Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of Energy in Southern Africa
versión On-line ISSN 2413-3051
versión impresa ISSN 1021-447X
J. energy South. Afr. vol.26 no.3 Cape Town ago. 2015
Comparative bioelectricity generation from waste citrus fruit using a galvanic cell, fuel cell and microbial fuel cell
Abdul Majeed Khan; Muhammad Obaid
Department of Chemistry, Federal Urdu University, Gulshan-e-Iqbal Campus, Karachi, Pakistan
ABSTRACT
This article demonstrates the new approaches for the generation of bioelectricity from waste citrus fruit using direct a galvanic cell (DGC), an indirect galvanic cell (IDGC), a conventional fuel cell (CFC) and a microbial fuel cell (MFC). The citrus fruit was used as whole for the preparation of DGC and their juices for the preparation of IDGC, CFC and MFC. The performance and bioelectrical parameters obtained were compared. The voltage found to be increased by increasing the number of cells in a series while, the current remains constant. Whereas the voltage remains constant and the current found to be increased with increasing the number of cells in parallel sequence. The power output of three units of citrus fruit connected together in a series found to be sufficient to turn on the LED light bulb in all cases. The result showed that lemons have the maximum power output by the DGC and MFC method, whereas grapefruit showed the maximum power output by IDGC, and thus considered as the best citrus fruit. Addition of NaCl solution in DGC and IDGC slightly increased the values of power output. The power output of citrus fruit was also determined by CFC and MFC before and after the inoculation of Escherichia coli. The detailed microscopic analysis of all the samples was carried out. It is found that all MFCs have higher power output as compared to their counterpart CFCs. However, maximum power output was displayed by DGCs. Moreover, a lemon fuel cell has the higher power output as compared to the fuel cells of other citrus fruit. This approach can be used to overcome the disadvantages of many non-renewable and conventional sources of energy including burning of fossil fuels to mitigate the major source of global warming and pollution by using such biodegradable and renewable sources.
Keywords: citrus fruit, bioelectricity, direct method, indirect method, galvanic cell, microbial fuel cell, E. coli
1. Introduction
Energy is the prime requirement of all sectors including industry, transportation, agriculture and domestic use without which advancement of technology and survival of life is not possible (Carvalho et al., 2011). Most of the energy around the world comes from non-renewable sources including: petroleum, coal, oil and natural gas which are being depleting at a high rate (Larhum, 2010). Fossil fuels are the major source of global warming and pollution due to increase in greenhouse gases, volatile matter and particles in the atmosphere (Khan et al., 2011). However, technologies of renewable energy are growing worldwide that can overcome these drawbacks (Christi, 2007; Goff et al., 2004; Ha et al., 2010). Biomass, which includes agricultural crops, seeds, algae and biowastes are major sources of renewable energy that replenish themselves through natural processes (Hossain and Mekhled, 2010; Mata et al., 2010; Dincer, 2000). Bioelectricity generation is reported from waste-water using a microbial fuel cell (Khan 2009, Khan et al., 2010, Khan et al., 2011, Khan and Naz, 2014). Lemon, orange and grapefruit are examples of biomass and commonly known as citrus fruit (Randhawa et al., 2014). They contain citric acid, sugar and other ingredients with sufficient chemical energy that can be converted into electrical energy by means of redox reaction with a specific condition and thus be utilized as batteries to light up LEDs and power up a clock or a calculator etc. (Kelter and Morgan, 1996; Goodisman, 2001; Swartling et al., 1998). Under certain conditions, the citric acid contained in citrus fruit may act as an electrolyte, which enables the generation of electricity just the same way as a galvanic battery (Oon, 2007).
