 (7.5) b/min (range 0 - 25), which was significantly higher than in the ASV group 2.9 (5.7) b/min (range 0 - 17) and the SIMV group 2.4 (4.5) b/min (range 0 - 18) (p=0.002) (Table 3). There was no difference in the total respiratory rate between the groups. The number of patients who were breathing completely spontaneously during the study period was higher in the Intellivent-ASV group (n=14 v. n=5 and n=7 in the ASV and SIMV groups, respectively (p<0.005). No significant differences were seen between groups for tidal volume, minute ventilation or peak inspiratory pressure (Table 3).
The PaO2/FiO2 ratio in the Intellivent-ASV mode was 250 (98), which was not significantly different compared with ASV (241 (80)) and SIMV (238 (62)) (p=0.073).
Arterial blood gas (ABG) results
There were no significant differences in ABG parameters between the SIMV and Intellivent-ASV groups. In the ASV group, partial pressure of carbon dioxide (PCO2)(41 mmHg) was significantly different from the 44 mmHg in the other groups (p=0.001). PaO2 trended lower in the Intellivent-ASV group (88 (18) mmHg) compared with 99 (32) mmHg and 97 (22) mmHg in the ASV and SIMV groups, respectively (p=0.129). However, the FiO2 was lower in the Intellivent-ASV group.
Haemodynamics
Mean arterial pressure (MAP) in the Intellivent-ASV group was 75 (14) mmHg compared with 77 (17) mmHg and 77 (18) mmHg in the ASV and SIMV groups, respectively (p=0.642) (Table 3). There were no significant differences in HR between the groups. There was also no statistically significant difference in the mean inotrope doses between the groups. For noradrenaline, the dose was 0.098 μg/kg/ min in the Intellivent-ASV group v. 0.096 μg/kg/min and 0.081 μg/ kg/min in the ASV and SIMV groups, respectively (p=0.5).
Discussion
The results of this study support the view that Intellivent-ASV was a safe mode of ventilation, at least as safe as ASV and SIMV. We had defined safety as: ABG results that were not worse than the baseline and that were not statistically different from the comparison modes (ASV and SIMV), not encountering high inspiratory pressures; and non-compromised haemodynamic parameters - all of which were measurable and would have been evident during the 2-hour study periods.
PEEP was significantly higher when using the Intellivent-ASV mode compared with the other modes. This was associated with a significantly lower FiO2 in the Intellivent-ASV group. This occurred without any significant haemodynamic consequence (i.e. no significant difference in MAP or required doses of inotropes). It is well known that PEEP may impair venous return and thereby reduce cardiac output and even lower BP; however, this level of change in PEEP was unlikely to affect BP in this stable population of patients. Indeed, haemodynamic parameters were unchanged between the three ventilation modalities tested.
The significantly lower FiO2 in the Intellivent-ASV group occurred without a significantly reduced PaO2, compared with the other modes, which implies better oxygenation efficiency. It should be noted that the lowest PEEP possible while using the Intellivent-ASV mode (without presetting) is 5 cm H2O.
We also saw that the PaO2/FiO2 ratio trended higher in the Intellivent-ASV mode (although not statistically significant), which means that oxygenation and the efficacy of oxygenation was not adversely affected by the setting algorithms in this mode. In agreement with our results, Clavieras et al.[4] showed that during the weaning phase of ventilation, the PaO2/FiO2 ratio was significantly higher in the Intellivent-ASV mode, compared with pressure support ventilation, further supporting the conclusion that oxygenation efficacy is better in this mode. Treatment with high concentrations of oxygen has negative consequences (e.g. creating free radicals) and therefore using the lowest suitable FiO2 is a reasonable therapeutic goal.[8] Using the Intellivent-ASV mode approached this goal more closely than the ASV or SIMV modes in our study population. PEEP level settings in the algorithm of the Intellivent-ASV mode were derived from the ARDS network table of PEEP and FiO2, previously proven to be beneficial in ARDS patients.[8-10]
In the Intellivent-ASV mode, the rate of spontaneous breathing was significantly higher compared with the other modes, even though the total respiratory rate did not differ between the modes. This indicates that Intellivent-ASV supports the patient, matched to his or her needs, but also perhaps promotes spontaneous breathing compared with the other modes. Breathing spontaneously may potentially result in a quicker weaning from ventilation as spontaneous breathing is a vital component of any spontaneous breathing trial on the way to weaning a patient.[5,6]
Linton[11] in his paper on ASV discussed the potential of ASV ventilation to be safer and succeed more often in weaning patients from ventilation. This contention was based on studies that showed knowledge-based computer-driven algorithms such as ASV to be better than physician-directed weaning.[5] Given our results, we speculate that Intellivent-ASV (a more advanced mode of ASV) adds another aspect of potentially faster and more successful weaning, because of increased rates of spontaneous breathing.
Most of the parameters of gas exchange (pH, bicarbonate, PCO2, SaO2) that we examined showed no significant differences between the SIMV and Intellivent-ASV groups. The ASV group showed a significant tendency toward alkalosis and lower PCO2 levels, which probably represents a tendency of overventilation in the ASV mode, or indeed perhaps more efficient ventilation. There was, however, no significant difference in parameters of ventilation, such as tidal volume, minute ventilation, respiratory rate and peak airway pressure between the groups. This reassures us that the Intellivent-ASV mode is a safe mode and that all three modes tested provided safe ventilation and stable haemodynamics.
