Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of Energy in Southern Africa
versión On-line ISSN 2413-3051
versión impresa ISSN 1021-447X
J. energy South. Afr. vol.32 no.2 Cape Town may. 2021
http://dx.doi.org/10.17159/2413-3051/2021/v32i2a8903
ARTICLES
Preparation of bio-oil from Scenedesmus acutus using thermochemical liquefaction in a 1 L reactor
H. BaloyiI, *; S. MarxII
IDepartment of Chemistry, Faculty of Science, Nelson Mandela University, Port Elizabeth 6031, South Africa
IIDST/NRF Research Chair in Biofuels, Centre of Excellence in Carbon Based Fuels, School of Chemical and Minerals Engineering, North-West University, Potchefstroom, South Africa
ABSTRACT
Biomass from microalgae is a potential feedstock for biofuels production. It poses no threat to food security as it does not compete with agricultural crops for arable land. Scenedesmus acutus was used as feedstock to produce bio-oil in a large liquefaction reactor. The influence of reaction temperature (280-360ºC), reaction atmosphere (N2 or CO2) and solvent on bio-oil yield, C-16 fatty acid yield and oil properties were investigated. Oils were characterised using gas chromatography, Fourier transform infrared (FTIR) spectroscopy and ultimate analysis. Higher bio-oil yields were obtained in a CO2 atmosphere (250 g.kg-1 dry microalgae) than in a N2 atmosphere (210g.kg-1 dry microalgae) whilst higher C16 fatty acid concentrations (600g.kg-1 bio-oil) were recorded in N2 atmosphere compared to oil prepared in a CO2 atmosphere (500 g.kg-1 bio-oil). The oil yield increased to a maximum at 320°C, after which there were no significant changes. Highest bio-oil yields (425 g.kg-1 dry microalgae) were obtained in ethanol as solvent. FTIR spectroscopy and ultimate analysis showed that proteins present in the feedstock were degraded by breakage of peptide linkages, and nitrogen present in the oils is peptide fragments from protein degradation. The carbon content of all produced oils was high, but the hydrogen content was low, leading to low hydrogen/carbon ratios. Energy consumption and energy efficiency calculations showed that liquefaction in both reaction atmospheres results in a net energy gain, and a CO2 atmosphere is best for high energy efficiency.
Highlights
• Higher bio-oil yields obtained in a CO2 atmosphere than N2.
• N2 atmosphere resulted in a higher C-16 content of the oil than in a CO2 atmosphere.
• Significantly higher bio-oil yields were obtained in ethanol as solvent.
• There was a lower energy consumption rate value for oils prepared in a CO2 atmosphere.
Keywords: microalgae, atmosphere, reaction temperature, yields, C-16 fatty acid
1. Introduction
There is a growing interest in the development of renewable and sustainable energy sources that could serve as an alternative to fossil-derived fuels. Microalgae biomass is a promising photosynthetic source of renewable energy and has several advantages as a biofuel feedstock, such as the ability to capture CO2, high oil productivity (exceeding some of the best oil crops) and having fast growth rates. Moreover, microalgae biomass is tolerant of marginal land and thus does not compete for arable land with agricultural crops (Vlaskin et al. 2018; Saber et al. 2016; Lam and Kee, 2012). A thermochem-ical conversion process, such as hydrothermal liquefaction, can be used for bio-oil production from algal biomass. Bio-oil from microalgae can be upgraded into useful liquid fuels and used for extraction of valuable chemicals (Galadima and Muraza, 2018; Saber et al. 2016). Hydrothermal liquefaction (HTL) is a decomposition reaction carried out in a water media at a lower temperature range (200-400°C) and pressures between 5 and 20 MPa, keeping the solvent still in a liquid state, often with alkaline catalysts present. Under these conditions, bio-oil is produced, and the oil can be used for heating or improved to liquid transport fuel (Chen et al., 2009). The key principle of the process is to produce oil products of increased hydrogen-to-carbon (H/C) ratios and decreased oxygen-to-carbon (O/C) ratios relative to those present in the original feedstock, and therefore high calorific values (Xu and Lancaster, 2008). Other liquefaction products are biochar and syngas; however, bio-oil has been considered here as the product of interest.
