Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of Energy in Southern Africa
On-line version ISSN 2413-3051
Print version ISSN 1021-447X
J. energy South. Afr. vol.28 n.3 Cape Town Aug. 2017
http://dx.doi.org/10.17159/2413-3051/2017/v28i3a2354
ARTICLES
Bioethanol production from lignocellulosic sugarcane leaves and tops
Charlie Marembo DodoI, *; Samphson MamphweliII; Omobola OkohI
IDepartment of Chemistry, University of Fort Hare, Private Bag X1314, Alice, 5700, South Africa
IIInstitute of Technology, University of Fort Hare, Private Bag X1314, Alice, 5700, Alice, South Africa
ABSTRACT
Bioethanol production is one of the most promising possible substitutes for fossil-based fuels, but there is a need to make available cost-effective methods of production if it is to be successful. Various methods for the production of bioethanol using different feedstocks have been explored. Bioethanol synthesis from sugarcane, their tops and leaves have generally been regarded as waste and discarded. This investigation examined the use of lignocellulosic sugarcane leaves and tops as biomass and evaluated their hydrolysate content. The leaves and tops were hydrolysed using concentrated and dilute sulphuric acid and compared with a combination of oxidative alkali-peroxide pre-treatment with enzyme hydrolysis using the enzyme cellulysin® cel-lulase. Subsequent fermentation of the hydrolysates into bioethanol was done using the yeast saccha-romyces cerevisae. The problem of acid hydrolysis to produce inhibitors was eliminated by overliming using calcium hydroxide and this treatment was subsequently compared with sodium hydroxide neutralisation. It was found that oxidative alkali pre-treatment with enzyme hydrolysis gave the highest yield of fermentable sugars of 38% (g/g) for 7% (v/v) peroxide pretreated biomass than 36% (g/g) for 5% (v/v) with the least inhibitors. Concentrated and dilute acid hydrolysis each gave yields of 25% (g/g) and 22% (g/g) respectively, although the acid required a neutralisation step, resulting in dilution. Alkaline neutralisation of acid hydrolysates using sodium hydroxide resulted in less dilution and loss of fermentable sugars, compared with overliming. Higher yields of bioethanol of 13.7 g/l were obtained from enzyme hydrolysates than the 6.9 g/l ethanol from dilute acid hydrolysates. There was more bioethanol yield of 13.7 g/l after 72 hours of fermentation with the yeast than the 7.0 g/l bioethanol after 24 hours.This research showed that it is possible to use sugarcane waste material to supplement biofuel requirements and that combining the chemical and biological methods of pretreat-ments can give higher yields at a faster rate.
Keywords: biomass pretreatment; biofuels; cellulose accessibility; alkali peroxide pretreatment
1. Introduction
The ever-rising cost of fossil fuel, coupled with it being non-renewable, put a great impetus on the science, engineering and technology research to examine alternative sources of fuel. Bioethanol production has the potential to support and perhaps even surpass current depletion of fossil fuel sources(Hui Li et al (2009). The greatest possible source of sugars for ethanol production is starch-based crops, such as those containing cellulose, which is one of the main energy-providing food sources (Sun and Cheng 2002). Population growth and droughts, however, led to severe food shortages and resulted in the need to find alternative sources of cellulose for a sustainable supply for bioethanol production (Taherzadeh et al 2007). Starch-based crops such as sugarcane produce large volumes of waste in the form of plant leaves and tops (Shields and Boopathy, 2011), which contain lignocellulose, which can be used to produce ethanol (Zhu et al, 2015). Research is being carried out in order to find efficient, cost-effective and environmentally friendly ways to produce lignocellu-lose-based bioethanol (Rabelo et al, 2011; Shields and Boopathy, 2011).
Bioethanol is used in blends with gasoline in varying proportions, such as 10% in the US or 22% in Brazil (Wyman, 1994). It is an oxygenated fuel with 35% oxygen that decreases particulate matter and NOX emissions from combustion reactions (Prasad et al., 2006). According to Lynd et al. (1991), bioethanol may be used directly (95% ethanol and 5% water) to the benefit of the environment. Bioethanol has better combustion and acts as an octane booster in fuel blends (Prasad et al., 2007) despite ethanol having two-thirds the heat and energy value of petrol. A similar booster role is played in diesel by the conventional additive meta-tertiary butyl ethanol, although it has been identified as a potential health hazard (Prasad et al., 2007). Bioethanol has the advantage of assisting the environment by reducing global warming through the use of carbon dioxide by plants. The global warming potential (GWP) in Brazil, in the pre-mechanisation situation (100% burning of biomass residue), and the current (~ 50% burning) and future (100% mechanisation) situations, was calculated by Galdos et al.(2013). Galdos et al.(2013) found that current methods of ethanol production have a GWP 46% smaller than pre-mechanisation and are projected to be 70% smaller with completely mechanised harvesting of food crops residues, with a subsequent reduction in emissions of black carbon (found in soot).