Arnal et al.[12] in a similarly designed study comparing ASV with Intellivent-ASV found that Intellivent-ASV was a safe mode in 50 fully sedated and passive patients. They also saw that the minute ventilation, tidal volume (Vt), FiO2 and PEEP were significantly lower in the Intellivent-ASV mode. Our study showed similar results in the safety of the mode and reduced FiO2. However, we did not find significant changes in the Vt and minute ventilation parameters, and in our study there was a significantly higher PEEP when using Intellivent-ASV. These differences from the study results of Arnal et al.[12] might be due to the use of different default ventilation parameters used in the ASV setting and in their use of passive patients. In our practice, we use low levels of PEEP as a routine. We found that in the Intellivent-ASV mode there was significantly more spontaneous breathing compared to in Arnal's study, probably due to our patients only being lightly sedated and active, while in Arnal's study the patients were passive. In 2013 Arnal et al.,[13] in a further study, again demonstrated the feasibility of the Intellivent-ASV mode. In agreement with our results, they found no safety issues during the study or need to switch to any other mode during use of Intellivent-ASV.
The Intellivent-ASV mode needs less intervention by the attending staff because it automatically changes the ventilation and oxygenation level of support to meet the patients' needs. Where staff resources are scarce, this capability is greatly advantageous. With Intellivent-ASV, we saw more spontaneous breathing and a tendency to lower resistance and higher compliance, which may indicate the possibility of easier weaning from ventilation. However, our study was not designed to answer this question. Weaning rate deserves another study designed specifically to address this question.
The main strength of this study is that it was designed as an in-group comparison, with each patient acting as his own control. The only parameter changed between subjects was the mode of ventilation, thus avoiding the bias of comparing different patients with different lung characteristics. The study period was relatively short, reducing the effect of temporal changes on our results.
Study limitations
The main limitations of the study were that the ventilator settings used in the ASV and SIMV groups were according to the standard of care in our ward and were used during the period of the study to meet patient need, but weren't changed breath by breath as occurs with Intellivent-ASV. These settings were not blinded and may represent a source of bias.
Conclusions
We found that Intellivent-ASV resulted in higher PEEP levels and a significant reduction in the FiO2 without haemodynamic detriment compared with ASV and SIMV. This mode may therefore be safer, but a larger study would be required to demonstrate this. Intellivent-ASV encouraged significantly more spontaneous breathing, which may translate to faster weaning. Further studies to examine this effect are warranted.
References
1. Laubscher TP, Frutiger A, Fanconi S, Jutzi H, Brunner JX. Automatic selection of tidal volume, respiratory frequency and minute ventilation in intubated ICU patients as start-up procedure for closed-loop controlled ventilation. Int J Clin Monit Comput 1994;11(1):19-30. [ Links ]
2. Linton DM, Potgieter PD, Davis S, et al. Automatic weaning from mechanical ventilation using an adaptive lung ventilation controller. Chest 1994;106(6):1843-1850. [ Links ]
3. Tehrani FT. The origin of adaptive support ventilation. Int J Artif Organs 2005;28(10):1051-1052. [ Links ]
4. Clavieras N, Wysocki M, Coisel Y, et al. Prospective randomised crossover study of a new closed- loop control system versus pressure support during weaning from mechanical ventilation. Anesthesiology 2013;119(3):631-641. [http://dx.doi.org/10.1097/ALN.0b013e3182952608] [ Links ]
5. Kollef MH, Shapiro SD, Silver P, et al. A randomised, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med 1997;25(4):567-574. [ Links ]
6. Brochard L, Harf A, Lorino H, Lemaire F. Inspiratory pressure support prevents diaphragmatic fatigue during weaning from mechanical ventilation. Am Rev Respir Dis 1989;139(2):513-521. [ Links ]
7. Iotti GA, Polito A, Belliato M. et al. Adaptive support ventilation versus conventional ventilation for total ventilatory support in acute respiratory failure. Intensive Care Med 2010;36(8):1371-1379. [http://dx.doi.org/10.1007/s00134-010-1917-2] [ Links ]
8. Allardet-Servent J, Forel JM, Roch A, et al. FiO2 and acute respiratory distress syndrome definition during lung protective ventilation. Crit Care Med 2009;37(1):202-207. [http://dx.doi.org/10.1097/CCM.0b013e31819261db] [ Links ]
9. Britos M, Smoot E, Liu KD, et al. The value of positive end-expiratory pressure and FiO2 criteria in the definition of the acute respiratory distress syndrome. Crit Care Med 2011;39(9):2025-2030. [http://dx.doi.org/10.1097/CCM.0b013e31821cb774] [ Links ]
10. The National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351(4):327-336. [http://dx.doi.org/10.1056/NEJMoa032193] [ Links ]
11. Linton DM. Adaptive lung ventilation. Respir Care Clin N Am 2001;7(3):409-424. [ Links ]
12. Arnal JM, Wysocki M, Novotni D, et al. Safety and efficacy of fully closed-loop control ventilation (IntelliVent-ASV) in sedated ICU patients with acute respiratory failure: A prospective randomised crossover study. Intensive Care Med 2012;38(5):781-787. [http://dx.doi.org/10.1007/s00134-012-2548-6] [ Links ]
13. Arnal JM, Garnero A, Novonti D, et al. Feasibility study on full closed-loop control ventilation (IntelliVent-ASV™) in ICU patients with acute respiratory failure: A prospective observational comparative study. Crit Care 2013;17(5):R196. [http://dx.doi.org/10.1186/cc12890] [ Links ]
Correspondence: P V van Heerden (vernon@hadassah.org.il)