Microalgae liquefaction in batch reactors has been studied by various researchers (Vlaskin et al., 2018; Wang et al., 2018; Saber et al., 2016; Xu and Savage, 2014) to produce bio-oil using various mi-croalgae species, under various reaction condition and variables (i.e., reaction temperature, catalyst type, holding time and reactor volumes). In general, these studies focused primarily on the liquefaction of microalgae in a water medium and inert (N2) atmosphere. Vlaskin et al. (2018) successfully obtained high bio-oil yields (44 wt.%) from the liquefaction of different samples of Arthrospira platensis (freshly harvest, dried and frozen samples) at 300°C, at 60 minutes holding time in small autoclaves as reactors. Wang et al. (2018) investigated the catalytic hydrothermal liquefaction of microalgae Nannochloropsis and observed maximum bio-oil yields (48,23 wt.%) using Ni/TiO2 as catalyst at 300°C, at 30 minutes holding time. Saber et al. (2016) performed hydrothermal liquefaction of Nannochloropsis sp. carried out in a 500 ml batch reactor, at a range of temperatures (210°C; 230°C; 250°C) for 60 minutes, with the addition of a nano catalyst (Ni/SiO2), zeolite and sodium carbonate (Na2CO3), and achieved highest bio-oil yields (30 wt. %) at 250°C using nano-Ni/SiO2 as catalyst. Xu and Savage (2014) performed the liquefaction of Nannochloropsis sp. (in slurry form) in mini-batch reactors at 350°C (20 minutes holding time) and obtained high bio-oil yields (44 wt.%). Other researchers (Jena et al., 2011; Ross et al. 2010; Shuping et al., 2010) have also demonstrated that reasonable oil yields with good fuel properties can be obtained from liquefaction of other algal species (Spirulina plantensis, Chlorella vulgaris and Dunaliella tertio-lecta, respectively) using alkali catalyst (i.e., Na2CO3) at 350°C, 60 minutes holding time.
The dielectric constant of water changes when it is heated under pressure, which changes its solubility and reactive properties (Kruse and Dinjus, 2007; Jena et al., 2011). The higher reactivity of water under sub-critical conditions can be used during hydrothermal liquefaction (HTL) to convert plant materials into solid, liquid and gaseous fractions through a combination of hydrolysis, decarboxylation, aromatisation and dehydration reactions (Marx and Piyo, 2014; Toor et al., 2011). The use of HTL is advantageous in that it takes place in the water phase, and hence requires no drying of feedstock, and the higher content of lipids, proteins, fibre and carbohydrates results in higher yields of biofuels (Jin et al., 2013; et al., 2008; Biller and Ross, 2011).
Bio-oil is an oxygen-rich, dark, viscous, crude oil-like liquid obtained as one of the products of HTL. It has been shown to contain phenols (Akhtar and Min, 2011), water soluble organic acids (Kruse et al., 2013), n-alkanes and polyaromatic hydrocarbons (Brown et al., 2010). Bio-oil yields are directly related to the lipid content in the feedstock (Jin et al., 2013). The glycerol backbone of lipids present in microalgae can be converted to several hydrocarbon components that will be distributed in the water and organic fractions of the oil as well as the gas phase (Barreiro et al., 2013). The lipids form fatty acids as part of the oils fraction, and, in the presence of methanol or ethanol as solvent during thermochemical liquefaction, the fatty acids can be converted to methyl or ethyl esters (Huang et al., 2011; Yuan et al., 2011). Proteins in the feedstock are hydrolysed to amino acids, which undergo decarboxylation to ammonia and organic acids, which in turn can further be converted through polymerisation to form a wide variety of nitrogen-containing hydrocarbons (Toor et al., 2011). Carbohydrates degrade mostly to water soluble fractions, but some of the smaller fractions can repolymerise to form heavier hydrocarbons that will contribute to the bio-oil fraction (Barreiro et al., 2013, Biller and Ross, 2011). The lignin fraction of the biomass contributes to the biochar fraction from thermochemical liquefaction (Marx and Piyo, 2014).
The characteristics of the crude bio-oil depend on the characteristics of the biomass feedstock (Shuping et al, 2010) as well as the processing conditions (temperature, pressure, reaction time, solvent, reaction atmosphere) (Barreiro et al., 2013). Many studies have reported the influence of temperature (200-380°C) on the bio-oil yield and composition (Jena et al., 2011; Huang et al., 2011; Duan et al., 2013; Jin et al., 2013). Liquefaction begins at temperatures above 280°C (depending on the feedstock), and below this temperature only water-soluble components, extractives, and volatile organics are obtained (Kruse et al., 2013). After the onset of liquefaction, the bio-oil yield increases up to a maximum temperature (usually around 360-380°C), after which a decrease in yield is observed as the temperature exceeds the supercritical temperature of water. The unique solvent properties of supercritical water benefit the formation of lighter organic fractions through cracking, condensation, and dehydration of longer oil molecules (Duan et al., 2013, Barreiro et al., 2013), causing a decrease in oil yield.
Most studies on the influence of reaction holding time on the bio-oil yield and composition have observed an initial increase in bio-oil yield with an increase in holding time from 5 to 30 minutes followed by a decrease in oil yield at longer holding times (Zou et al., 2009; Shuping et al., 2010; Akhtar and Amin; 2011; Li et al., 2012). During liquefaction, longer holding times are necessary at low temperatures and shorter holding times at high temperatures to achieve the same bio-oil yield (Barreiro et al., 2013). The composition of the bio-oil is dependent on the holding time (Jin et al., 2013). The decrease in bio-oil yield at longer holding times have been attributed to repolymerisation and cracking reactions to form gas and biochar (Zou et al., 2010; Jin et al., 2013), decomposition of bio-oil (Li et al., 2012; Akhtar and Amin, 2011), and loss of light fractions from the bio-oil during the bio-oil recovery steps (Zhou et al., 2010). Alkali metal earth catalysts (NaOH, KOH, Na2CO3) have been reported to suppress biochar formation during liquefaction, but this does not increase the bio-oil yield significantly (Huang et al., 2011; Shuping et al., 2010). Biller and Ross (2011) showed that Na2CO3 selectively increased the oil yield from carbohydrates, but not from proteins. The addition of an alkali catalyst increases the light water-soluble fractions and gas fractions, which could lead to decreased bio-oil yields (Li et al., 2012; Anastasakis and Ross, 2011).