The United States and Brazil together are responsible for 89% of the world's bioethanol production (Limayem et al., 2012). The main raw material used in bioethanol production in the United States is corn, with up to 48.52 billion litres of bioethanol produced, while sugarcane juice and molasses are used by Brazil (Limayem et al 2012). Different feedstocks across the world are being investigated, including crops such as rice and sugar beets. The current production of bioethanol is, however, not enough to replace a substantial part of the one trillion gallons of fossil-based fuel consumed globally each year (Limayem et al., 2012). One of the main problems the world is faced with is population growth, coupled with food shortage, and using food sources for bioethanol production carries an ethical challenge, as does prioritising biofuel crops over basic food crops. Research has focused on food crop residues, such as corn stover, rice hulls and sugarcane bagasse, which are lignocellulosic materials. Using such residues as raw material for bioethanol production would not compete with food sources such as sugar from a sugarcane plant. Mechanised harvesting can produce huge amounts of waste materials that can be used in bioethanol production (Sant'ana da Silva et al., 2010), which would be beneficial through increasing utilisation of the whole sugarcane plantation. Lignocellulosic material is renewable, unlike the current fossil-based fuels and helps environmental management. Sugarcane leaves are usually either burnt to enable manual harvest, adding to environmental pollution and greenhouse gases, or they are left in the field as part of manure, providing soil nutrients (Sant'ana da Silva et al., 2010). It could be beneficial to convert most of the sugarcane waste into biomass to be used for bioethanol production rather than burn it.
The use of lignocellulosic materials has its own challenges, one of which is the recalcitrance of the biomass. Lignocellulose contains a matrix of lignin, cellulose and hemicellulose (Talebnia et al., 2010). Cellulose is a polymer of glucose units connected through b-1,4-glycosidic bonds. The breaking of these glycosydic linkages is what is referred to as hydrolysis. Steps involved in bioethanol production include pretreatment, hydrolysis, fermentation and distillation (Prasad et al., 2007; Talebnia et al., 2010). Different pretreatment methods are being examined from milling, through the use of hot water, chemical treatment to biological treatment, and mechanised treatments such as microwaves. The development of environmentally friendly, efficient and cost-effective methods is the basis of modern research in lignocellulose bioethanol production. This investigation focused on the production of bioethanol from sugarcane waste material in the form of leaves and tops using environmentally friendly and cost-effective methods.
2. Experimental methods
Methodologies followed during pretreatment and treatment of biomass through to bioethanol production were grouped into three basic categories: pre-treatment, treatment and characterisation (Talebnia et al., 2010). Treatment methods used were acid hydrolysis and treatment with enzyme hydrolysis. Both dilute acid pretreatment and alkali-peroxide pretreatment were followed by enzyme hydrolysis. Hydrolysis steps were subsequently followed by fermentation and characterisation steps. The material used was sugarcane leaves and tops supplied by Malalane Mill, at Mhlathi Farm.
Pretreatment of biomass
The lignocellulosic material was washed by triple rinsing with deionised water, followed by drying in an oven at 60 for 24 hours to remove residual sucrose and water soluble, and then left at room temperature (Ou et al., 2007). A blender was used to cut the material into small particles; this was sifted through a 2 mm screen, to increase material surface area, before the material was packed in sealed plastic bags and stored frozen, to ensure the materials remained in a more or less similar condition before being subjected to different pretreatment and treatment conditions.
Concentrated acid hydrolysis method
High acid concentrations of 30% and 72% sulphuric acid (v/v H2SO4) were used for purposes of comparison with dilute acid treatment yield. Since both concentrations were high, the subsequent step of fermentation was not carried out on hydrolysates. A range of residence times from one to twelve hours were considered. Up to 300 mg of the sample was weighed and placed into 100 ml glass bottles. This was followed by the addition of 3 ml of the 30% acid concentration. The same procedure was repeated for the 72% acid concentration, as well as deionised water blanks. All experiments were carried out in triplicate. Acid hydrolysis requires a neutralisation step and this was carried out using alkali neutralisation using sodium hydroxide and overliming.
Dilute acid hydrolysis and enzyme fermentation method
The dilute acid method involved the preparation of 1.6 and 2M H2SO4for hydrolysis. A dry substrate amount of 3 g was weighed and placed into nine 300 ml glass bottles, with 200 ml of 1.6M sulphuric acid was added to each, and the process repeated for 2M sulphuric acid and deionised water control. The mixtures were then left to soak for two hours at room temperature, followed by autoclaving at 121 °C for 20 minutes, then left to cool at room temperature. The inhibitors removal/detoxification of lignocellulosic hydrolysates was achieved by the use of calcium hydroxide for over liming and sodium hydroxide (NaOH) for neutralisation.