The population around the globe is continuously increasing, which is demanding not only more food but also the energy to fulfil the requirement of the latest needs and technology. Some crops may be produced and consumed for both purposes like corn, sugarcane, fruit and vegetable oils. Therefore,
there is an urgent need to develop the food vs. energy priority for a sustainable future. However, the increase in crop yield with the passage of time is not as per the desired level, which may increase the price of food grain but on the other hand, hectors of arable land is available for additional harvesting of crops for both food and fuel (energy). This manuscript demonstrates the conversion of waste to energy using waste citrus fruit as a source of biofeed-stock. Large quantities of waste citrus fruit are generated from agricultural processes and in the retail markets worldwide. This waste is often simply dumped into landfills or the ocean. Therefore, there is no doubt in easy availability and cheap prices of such waste biofeedstock. Waste citrus fruit has sufficient content of acid and sugar that can be used for the production of bioelectricity using a galvanic cell and microbial fuel cell technology at a laboratory scale. This will not only reduce the disposal cost of waste but also increase a total of the production of bioenergy with nominal investment. Moreover, the production of citrus fruit is increasing gradually and there is no evidence that the supply of citrus fruit will face a shortage in the near future. Therefore, there is no significant impact of food vs energy due to the generation of bioelectricity from waste citrus fruit.
2. Experimental
2.1. Materials and instruments
The materials and instruments applied during this research included analytical weighing balance TE 3135-DS (Sartoris, Germany), digital multimeter, CD771 (Sanawa, Japan), pH meter, Hi-9810 (Hanna, Rhode Island), TDS/ conductivity meter, LF12 (Schott, Germany), Karl Fisher (Mettler, USA), Binocular Microscope, 107BN (Jinhua Huiyou Equipment and Instrument Co. Ltd.), incubator (MMM Medcenter Einrich-tungen, Germany), autoclave YX 280B (China), Cumber test kit (Roche), copper electrode, zinc electrode, LED (light bulb), copper electrical wire, connector, alligator clip, fruit (lemons, oranges, grapefruit), sodium chloride solution (1%), distilled water, potassium chloride (KCl), volumetric flask, beaker, glass vessel, container, PVC pipe and microorganism (E. coli), glass slide, crystal violet, Gram's stain, safranin, ethanol, and a wire loop.
2.2. Direct galvanic cell (DGC)
In this experiment, four series of fruit were studied for the generation of bioelectricity. The three series contain a single type of either of the waste fruit (lemon, orange and grapefruit) and the fourth series contain mixed fruit arranged in alternate arrangement in a successive manner. A zinc electrode of dimension 5 cm x 1 cm was inserted into one side of the fruit and a copper electrode of the same dimension was inserted into another side of the fruit. A copper wire was connected to the zinc electrode on one end and the other end was connected to one end of the electric socket. Similarly, a copper wire was connected to the copper electrode on one end and the other end was connected to another end of the electric socket to complete the circuit.
The voltage, current and other parameters of this electric circuit were determined with a digital multimeter with a positive terminal connected to the zinc electrode and a negative terminal connected with copper electrode. The LED was connected to the circuit with zinc electrode to its short leg and copper electrode to its long leg. Then the numbers of citrus fruit were increased in series by connecting the zinc electrode of one fruit to the copper electrode of the next fruit via copper wire using alligator clips. In addition to the above series combination, the cells were also connected in parallel sequence by connecting the anode electrodes (zinc) of all cells together and the cathode electrodes (copper) of all cells together. The electrical parameters were determined using the same multimeter. Afterwards, 1% solution (1 ml) of NaCl was injected into each fruit and the electrical parameters were measured (see Figure 1).