Li et al. (2012) suggested that the ash content of the feedstock influences the effect the addition of an alkali catalyst will have on the bio-oil yield from microalgae. Li et al. (2012) found that an increase is biomass to water ratio leads to an increase in overall product yield, but a decrease in bio-oil yield during the liquefaction of marine microalgae. Akhtar and Amin (2011) suggested that the hydrothermal liquefaction process behaves like pyrolysis at high biomass to water ratios. Jena et al. (2011) reported an increase in bio-oil yield with an increase in biomass to water ratio from 10% to 20% during the liquefaction of microalgae.
Many different micro and macro algae species have been investigated for bio-oil production. In this study the influence of temperature, reaction atmosphere and solvent on bio-oil yields and characteristics during the thermochemical liquefaction of Scenedesmus acutus in a one-litre reactor was investigated. To the author's knowledge, this is the first study to evaluate the liquefaction of Scenedesmus acutus as feedstock for bio-oil production and in a large reactor.
2. Materials and methods
2.1 Materials
Scenedesmus acutus used in this study was obtained from the Institute of Chemical Technology at the Nelson Mandela University (33°59'54.88" S 25°40"19.90" E) in dry powder form. The microalgae powder was kept in airtight containers at room temperature for the duration of the study. Nitrogen (N2) (ultra high purity) and carbon dioxide (CO2) (technical grade) were obtained from Afrox gas company. Solvents used in this study were all of 99% purity and were obtained from ACE chemical company. Solvents were used as received without prior purification. Some properties of solvents used in this study are listed in Table 1.
2.2 Experimental setup and procedure
Liquefaction experiments were carried out in a standard SS316 stainless steel high-pressure autoclave (Sawayama et al., 1995; Dote et al., 1996). The autoclave has a working volume of 950 mL, an inside diameter of 90 mm, and a height of 150 mm, and is equipped with a high-pressure stirrer and heated with an electrical heating jacket. In each experiment, a desired amount of Scenedesmus acutus, distilled water (or alcoholic solvent) and potassium hydroxide (KOH) were fed into the autoclave. The dosage of KOH was equal to 5 wt.% of microalgae. In all experiments, the autoclave was agitated using a magnetic stirrer drive at 720 rpm speed, which was set by a variable speed controller to ensure homogeneous reactions. After the completion of the experiment, the heating jackets were removed, and the autoclave could cool to room temperature Following liquefaction, chloroform was used to dissolve all organic compounds in the crude extract in the autoclave whilst stirring. The mixture was vacuum filtered using Whatman no. 3 filter paper to remove solid residues. A separating funnel was used to separate the aqueous and organic phases. The organic phase containing the oil extract was decanted into a pre-weighed round-bottom flask and the flask was used in a vacuum distillation set-up to evaporate the chloroform from the oil extract at 70°C. The round-bottom flask containing the purified oil sample was then weighed to determine the oil weight by mass difference. The influence of temperature was investigated by varying the reaction temperature from 280°C to 360°C with 20°C intervals. Experiments were conducted in either a N2or a CO2atmosphere.
2.3 Calculation of energy and mass balance parameters
The bio-oil yield was calculated as the ratio between the mass of dry bio-oil obtained and the mass of dry microalgae powder used in the experiment. An energy efficiency parameter (ER) and an energy consumption rate (ECR), as defined by Jena et al., (2011), were calculated according to Equations 1 and 2 (Minowa et al., 1995).
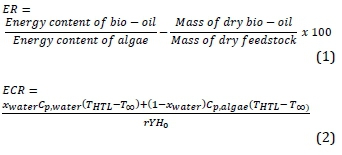
where ER is the liquefaction energy efficiency, ECR is the energy consumption, Y is the bio-oil yield, and H0 is the higher heating value of Scenedesmus acutus. The value of r was taken to be 0.6 for calculation purposes (Jena et al., 2011). Xwater is the fraction of water in the liquefaction reactor, THTL is the reaction temperature and Cp, water and Cp, microal-gae are the heat capacities of water and microalgae, respectively. The heat capacity of microalgae was taken to be equal to that of water (4.18 kJ.kg-1k-1) due to the low biomass loadings used in this study.
The higher heating values (HHV) were calculated according to Beckman's equation (Equation 3) (Channiwala et al., 2002).