Alkali/oxidative pretreatment method
Solutions of 0.5%, 3%, 5%, and 7% (v/v) hydrogen peroxide (H2O2) were prepared. Sodium hydroxide was added to each of these solutions to adjust the pH to 11. Aliquots of 4 g dry sugarcane leaves and tops (material) were prepared into 500 ml Erlenmeyer flasks to which 100 ml of solutions were subsequently added. These 100 ml mixtures of samples and concentrations were each soaked for 48 hours, during which sampling at 24-hour intervals took place. In addition, a deionised water control was conducted without adjusting the pH. Each H2O2, pH, and the time-treatment combination was repeated three times. After the allotted amount of time for soaking, the mixtures were filtered and residues collected. The residues were each triple-rinsed for 30 minutes in deionised water to wash off the pretreatment chemicals and any soluble matter then oven-dried at 100 °C for approximately 12 hours. The dried residue was then soaked in a buffer pH 4.8 to prepare for the next step of hydrolysis.
Enzyme hydrolysis method
The enzyme cellulase was used to hydrolyse products of dilute acid and alkali/oxidative pretreatments to produce the reducing sugars whose concentration were determined using the dinitrosalicylic (DNS) acid method. A buffer of pH 4.8 was prepared according to the International Union of Pure and Applied Chemistry (IUPAC) method (Ghose, 1987). A solid loading of 15% (w/v) of pretreated hydrolysates was used. Enzyme hydrolysis was conducted at mild conditions of temperature (45-50) and pH 4.8. The advantage of enzyme hydrolysis over acid hydrolysis is that it does not have a corrosion problem (Duff and Murray, 1996). A cellulase dosage of 10 filter paper units/g (Ghose, 1987) was followed as it provides high levels of glucose in a reasonable time of 48-72hours (Gregg and Saddler, 1996).
Fermentation method
Fermentation was carried out using the yeast sac-charomyces cerevisae. The growth medium consisted of 20 grams per litre (g/l) of saccharose, 5 g/l of yeast extract, 5 g/l of dipotassium phosphate, 1.5 g/l of ammonium chloride, and 1.15 g/l of potassium chloride and 0.65 g/l of magnesium sulphate hep-tahydrate.A 10% volume per volume (v/v) yeast inoculum was charged into the 250 ml stopped conical flasks together with the nutrients. Reaction temperatures of about 30 °C were maintained throughout the fermentation process. A 30 ml sample was taken at 24-hour, 5-day and 7-day intervals and micro-centrifuged at 5000 revolutions minute for 15 minutes, then filtered. The supernatant was taken to a gas chromatography Perkin Elmer XL model equipped with a flame ionisation detector at 250 °C to measure the ethanol content. Separate hydrolysis and fermentation was used to ferment the hydrolysates and all fermentations were carried out in triplicate. The batch fermentation method was followed to ferment hydrolysates into bioethanol.
3. Characterisation
A variety of instruments were used to characterise the lignocellulose biomass in terms of surface morphology, crystalline structure, reactivity and substrate concentrations. These instruments were kindly made available by the departments of Chemistry and Biochemistry and the Fort Hare Institute of Technology, all at the University of Fort Hare, Alice Campus, South Africa.
Scanning electron microscopy (SEM) and Energy dispersive x-ray spectroscopy
The imagery was produced by a JEOL JSM 6390 scanning electron microscope (SEM) equipped with a secondary electron detector to compare the effectiveness of pretreatment and treatment methods. The images of the samples were produced by focusing a beam of electrons on the sample. This gave the sample's surface topography. Energy dispersive spectroscopy is a chemical microanalysis technique which was used in conjunction with SEM to analyse the elemental composition of the material. A gold/palladium coating was applied to the material to enhance responsiveness and readability to the instruments.
Enzyme activity - Filter paper assay for saccharifying cellulase
There are a number of challenges faced when characterising cellulase, including the varying nature of the substrate and its insolubility; the little literature and understanding of processes and activities involved when endo- and exo-glucanases coordinate to break the substrate; and the variety of end products produced (Ghose, 1987). Hence a series of empirical assays were developed to try and standardise the characterisation procedures. The filter paper assay for saccharifying cellulase from the IUPAC Applied Chemistry Division Commission on Biotechnology was used (Ghose, 1987). The uv-vis-spectrophotometer was used to measure absorbance at 540nm with an objectiveto determine the amount of enzyme that will produce 2mg of glucose as filter paper units (FPU) per ml. Reducing sugar estimation by DNS method (Ghose, 1987) was then used to determine the amount of reducing sugars present. All enzyme concentrations were prepared and analysed in triplicate and the results averaged. The results were then used to estimate the concentration of enzyme that would produce 2mg of glucose in 1h from the appropriate substrate. This concentration is equivalent to one FPU. Subsequent enzyme hydrolysis experiments were carried out using an enzyme concentration of 10 FPU.