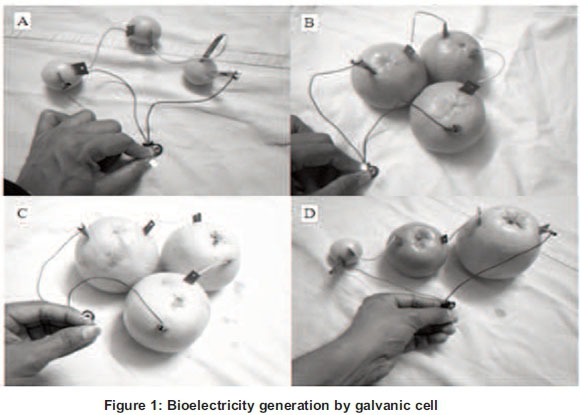
2.3. Indirect galvanic cell (IDGC)
During this experiment, the juice of waste citrus fruit (lemon, orange, grapefruit and mixed fruit), were collected into a separate glass vessel. The parameters of the fruit juices including pH, total dissolved solids (TDS), water content (Karl Fisher), acid content, salinity were determined and compared. The juices of citrus fruit were transferred into 1 to 4 glass vessels. One electrode of each metal (zinc and copper) of dimension 5 cm x 1 cm was inserted into the fruit juice distant (2 to 4 cm) to each other. A copper wire was connected to the zinc electrode on one end and the other end was connected to an electric socket. Similarly, a copper wire was connected to the copper electrode on one end and the other end was connected to the electric socket to complete the circuit. The voltage and current were noted by connecting a digital multimeter. The positive terminal was connected to the zinc electrode and negative terminal to the copper electrode. The LED was connected to the zinc electrode to its short leg and the copper electrode to the long leg of the LED bulb. The cell units were also connected in a series and parallel combination and the electrical parameters were determined using the same multimeter. Afterwards, 1% solution (1 ml) of NaCl was injected into each fruit juice and the electrical parameters were measured.
2.4. Conventional fuel cell
The construction of a conventional fuel cell (CFC) consists of two chambers. One is the Anodic chamber containing either of the fruit juice of waste lemon, orange, grapefruit and mixed fruits. The other is a cathodic chamber containing water in which air was continuously pumped by an aquarium pump. The two chambers were connected with a salt bridge (25% NaCl: 75% sand). One zinc electrode was submerged in the fruit juice of the anodic chamber and another electrode of copper metal was submerged in water. The two metal electrodes were connected together with a copper wire to compete the one unit of CFC. Then the numbers of the CFC unit were increased from one to four in a series by connecting the zinc electrode of one CFC to the copper electrode of the next CFC via copper wire. The electrical parameters with increasing number of CFC units were determined using the digital multimeter for each fruit juice (see Figure 2).
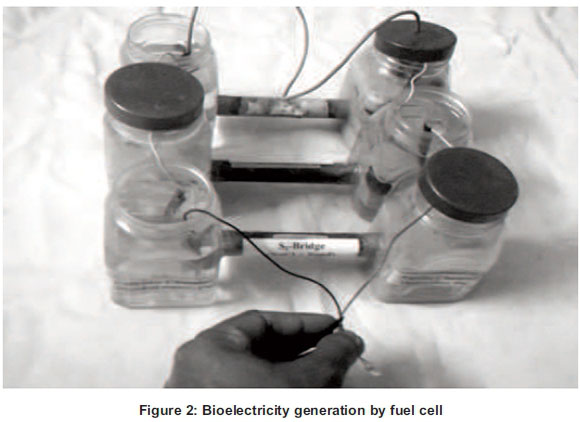
2.5 Microbial fuel cell (MFC)
2.5.1 Microscopic examination
The apparatus and glass wares used were sterilized either by autoclaving or a flame (wire loop) where required. The extract of fruit juices were subjected to microscopic examination before and after inoculation of microorganism. A reference slide with E. coli culture was prepared for comparison. The samples were incubated at 37°C for 7 days and the smears on glass slides were stained with standard procedure of gram's staining technique (Khan and Naz, 2014). The slides were examined through a lens having a resolution of 10/ 0.25 (160/0.17).
2.5.2. MFC construction
The MFC was constructed with the same electrical connection chambers as of CFC however, the microorganism (E. coli) was added to either of the fruit juice of lemon, orange, grapefruit and mixed fruits filled in the anodic chamber, which was sealed to prevent the entrance of air and thus forced the microorganism to aid the fermentation of the sugar contents of the fruit juice. The cell units were also connected in a series and parallel combination and the electrical parameters were determined using the digital multimeter for each sample.