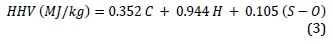
2.4 Analytical methods
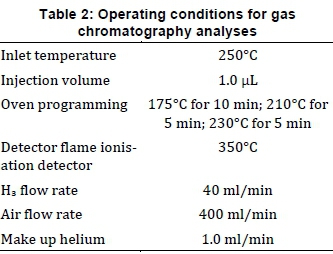
An Agilent 7890 GC equipped with an Agilent 7683B auto-injector, a HP-5 column (100 m x 320 |im x 0.25 |im) and a flame ionisation detector were used to determine the composition of bio-oil obtained during liquefaction. The bio-oil was methylated into the methyl esters by a trimethyl sulfonium hydroxide solution. The amount of fatty acid from the bio-oil was determined through standard calibration curves and the amount of fatty acid yield (wt. %) was calculated as the ratio of the mass of measured fatty acid to the initial mass of Scenedes-mus acutus used. The conditions under which the oils were analysed on the gas chromatography are summarised in Table 2.
FTIR analyses were used to determine the main organic constituents of the bio-oil samples, based on the absorption peaks of the functional groups present in the bio-oil. The bio-oil was applied as a droplet to a potassium bromide pellet and 30 scans were obtained and processed on a Bruker spectrometer. The carbon (C), hydrogen (H). nitrogen (N), sulphur (S) and oxygen (O) present in the extracted bio-oil were determined by a FLASH organic 2000 elemental analyser.
3. Results and discussion
3.1 Bio-oil yield and composition
The influence of reaction temperature on the total bio-oil yield at different reaction atmospheres is shown in Figure 1. The graph shows that the bio-oil yield stabilised rapidly in a CO2atmosphere with only an insignificant increase in oil yield with an increase in temperature, whereas in a N2 atmosphere, a steady increase in oil yield was observed and peaked at 320°C. A similar trend was observed by Wang et al. (2018), who noted an increase in bio-oil yield that reached a peak at 300°C and no significant increase was observed beyond that temperature. In contrast, Jena et al. (2011) observed an increasing trend in oil yields with an increase in reaction temperature (from 200°C to 350°C). At subcritical temperatures, the unique ionic properties of water allow for the hydrolysis of a wide range of compounds (Shuping et al., 2010). As the temperature is increased, the free radical fractions formed during hydrolysis start to repolymerise to form biochar and/or gas.
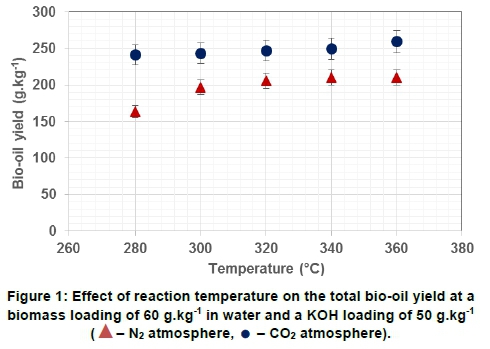
The role of the reaction atmosphere during liquefaction is to stabilise the free radical fragments and inhibit polymerisation reactions from taking place, thereby increasing the oil yield. This was observed in this study, with higher bio-oil yields obtained with CO2 than with N2 as atmosphere. Lower bio-oil yields in a N2atmosphere could be attributed to the low reactivity of the N2comparatively (Akh-tar and Amin, 2011). Liquefaction in a CO2 environment is thus conducive to the formation of bio-oil, as it promotes the deoxygenation and hydrodeoxy-genation pathways for the degradation of the biomass. The ultimate analysis will also show the lower oxygen content of bio-oil prepared in a CO2 atmosphere compared to the oxygen content of the original biomass and bio-oils prepared in a N2atmosphere.
Palmitic fatty acid (C-16:0) was the main fatty acid detected in the prepared bio-oils. The influence of reaction temperature and atmosphere on the Palmitic (C-16:0) lipid content of the prepared bio-oils is shown in Figure 2.
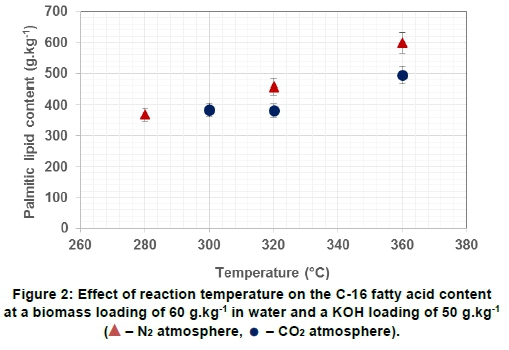
As shown in Figure 2, C-16 fatty acid content increases as temperature increases, and a N2atmosphere resulted in a higher C-16 content of the oil than did a CO2atmosphere. The high concentrations of C-16 present in the bio-oils supports the notion that cellulose and hemicellulose in the plant material degrade to form part of the bio-oils. As the temperature is increased, the ionic nature of the solvent is increased, leading to increased hydrolysis. C-16 fatty acids are formed during the hydrolysis stage, prior to depletion of hydrogen that supports the ongoing formation of free radical fragment. CO2acts as a stabiliser for the free radicals and inhibits the formation of heavier fragments that could condensate to the biochar fraction. Consequently, higher yield of C-16 fatty acid concentrations is obtained in a N2atmosphere, as the hydrolysis products are less stable than in a CO2 atmosphere.