X-ray diffraction
The ideal sample has to be homogeneous to ensure that the crystallites are randomly distributed. The sample was then pressed into a sample holder and analysed by X-ray diffraction (XRD) on the D8 advance diffractometer (Bruker AXS, Germany). The XRD had CuKa radiation, wavelength of 0.1542 nm at 40 kV and 40 mA with goniometer radius of 280 mm. The diffraction intensity was measured in the range of 2Θ = 10-45° with a step size of 0.02° per second. The method of Segal et al. (1959) was used to calculate the crystallinity indices (CrI). Nickel-filtered CuKa radiation (λ =0.1542 nm) was used at 40 kV and 40 mA. The diffraction intensity was measured in the range of 2Θ =10-60°, with a step size of 0.02° at a rate of 2°/min. The CrI calculations were based on Segal et al.'s (1959) method, given by Equation 1.

whereI002 is the intensity of the 002 crystalline peak at 2Θ = 22.4° and Iam corresponds to the amorphous cellulose region for cellulose, hemicellulose and lignin at 2Θ = 18.0° (Sindhu et al., 2011).
Gas chromatography
This technique was used to characterise the presence of bioethanol with gas chromatogram spectrometer Perkin Elmer XL model equipped with flame ionisation detector. The initial oven temperature was 65 °C and it was held there for five minutes. It was then increased to 150 °C at a rate of 4 °C/min and held for five minutes before being raised to 250 °C at a rate of 4 °C/min and held for a further five minutes and then set on split. The injector temperature was set at 175 °C and detector temperature set at 250 °C.
A UV-VIS spectrometer was used for DNS assays and was set at a wavelength of 540 nm to analyse the hydrolysates. The FPU method for saccharifying cellulase from the IUPAC Applied Chemistry Division Commission on Biotechnology was used, particularly to estimate the amount of reducing sugars in the hydrolysates. The DNS reagent was prepared according to IUPAC method. Once mixed with the hydrolysates, the DNS reagent attaches itself to glucose molecules. Glucose standards of 0.2-5.0mg of glucose per millilitre were prepared together with a DI water blank for 100% transmittance. The results were used to plot a graph of mg of glucose against absorbance at 540 nm (A540). The graph was then used to estimate the amount of glucose produced. The XRD was used to analyse the change in crystallinity of the material. The method of Segal et al. (1959) was then used to calculate the crystallinity indices.
4. Results and discussion
Pretreatment and treatment
Scanning electron microscope images indicate the effectiveness of the pretreatment imposed. In Figure 1, SEM images E and F are of the raw sugarcane leaves and tops before pretreatment had been effected. Not much flaking can be seen on the surfaces of the material. This is in contrast to the images labelled A-B and C-D, which are images of 72% and 30% concentrated sulphuric acid pretreat-ed sugarcane laves and tops respectively, where there is a high degree of surface flaking and rupturing. The rupturing is more pronounced for 72% sulphuric acid pretreatment than it is for 30% sulphuric acid pretreament. The SEM images in Figure 1 are given at two resolutions of 500 and 2000 and illustrate the surface rupture and lignocellulosic rupture that occurred at high acid concentration compared with material without pretreatment.
The flakes and rough surfaces in Figure 1 (A and B) are indications that 72% H2SO4 resulted in the highest disruption and destruction of the lignin-hemicellulose-cellulose complex, while 30% H2SO4 also resulted in nearly as much disruption and part destruction of the complex. The SEM was also used to assess the effectiveness of dilute acid hydrolysis and hydrogen peroxide pretreatment and the images are in Figure 2. The raw untreated material image A shows relatively undisturbed surfaces whereas 1.6M sulphuric acid (B) had a slightly damaged lignocellulose structure. The structural damage was more for 2M sulphuric acid pretreated lignocel-lulose material, image D than 1.6M acid. Hydrogen peroxide pretreated sugarcane leaves and tops also exhibited some degree of surface disruption.
Figure 2 also shows that, despite some degree of flaking and rupturing caused by blending of the untreated material, this occurrence became even more pronounced for 7% hydrogen peroxide and 2M acid pretreated material.
Electron dispersive spectroscopy values indicate that there was a change in the concentrations of carbon and oxygen, as can be seen in Table 1. This change was more pronounced for oxygen present in hydrogen peroxide and 72% acid pretreated material, with averages of 56.33% and 53.77% respectively up from 48.81% for the untreated material. This could possibly be attributed to the presence of more glucose and oligosaccharides at the surfaces of the material. Much of the lignocellulosic material upon hydrolysis is broken down exposing more surfaces with COH bonds.The amount of carbon element, however, decreased slightly from 32% in untreated material to 27.4% for 2M acid pretreated and 27.91% for hydrogen peroxide pretreated material. Table 1 presents mean values after analyses were done in triplicate.
The SEM values also gave evidence of availability of high cellulose surface area for hydrolysis after blending. This was crucial for the next steps of enzyme hydrolysis and yeast fermentation. Dilute acid treatment did not appear to have significantly altered the surface of the material in favour of hydrolysis steps, when1.6M acid treated material decreased in oxygen amounts.
The X-ray diffractograms of pretreated and treated material
The results showed that treating the raw blended material significantly reduced the crystallinity of the lignocellulose, resulting in the more amorphous cellulose being made available. The XRD diffractograms in Figure 3, however, show that concentrated acid hydrolysis significantly reduced the crys-tallinity of the lignocellulose material, while 72% sulphuric acid gave the resultant residue with the least crystallinity.