The role of the solvent in thermochemical liquefaction is to dissolve the biomass and act as a hydrogen donor for the hydrolysis reactions (Baloch et al. 2018; Singh et al. 2015). The bio-oil obtained from different feedstocks using different solvents during liquefaction is determined to a large extent by the properties of the solvent as well as the process parameters. The influence of different solvents on the bio-oil yield during thermochemical liquefaction of Scenedesmus acutus was investigated using different solvents in a CO2 atmosphere. The results are shown in Figure 3.
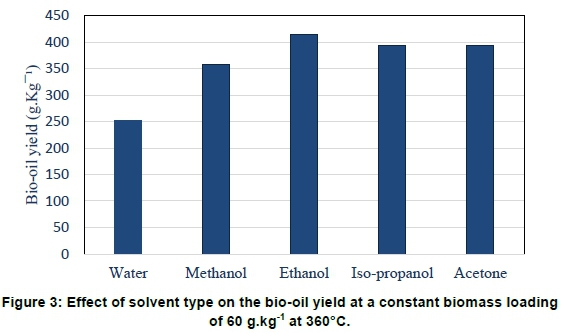
As shown in Figure 3, significantly higher bio-oil yields were obtained in ethanol as solvent than in any of the other solvents investigated. Water as solvent resulted in the lowest bio-oil yield. These results are consistent with findings by Baloch et al. (2018). Solvents act as hydrogen donors and, as such, the presence of active hydrogen from the solvent plays a role in further stabilisation of liquefaction intermediates, thereby inhibiting them from forming compounds that are more difficult to decompose, leading to high bio-oil yields (Singh et al., 2018). Comparing the properties of the various solvents, all solvents except water were in a supercritical state during liquefaction, and this explains the much lower yield obtained in water as solvent. A comparison of the different listed properties of the solvents (see Table 1) showed that there is no clear relationship between the bio-oil yields and most of the properties, although the di-electric constant and the number of hydrogen atoms in the molecule has a weak linear relationship with the bio-oil yields. Hydrogen from the solvent and the polarity of the solvent both play a role in the initiation of solvolysis and hydrolysis and it thus makes sense that there should be some relationship, albeit weak, between these properties and the amount of oil formed. The polar nature of the solvent during liquefaction can both sustain fragmentation as well as stabilise fractions to produce more oil.
3.2 Compositional analysis of oils
3.2.1 FTIR analysis
The main functional groups and the related classes of compounds present in the Scenedesmus acutus feedstock and the bio-oil are represented in Table 3.
The C-N and primary amine peaks at 3325, 3450, 3500, 1550 and 1300 cm-1 associated with proteins and polypeptides in the feedstock is not present in the bio-oil spectra, and a peak at 1377 cm-1 that is associated with peptide fraction is only present in the bio-oils. This indicates that the proteins present in the microalgae feedstock were completely broken down through the breakage of peptide linkages, leading to the -C-N fractions in the oils (Yuan et al., 2011). This is confirmed by ultimate analysis (in Table 4) that showed high concentrations of nitrogen in the feedstock, but less in the bio-oils. Weak aliphatic methyl and methylene peaks at 2850 and 2925 cm-1 in the feedstock are present as prominent peaks in the oils. This is linked to -CH3 groups attached to fatty acid chains. Single peaks between 1600 and 1700 cm-1 in the feedstock become broader, stronger peaks in the bio-oil. These peaks are associated with the -C=O and -C-O vibrations of fatty acids. There are some oils present in the microalgae feedstock as C-16 fatty acids, but as the organic material is liquefied and some fatty acid methyl esters are forming, the peaks become more prominent. The fatty acids present in the bio-oils are also evidenced by the peaks at 2300-2400, 1400, 1270 and 940-950 cm-1. The presence of organic iodine in microalgae have been reported (Yang et al., 2014; Gómez-Jacinto et al., 2010). In this study, the presence of an iodide compound in the microalgae was observed at a wave number of 550 cm-'. The oils were not analysed for iodine presence in this study, so it is not clear if the iodine was distributed to the oils or the chars. Polysulfide stretching was observed at wave numbers of 400-500 cm-1 in the feedstock, but not in the oils. This could indicate the organic sulphur present in the microalgae was removed as SOx during liquefaction (Barreiro et al., 2014). Silicone normally associated with cell walls was observed at a wave number of 1050 cm-1 in the feedstock but was not present in the bio-oil spectra. This is an indication that cell walls were completely broken down during the liquefaction process. Comparison of the spectra of the feedstock and the oils thus confirmed that plant cell walls were broken down completely and organic matter was liquefied to fatty acids (primarily C-16) and gas components containing SOx and NOX.
3.2.2 Ultimate analysis
The ultimate analysis of the raw microalgae and the bio-oil product was conducted to evaluate the extent of deoxygenation in the liquefaction of Scenedesmus acutus and the HHV of the bio-oil products. The elemental composition of the raw microalgae and of the bio-oil are presented in Table 4. The elemental composition of the bio-oil shown were obtained at 360°C, at a 60 g.kg-1 biomass loading in water under N2 and CO2 atmospheres. The HHV, energy efficiency (ER) and energy consumption (ECR) parameters for each of the oils are presented in Table 5.