The XRD spectra of dilute acid hydrolysis in Figure 4 show that, despite the ability of both the 1.6 and 2M H2SO4 to reduce the crystallinity of the lignocellulose, there was relatively not much difference in the overall effects of the two concentrations. There was a significant decrease in the crystallinity index after acid pretreatment with both the 1.6 and 2M dilute sulphuric acid concentrations. The peaks at 2Θ = 18 and 2Θ = 22.4 decreased after pretreatment but significantly so for the latter (Chen et al., 2011; Sindhu et al, 2011; Segal et al, 1959). This indicates an increase in successful breakdown of the cellulose crystal lattice within the lignocellulose.
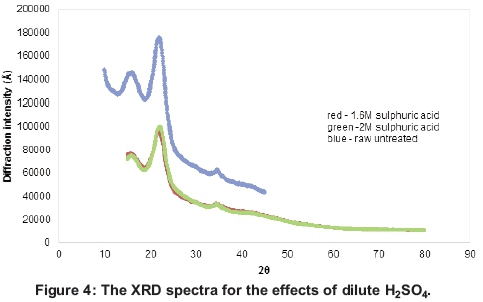
The XRD spectra for the alkali peroxide pretreat-ment in Figure 5 indicate that there was a significant increase in the crystallinity of the material after pretreatment, while there was a little difference between pretreatment for 24 and 48 hours. The increase in crystallinity was attributed to hemicellu-lose solubilisation and partial lignin degradation, as reported by Sun and Cheng (2002), where cellulose was then more exposed in preparation for enzyme hydrolysis.
The crystallinity indices (CrI) calculated using the method of Segal et al. (1959) also indicate a sharp increase upon pretreatment from CrI values of 28% for the raw untreated material to 45.6% and 46.4% after 24 and 48 hours treatment respectively.
Dinitrosalicylic acid assay results for concentrated acid hydrolysis show that there was a reduction in the amount of reducing sugars present in the hydrolysates. This was probably because of the dilution that took place during detoxification. The results of the glucose quantities indicate that there were more reducing sugars present in the 72% sulphuric acid hydrolysates than there were in the 30% sulphuric acid hydrolysates.
The formula used for calculation of glucose is shown in Equation 2.

Pretreatment with 72% sulphuric acid gave the highest yield of reducing sugars per gram biomass of 76.5% (g/g) with 30% acid pretreated material producing 46.9% (g/g) reducing sugars. Overall more reducing sugars were present after neutralising with sodium hydroxide (NaOH) than after neutralising the acid with overliming (CaOH)2. This can be attributed to the high Ca(OH)2 solid residue present during overliming that results in some reducing sugar particles being adsorbed to the Ca(OH)2 residue. Dilute acid pretreatment yielded lower amounts of reducing sugars of 22% and 25% for 1.6 and 2M sulphuric acid respectively. All experiments were carried out in triplicate and mean results are as shown in Table 2.
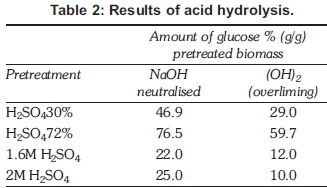
It would be beneficial to use 1.6M H2SO4 rather than 2M H2SO4 since there was not much of a difference in the reduction of the degree of crystallinity and the yield of sugar with 22% (g/g) yield of glucose per gram of biomass. This will in turn reduce costs incurred in the neutralisation step without affecting the sugar yield. Lower temperatures were employed in dilute acid hydrolysis. The set back with acid hydrolysis remains the further dehydration of sugars into furfural and hydroxyhemifurfural. Overliming requires a concentration step in order to increase the sugar concentration before the succeeding step of fermentation so as to make it viable.
The DNS assay was also used to calculate the amount of reducing sugars present in the alkali peroxide pretreatment hydrolysates. The highest yield for glucose was obtained from 7.0% peroxide pre-treatment with 380 mg/g of glucose. There was, however, a significant efficiency in yield from 3.0% peroxide pretreatment of 326mg/g.
Fermentation
There was not much of a difference in terms of ethanol produced after 24 hours of fermentation. The difference was, however, seen after 72 hours of fermentation when peroxide-treated hydrolysates had slightly more ethanol than acid hydrolysed hydrolysates, as shown in Table 3. The highest yield of bioethanol 13.7 g/l was obtained from hydro-lysates produced using enzyme hydrolysis method compared with dilute acid yield of 11.7 g/l.

Alkali neutralisation with sodium hydroxide resulted in the highest amount of reducing sugars being present in the hydrolysates without requiring a further step of concentration which would have increased the costs. Enzyme hydrolysis gave a high amount of hydrolysates 380 mg/g biomass without requiring any neutralisation. The absence of fermentation inhibitors resulted in more bioethanol being produced through enzyme hydrolysis of 13.7 g/l than from acid hydrolysis 11.7 g/l after 72 hours of fermentation. Ethanol yield could have been higher if the yeast saccharomyces cerevisiae could ferment both pentose and hexose sugars. Alkali hydroxide pretreatment has the advantage of being environmentally friendly and not requiring equipment and neutralisation steps.
5. Conclusions
In this investigation, pretreatment of lignocellulosic sugarcane leaves and tops was carried out using cheaper methods of blending, unlike the energy-intensive ball milling. Concentrated acid hydrolysis resulted in the production of large amounts of reducing sugars, which was evidence of a remarkable hydrolysis success. The problem associated with high concentrations was that inhibitors were inevitably produced, therefore required a detoxification step which reduced yield. Dilute acid hydrolysis greatly improves the accessibility of cellulose to hydrolysis enzymes in the next step. Alkali peroxide gave the highest bioethanol yield since it did not produce inhibitors. Acid hydrolysis offers the advantage of taking a shorter time than enzyme hydrolysis. Pretreatment is an important step in lig-nocellulosic bioethanol production. The main aim of pretreating the lignocellulosic material before hydrolysis is to remove the lignin and hemicellu-lose. This also helps in reducing the crystallinity of cellulose resulting in the more amorphous cellulose which is more accessible to hydrolysis.
Future work in bioethanol production needs to be focussed on producing enzymes that are more tolerant to harsh acid conditions or produce reducing sugars faster. The same also applies to fermentation yeasts.
References
Adapa PK., Schoenau G.J., Canam T. and Dumonceaux T. 2011. Quantitative analysis of ligno-cellulosic components of non-treated and steam exploded barley, canola, oat and wheat straw using Fourier transform infrared spectroscopy. Faculty Research and Creative Activity. Paper 107. [ Links ]
Ahring B.K., Jensen K., Nielsen.,Bjerre A.B. and Schmidt A.S. 2006. Pre-treatment of wheat straw and conversion of xylose and xylan to ethanol by thermophilic anaerobic Bacteria. Bioresource 58: 107-113. [ Links ]
Alvira P, Tomas-Pejo E., Ballesteros M. and Negro M.J.2010. Pre-treatment technologies for an efficient bioethanol production process based on enzyme hydrolysis. A review. Bioresource Technology 101:4851-4861. [ Links ]
Balat M. 2011. Production of bioethanol from lignocel-lulosic materials via the biochemical pathway. A review. Energy Conversion and Management 52: 858-875. [ Links ]
Belkacemi K., Turcotte G., Savoie Pand Chornet E.1997. Ethanol production from enzymatic hydrolyzates of cellulosic fines and hemicellulose-rich liquors derived from aqueous/steam fractionation of forages. Industrial & Engineering Chemistry Research 36: 4572-4580. [ Links ]
Boopathy R. and Shields S. 2011. Ethanol production from lignocellulosic biomass of energy cane. International Biodeterioration and Biodegradation 65: 142-146. [ Links ]
Brethauer S., and Wyman C. E. 2010.Continuous hydrolysis and fermentation for cellulosic ethanol production. BioresourceTechnology: 4862-4874. [ Links ]
Buaban B., Inoue H., Yano S., Tnapongpipat S., Ruanglek v., Champreda V, Pichyangkura R., Rengpipat S. andEurwilaichitr L.2010. Bioethanol production from ball milled bagasse using an on-site produced fungal enzyme cocktail and xylose- fermenting pichiastipitis. Journal of Bioscience and Bioengineering 110(1): 18-25. [ Links ]
Cardona C. A. and Sánchez O. J. 2007. Fuel ethanol production: Process design trends and integration opportunities. Bioresource Technology 98: 2415-2457. [ Links ]
Chen, W., Tu, Y. and Sheen, H. 2011. Disruption of sugarcane bagasse lignocellulosic structure by means of dilute sulphuric acid pre-treatment with microwave-assisted heating. Applied Energy 88: 2726-2734. [ Links ]
Colthup, N. B., Daly L.H. and Wiberley S.E.1990.Introduction to infrared and raman spectroscopy. 3rd ed. Boston, MA: Academic Press. [ Links ]
Dale B. E., Leong C. K., Pham T. K., Esquivel V. M., Rios I.and Lalitimer V. M. 1996. Hydrolysis of ligno-cellulosics at low enzyme levels. Application of the AFEX process. Bioresource Technology, 56:111-116. [ Links ]
Dawson L., Boopathy R. 2007. Use of post-harvest sugar cane for ethanol production. Bioresource Technology 98: 1695-1699. [ Links ]
De Bari I., Viola, E., Barisano D., Cardinale M., Nanna F, Zimbardi F, Cardinale G.andBraccio G.2002. Ethanol production at flask and pilot scale from concentrated slurries of steam-exploded aspen. Industrial & Engineering Chemistry Research 41: 1745-1753. [ Links ]