In Table 4, it can be observed that the oxygen and sulphur contents were lower in the bio-oil than in the raw microalgae; this is an indication that de-oxygenation and desulphurisation of the bio-oils took place and agrees with the FTIR compositional analysis that showed the absence of sulphur peaks in the oils. The lower nitrogen content in the oils compared to the feedstock agrees with the FTIR analysis. The carbon content of the oils was significantly improved compared to the microalgae feedstock, but the H/C ratio was not significantly increased. From Table 5, the HHV of both oils are significantly higher than that of the feedstock. The energy efficiency calculations showed that more energy of the original feedstock was retained in the oils prepared in a CO2 atmosphere than in those prepared in a N2atmosphere. The energy consumption ratio for oils prepared in both atmospheres was below 1, indicating net energy being produced by the liquefaction process. The lower ECR value for oils prepared in a CO2atmosphere showed that CO2is better for bio-oil production than N2, even though the HHV of the oils produced in N2were slightly higher.
4. Conclusions
Scenedesmus acutus was successfully degraded in a one-litre liquefaction reactor. Both carbohydrates and fats were converted to a C-16 fatty acid rich oil. It was shown that not much is gained in terms of bio-oil yield by increasing the liquefaction temperature beyond 320°C, indicating that the liquefaction process is essentially finished at 320°C. Higher bio-oil yields were obtained in a CO2 atmosphere than in a N2 atmosphere, but higher C-16 fatty acid yields were obtained in a N2 atmosphere. Higher bio-oil yields were obtained when using ethanol as solvent compared to methanol, iso-propanol, or acetone. Deoxygenation and hydrodeoxygenation pathways were confirmed with Fourier transform infrared spectroscopy and ultimate analysis and it was shown that all oils produced had a high carbon content. The low hydrogen content in the original mi-croalgae feedstock and the oils resulted in low hy-drogen/ carbon ratios. However, high yield of C-16 fatty acid could make the upgrading of oils to improve the hydrogen/carbon ratio an economical prospect. Overall, it has been demonstrated that thermochemical liquefaction results in positive energy balance and that a CO2 atmosphere is ideal for high oil yields and high energy efficiency.
Author contributions
H. Baloyi: Primary research, data analysis and analytical discussions, and preparing final article.
S. Marx: Project supervision, technical editing and review of the draft paper prepared from an original mini dissertation.
Acknowledgement
This work is based on the research supported by the South African Research Chairs Initiative of the Department of Science and Innovation and National Research Foundation of South Africa. Any opinion, finding and conclusion or recommendation expressed in this material is that of the authors and the NRF does not accept any liability in this regard.
References
Akhtar, J., Amin, N.A.S. 2011. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renewable and Sustainable Energy Reviews, 15:1615-1624. https://doi.org/10.1016/i.rser.2010.11.054 [ Links ]
Anastasakis, K., Ross, A.B. 2011. Hydrothermal liquefaction of the brown macro-microalgae Laminaria Saccharina: Effect of reaction conditions on product distribution and composition. Bioresource Technology, 102:4876-4883. https://doi.Org/10.1016/j.biortech.2011.01.031 [ Links ]
Baloch, H.A., Nizamuddin, S., Siddiqui, M.T.H., Mubarak., Dumbre, D.K., Srinivasan, M.P., Griffin, G.J. 2018. Sub-supercritical liquefaction of sugarcane bagasse for production of bio-oil and char: Effect of two solvents. Journal of Environmental Chemical Engineering. 6 (5). 6589-6601. https://doi.org/10.1016/j.jece.2018.10.017 [ Links ]
Barreiro, D.L., Samori, C., Terranella, G., Hornung, U., Kruse, A., Prins, W. 2014. Assessing microalgae biorefinery routes for the production of biofuels via hydrothermal liquefaction. Bioresource Technology, 174:256-265. https://doi.org/10.1016/j.biortech.2014.10.031 [ Links ]
Barreiro, O.L., Prins, W., Ronsse, F., Brilman, W. 2013. Hydrothermal liquefaction (HTL) of microalgae for biofuel production: State of the art review and future prospects. Biomass and Bioenergy, 53:113-127. https://doi.org/10.1016/j.biombioe.2012.12.029 [ Links ]
Biller, P., Ross, A.B. 2011. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresource Technology, 102:215-225. https://doi.org/10.1016/j.biortech.2010.06.028 [ Links ]
Brown, T.M., Duan, P., Savage P.E. 2010. Hydrothermal liquefaction and gasification of Nannochloropsis sp. Energy and Fuels, 24:3639-3646. https://doi.org/10.1021/ef100203u [ Links ]
Channiwala, S.A, Parikh, P.P. 2002. A unified correlation for the estimation HHV of solid, liquid and gaseous fuels. Fuel, 81(2):1051-1063. https://doi.org/10.1016/S0961-9534(96)00045-1 [ Links ]
Chen, P., Min, M., Chen, Y., Wang, L., Li, Y., Chen, Q. 2009. Review of the Biological and engineering aspects of algae to fuels approach. International Journal of Agriculture and Biological Engineering. 2 (4). 1-30. https://doi.org/10.3965/j.issn.1934-6344.2009.04.001-030 [ Links ]
Das, D.D., Schnitzer, M.I., Monreal, C.M., Mayer, P., 2009. Chemical composition of acid-base fractions separated from bio-oil derived by fast-pyrolysis of chicken manure. Bioresource Technology, 100, 6524-6532. https://doi.org/10.1016/j.biortech.2009.06.104 [ Links ]
Dote, Y., Inoue, S., Ogi, T., Yokoyama, S., 1996. Studies on the direct liquefaction of protein-contained biomass: The distribution of nitrogen in the products. Biomass and Bioenergy, 11(6), 491 - 498. https://doi.org/10.1016/S0961-9534(96)00045-1 [ Links ]
Duan, P., Jin, B, Xu, Y., Yang, Y., Bai, X., Wang, F., Zhang, L., Miao, J. 2013. Thermo-chemical conversion of Chlorella Pyre-noidosa to liquid fuels. Bioresource Technology, 133:197-205. https://doi.org/10.1016/j.biortech.2013.01.069 [ Links ]
Duan, P., Savage, P.E. 2011. Hydrothermal liquefaction of a microalga with heterogeneous catalysts. Industrial and Engineering Chemistry Research, 50:52-61. https://doi.org/10.1021/ie100758s [ Links ]
Galadima, A., Muraza, O. 2018. Hydrothermal liquefaction of algae and bio-oil upgrading into liquid fuels: Role of heterogeneous catalysts. Renewable and Sustainable Reviews. 81. 1037-1048. http://dx.doi.org/10.1016/j.rser.2017.07.034 [ Links ]
Giordano, M., Jansiz, M., Heraud, P., Beardall, J., Wood, B., McNaughton, D. 2001. Fourier transform infrared spectroscopy as a novel tool to investigate changes in the intracellular macromolecular pools in the marine microalga Chaetoceros muellerii (Bacillanophyceae). Journal of Phycology, 37:271-279. https://doi.org/10.1046/j.1529-8817.2001.037002271.x [ Links ]
Gómez-Jacinto, V., Arias-Borrego, A., Garcia-Barrera, T., Garbayo, I., Vilchez, C., Gómez-Ariza, L. 2010. Iodine speciation in iodine enriched microalgae Chlorella vulgaris. Pure and Applied Chemistry, 82(2):473-481. https://doi.org/10.1351/PAC-CON-09-08-01 [ Links ]
Gushina, I.A., Harwood, J.L. 2006. Lipids and lipid metabolism in eukaryotic microalgae. Progress in Lipid Research, 45:160-186. https://doi.org/10.1016/j.plipres.2006.01.001 [ Links ]
Huang, H., Yuan, X., Zeng, G., Wang, J., Li, H., Zhou, C., Pei, X., You, Q., Chen, L. 2011. Thermochemical liquefaction characteristics of microalgae in sub- and supercritical ethanol. Fuel Processing Technology, 92:147-153. https://doi.org/10.1016/j.fuproc.2010.09.018 [ Links ]
Jena, U., Das, K.C., Kastner, J.R. 2011. Effect of operating conditions of thermochemical liquefaction on biocrude production from Spirulina platensis. Bioresource Technology, 102:6221-6229. https://doi.org/10.1016/j.biortech.2011.02.057 [ Links ]
Jin, B., Duan, P., Xu, Y., Wang, F., Fan, Y. 2013. Co-liquefaction of micro- and macroalgae in subcritical water. Bioresource Technology, 149:103-110. https://doi.org/10.1016/j.biortech.2013.09.045 [ Links ]
Kruse, A., Dinjus, E. 2007. Hot compressed water as reaction medium and reactant - Properties and synthesis reactions. Journal of Supercritical Fluids, 39:362-380. https://doi.org/10.1016/j.supflu.2006.03.016 [ Links ]
Kruse, A., Funke, A, Tritirici, M-M. 2013. Hydrothermal conversion of biomass to fuels and energetic materials. Current Opinions in Chemical Biology, 17:515-521. https://doi.org/10.1016/j.cbpa.2013.05.004 [ Links ]
Lam, M.K., Lee, K.T., 2012. Microalgae biofuels: a critical review of issues, problems and the way forward. Biotechnology Advances. 30 (3). 673-690. https://doi.org/10.1016/j.biotechadv.2011.11.008 [ Links ]
Li, D., Chen, L., Xu, D., Zhang, X., Ye, N., Chen, F., Chen S. 2012. Preparation and characteristics of bio-oil from marine brown alga Sargassum patens C. Agardh. Bioresource Technology, 104:737-742. https://doi.org/10.1016/i.biortech.2Q11.11.011 [ Links ]