Duff S.J.B. and Murray W.D.1996. Bioconversion of forest products industry wastes cellulosics to fuel ethanol: a review. Bioresource technology 55:1-33. [ Links ]
Galdos M, Cavalett O., Seabra J.E.A., Nogueira L.A.H. and Bonomi A.2013.Trends in global warming and human health impacts related to Brazilian sugarcane ethanol production considering black carbon emissions. Applied Energy 104: 546 - 582. [ Links ]
Ghose T.K. 1987. Measurement of cellulose activities. Pure and Applied Chemistry 59(2): 259-268. [ Links ]
Ghosh, P. and Ghose, T.K. 2003. Bioethanol in India: recent past and emerging future. Advances in Biochemical Engineering/Biotechnology 85: 1-27. [ Links ]
Gong, C.S., Cao, N.J., Du, J.and Tsao, G.T. 1999. Ethanol production from renewable resources. Advances in Biochemical Engineering/Biotechnology 65: 207-241. [ Links ]
Gregg D. J., Boussaid A. and Saddler J. N. 1998. Techno-economic evaluations of a generic wood-to-ethanol process. Effect of increased cellulose yields and enzyme recycle. Bioresource Technology 63: 712. [ Links ]
Hamelinck C.N., van Hooijdonk G.and Faaij A.P.C. 2005. Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term. Biomass and Energy 28: 384-410. [ Links ]
Hsu C., Chang K., Lai M., Chang T., Chang Y. and Jang H. 2011. Pre-treatment and hydrolysis of cellulosic agricultural wastes with a cellulase producing strep-tomyces for bioethanol production. Biomass and Bioenergy 35: 1878-1884. [ Links ]
Hui Li, Nag Jong Kim, Min Jiang, Jong Won Kang andHo Nam Chang. 2009. Simultaneous saccharifi-cation and fermentation of Lignocellulosic residues pretreated with acid-acetone for bioethanol production. Bioresource Technology, 100: 3245 - 3251 http://www.bing.com/images/search?q=lignocellulose+diagram&qpvt=lignocellulose+diagram,2011/11/05. [ Links ]
Ingram L.O., Gomez PF, Lai X, Moniruzzaman M. and Wood B.E. 1998. Metabolic engineering of bacteria for ethanol production. Biotechnology and Bioengineering 58:204-14. [ Links ]
Karimi K., Kheradmandinia S. andTaherzadeh M. J. 2006.Conversion of rice straw to sugars by dilute acid hydrolysis. Biomass and Bioenergy 30. 247-243. [ Links ]
Laser M., Schulman D., Allen S. G., Lichwa J., Antal Jr M. J. and Lynd L. R. 2002. A comparison of liquid hot water and steam pretreatments of sugar cane bagasse for bioconversion to ethanol. Bioresource Technology 81:33-44. [ Links ]
Lee J. 1997.Biological conversion of lignocellulosic biomass to ethanol. Journal of Biotechnology; 56: 1-24. [ Links ]
Lenthan P, Orozco A., O'Neill E., Ahmad M.N.M., Rooney D.W. and Walker G.M. 2009. Dilute acid hydrolysis of lignocellulosic biomass. Chemical Engineering Journal 156: 395-403. [ Links ]
Limayem A. andRicke S.C. 2012. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Progress in Energy and Combustion Science 38: 449-467. [ Links ]
Lynd L.R., Cushaman J.H., Nichols R.J. and Wyman C.E.1991. Fuel ethanol from cellulogic biomass. Science 251:1318. [ Links ]
Margeot A., Hahn-Hagerdal B., Edlund M., Slade R.and Monot F. 2009. New improvements for lignocellu-losic ethanol. Biotechnology 20: 372-380. [ Links ]
Nakamura Y, Sawada T. and Inoue E. 2001. Enhanced ethanol production from enzymatically treated steam-exploded rice straw using extractive fermentation. Journal of Chemical Technology and Biotechnology 76: 879-884. [ Links ]
Negro M.J., Manzanares P, Ballesteros I., Oliva J.M., Cabanas A. and Ballesteros M. 2003. Hydrothermal pre-treatment conditions to enhance ethanol production from poplas biomass. Applied Biochemistry and Biotechnology 105-108: 87-100. [ Links ]
Olsson L. and Hahn-Hagerdal B. 1993. Fermentative performance of bacteria and yeasts in lignocellulose hydrolysates. Process Biochemistry 28: 249-57. [ Links ]
Olsson L., Linden T. and Hahn-Hagerdal B. 1992. Performance of microorganisms in spent sulfite liquor and enzymatic hydrolysate of steam-pretreat-ed Salix. Applied Biotechnology Biochemistry 34/35: 359-67. [ Links ]
Olsson L. and Hahn-Hagerdal B.1996. Fermentation of lignocellulosic hydrolyzates for ethanol production. Enzyme Microbiology Technology 18: 312-31. [ Links ]