Marx, S., Piyo, N. 2014. Influence of reaction temperature and solvent on biochar yield and characteristics. Bioresource Technology, 164:177-183. https://doi.Org/10.1016/i.biortech.2014.04.067 [ Links ]
Minowa, T., Yokoyama, S-Y., Kishimoto, M., Okakura, T. 1995. Oil production from algal cells of Dunaliella tertiolecta by direct thermochemical liquefaction. Fuel, 74:1735-1738. https://doi.org/10.1016/0016-2361(95)80001-X [ Links ]
Parshetti, G.K., Hoekman, S.K., Balasubramanian, R., 2013. Chemical, structural and combustion characteristics of carbonaceous products obtained by hydrothermal carbonization of palm empty fruit bunches. Bioresource Technology. 135, 683-689. https://doi.org/10.1016/i.biortech.2012.09.042 [ Links ]
Patil, V., Tran, K.Q., Giselr0d, H.R. 2008. Towards sustainable production of biofuels from microalgae. International Journal of Molecular Science, 9:118-1195. https://doi.org/10.3390/iims9071188 [ Links ]
Saber, M., Golzary, A., Hosseinpour, M., Takahashi, F., Yoshikawa. 2016. Catalytic hydrothermal liquefaction of microalgae using nanocatalyst. Applied Energy. 183 (1). 566-576. http://dx.doi.org/10.10167j.apenergy.2016.09.017 [ Links ]
Sawayama, S., Inoue, S., Dote, Y. & Yokoyama, S., 1995. CO2 fixation and oil production through microalga. Energy. Conservation and Management, 36(6-9), 729 - 731. https://doi.org/10.1016/0196-8904(95)00108-P [ Links ]
Shuping, Z., Yulong, W., Mingde, Y., Kaleem, I., Chun, L., Tong, J. 2010. Production and Characterisation of bio-oil from hydrothermal liquefaction of microalgae Dunaliella tertiolecta cake. Energy, 35:5406-5411. https://doi.org/10.1016/i.energy.2010.07.013 [ Links ]
Singh, R., Bhaskar, T., Balagurumurthy, B. 2015. Effect of solvent on the hydrothermal liquefaction of macro algae Ulva fasciata. Process Safety and Environmental Protection. 93. 154-160. https://doi.org/10.1016/i.psep.2014.03.002 [ Links ]
Toor, S.S., Rosendahl, L., Rudolf, A. 2011. Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy, 36:2328-2342. https://doi.org/10.1016/i.energy.2011.03.013 [ Links ]
Vieler, A., Wilhelm, C., Goss, R., Süß, R., Schiller, J. 2007. The lipid composition of the unicellular green alga Chlamydomonas reinhardtii and the diatom Cyclotella meneghiniana investigated by MALDI-TOF MS and TLC. Chemistry and Physics of Lipids, 150:143-155. https://doi.org/10.1016/i.chemphyslip.2007.06.224 [ Links ]
Vlaskin, M.S., Grigorenko, A.V., Chernova, N. I., Kiseleva, S. V. 2018. Hydrothermal liquefaction of microalgae after different pre-treatments. Energy Exploration & Exploitation. 36 (6). 1546-1555. https://doi.org/10.1177/0144598718777107 [ Links ]
Xu, C., Lancaster, J. 2008. Conversion of secondary pulp/paper sludge powder to liquid oil products for energy recovery by direct liquefaction in hot-compressed water: Water Research. 42 (6-7). 1571 - 1582. https://doi.org/10.1016/i.watres.2007.11.007 [ Links ]
Xu, D., Savage, P.E. 2014. Characterization of biocrudes recovered with and without solvent after hydrothermal liquefaction of algae. Algae Research. 6. 1-7. https://doi.org/10.1016/i.algal.2014.08.007 [ Links ]
Yang, M., Her, N., Ryu, J., Yoon, Y. 2014. Determination of perchlorate and iodide concentrations in edible seaweed. International Journal of Environmental Science and Technology, 11:565-570. https://doi.org/10.1007/s13762-013-0263-7 [ Links ]
Yuan, X., Wang, J., Zeng, G., Huang, H., Pei, X., Li, H., Liu, Z., Cong, M. 2011. Comparative studies of thermochemical liquefaction characteristics of microalgae using different organic solvents. Energy, 36 :6406-6412. https://doi.org/10.1016/i.energy.2011.09.031 [ Links ]
Zepke, H.D., Heinz, E., Radunz, A., Linscheid, M., Pesch, R. 1978. Combination and positional distribution of fatty acids in lipids from blue-green microalgae. Archives of Microbiology, 119:157-162. https://doi.org/10.1007/BF00964267 [ Links ]
Zhou, D., Zhang, L., Zhang, S., Fu, H., Chen, J. 2010. Hydrothermal liquefaction of macroalgae Enteromorpha prolifera to bio-oil. Energy and Fuels, 24:4054-4061. https://doi.org/10.1021/ef100151h [ Links ]
Zou, S., Wu, Y., Yang, M., Li, C., Tong, J. 2009. Thermochemical catalytic liquefaction of the marine microalgae Dunaliella tertiolecta and characterisation of bio-oils. Energy and Fuels, 23:3753-3758. https://doi.org/10.1021/ef9000105 [ Links ]
* Corresponding author: Tel +27 72 217 4882 / +27 41 504 2380; email: baloyih82@gmail.com;