Ou S., Luo Y., Xue F, Huang C., Zhang N. and Liu Z. 2007. Separation and purification of ferulic acid in alkaline-hydrolysate from sugarcane bagasse by activated charcoal adsorption/anion macroporous resin exchange chromatography. Journal of Food Engineering 78: 1298-1304. [ Links ]
Prasad S., Sing A. and Joshi H.C. 2007. Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resources, Conservation and Recycling 50: 1-39. [ Links ]
Rabelo S.C., Amezquita Fonseca N.A., Andrade R.R., Macielfilho R and Costa A.C. 2011. Ethanol production from enzymatic hydrolysis of sugarcane bagasse pre-treated with lime and alkaline hydrogen peroxide. Biomass and Bioenergy: 1-8. [ Links ]
Saad M.B.W., Oliveira L.R.M., Candido R.G., Quintana G., Rocha G.J.M. andGoncalves A.R. 2008. Preliminary studies on fungal treatment of sugarcane straw for organosolv pulping. Enzyme and Microbial Technology 43: 220-225. [ Links ]
SánchezO.J. and Cardona C.A. 2008. Trends in biotech-nological production of fuel ethanol from different-feedstocks.Bioresource Technology 99: 5270-5295. [ Links ]
Sant'ana da Silva A., Inoe H., Endo T., Yano S. and Bon E.P.S. 2010. Milling pre-treatment of sugarcane bagasse and straw for enzymatic hydrolysis and ethanol fermentation. Bioresource Technology: 7402-7409. [ Links ]
Schell, D.J., Farmer, J., Newman, M. and McMillan, J.D. 2003. Dilute sulphuric acid pre-treatment of corn stover in pilot-scale reactor. Investigation of yields, kinetics, and enzymatic digestibilities of solids. Applied Biochemistry and Biotechnology 105(1-3): 69-85. [ Links ]
Segal L., Creely J.J., Martin A. E. and Conrad C.M. 1959. An empirical method for estimating the degree of crystallinity of native cellulose using X-ray Diffractometer. Textile Research Journal 29: 786-794. [ Links ]
Sindhu R., Kuttiraja M., Binod P, Janu K.U., Sukumaran R.K. and Pandey A. 2011. Dilute acid pretreatment and enzymatic saccharification of sugarcane tops for bioethanol production. Bioresource Technology 102: 10915-10921. [ Links ]
Sun Y. And Cheng J.2002. Hydrolysis of lignocellulosic materials for ethanol production: A review. Biotechnology Resource 83: 1-11. [ Links ]
Taherzadeh, M.J. and Karimi K. 2007. Acid based hydrolysis processes for ethanol from lignocellulosic materials: a review. Bioresources 2: 472-499. [ Links ]
Talebnia F, Karakshev D.andAngelidaki I. 2010. Production of bioethanol from wheat straw: An overview on pre-treatment, hydrolysis and fermentation. BioresourceTechnology 101: 4744-4753. [ Links ]
Wyman C.E., Dale B.E., Elander R.T., Holtzapple M., Ladisch M.R. and Lee Y.Y. 2005. Coordinated development of leading biomass pretreatment technologies. Bioresource Technology 6: 1959-66. [ Links ]
Wyman C.E., Dale B.E., Elander R.T., Holtzapple M., Ladisch M.R. and Lee Y.Y. 2005. Comparative sugar recovery data from laboratory scale application of leading pretreatment technologies to corn stover. Bioresource Technology 96: 2026-32. [ Links ]
Wyman C.E., Dale B.E., Elander R.T., Holtzapple M., Ladisch M.R. and Lee Y.Y. 2005.Coordinated development of leading biomass pretreatment technologies. Bioresource Technology 96: 1959-66. [ Links ]
Wyman C. E. and Goodman B. J. 1993. Biotechnology for production of fuel, chemicals and materials. Applied Biochemistry and Biotechnology 39/40: 41-59. [ Links ]
Wyman C. E. 1995. Economic fundamentals of ethanol production from lignocellulosic biomass. In: Saddler J., Penner M., eds. ACS Symposium Series. Washington, D.C.: American Chemical Society 618: 272-90. [ Links ]
Wyman C. E. 1999. Biomass ethanol: technical progress, opportunities, and commercial challenges. Annual Review of Energy and the Environment 24:189-226. [ Links ]
Wyman, C.E. 1994. Ethanol from lignocellulosic biomass: technology, economics and opportunities. BioresourceTechnology 50:3-16. [ Links ]
Zheng Y., Lin H.M. and Tsao G.T. 1998. Pre-treatment for cellulose hydrolysis by carbon dioxide explosion. Biotechnology Progress 14(6):890-896. [ Links ]
Zhu M-Q., Wen J-L., Wang Z-W., Su Y-Q., Wei Q. and Sun R-C. 2015. Structural changes in lignin during intergrated process of steam explosion followed by alkaline hydrogen peroxide of Eucommiaulmoides Oliver and its effect on enzymatic hydrolysis. Applied Energy, 158: 233-242. [ Links ]
* Corresponding author: Tel.: +27 73 725 8772, +27 72 343 2006; Email: 201113835@ufh.ac.za, charliedodo@gmail.com