3. Results and discussion
The drawbacks of conventional technologies of energy like fossil fuels which are non-renewable, being depleted and also considered as the major source of global warming and pollution stimulated us to conduct this research in which some basic parameters to generate the electricity from citrus fruit including lemon, orange, grapefruit and mixed fruit were investigated. Experiments were carried out using a galvanic cell (with two approaches namely direct method and indirect method), a fuel cell and a microbial fuel cell. In the direct method, whole fruit was used as a unit for the construction of a direct galvanic cell (DGC), whereas in the indirect method, fruit juices were used for the preparation of an indirect galvanic cell (IDGC, CFC and MFC). The galvanic cells (DGC and IDGC) were tested with and without the addition of NaCl solution as electrolyte. Furthermore, a conventional fuel cell (CFC) and a microbial fuel cell (MFC) were tested before and after the addition of microorganism (E. coli).
3.1 Galvanic cell
In a galvanic cell, proton (H+) of citric acid dissolves the zinc electrode of the anodic chamber to produce zinc ions (Zn2+) along with the liberated electrons which travel via copper wire to the copper electrode at the cathodic chamber and reacted with H+ ions from the citrus fruit that generated bio-H2 gas. This supply of electron generates electric current due to the potential difference of the two electrodes (Oon, 2007; Naidu and Kamakshiaih, 1995; Franco, 2005) (see Figure 3).

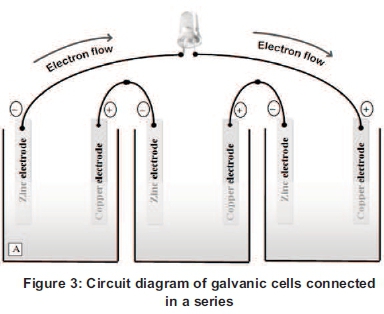
In the indirect method, the fruit juices were extracted and analysed for the determination of acid content, sugar, pH, total dissolved solids (TDS), salinity, water content, refractive index, conductivity etc. (see Table 1). The value of voltage and current in the indirect method was found to be lower but more stable than the direct method. This may be due to better homogeneity and less hindrance faced by ions in free flowing liquid medium as compared to the pulp of the whole fruit in the direct method. In the indirect method, the voltage and current does not get altered by increasing the amount (volume) of fruit juices in a glass container. When the same juices were divided up into four separate glass containers (galvanic cells) connected in a series, the voltage and power output were increased in a typical manner with an increasing number of cells and thus the LED light turn on.
3.2. Conventional fuel cell
In a conventional fuel cell, carbohydrates of the citrus fruit juices in the anodic chamber were to undergo the self-fermentation by environmental microorganisms to produce biogases (CH4, H2, CO2> N2 etc.), where CH4 and H2 were utilized as fuel along with liberation of H+ ions and electrons at the anodic chamber. The electrons moves from zinc electrode of the anodic chamber via copper wire and reach the copper electrode at the cathodic chamber in the form of current and the protons librated at the anodic chamber were transferred via a salt bridge to the cathodic chamber containing tap water, where it reacted with the air oxygen to produce water (John, 1983; Leon and Mugrwa, 1993; Badwal et al, 2015).
Anodic reactions:

Cathodic reactions:
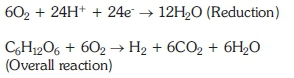
The conventional fuel cell (CFC) and microbial fuel cells (MFCs) were arranged in series. The anodic chamber of each fuel cell contain either of the fruit juice (lemon, orange, grapefruit and mixed fruit) and the cathodic chamber contains tap water with continuous flushing of air. The electrical parameters were measured before and after the inoculation of the microorganism in a closed anodic chamber to prevent the entrance of air oxygen.
3.3. Microbial fuel cell
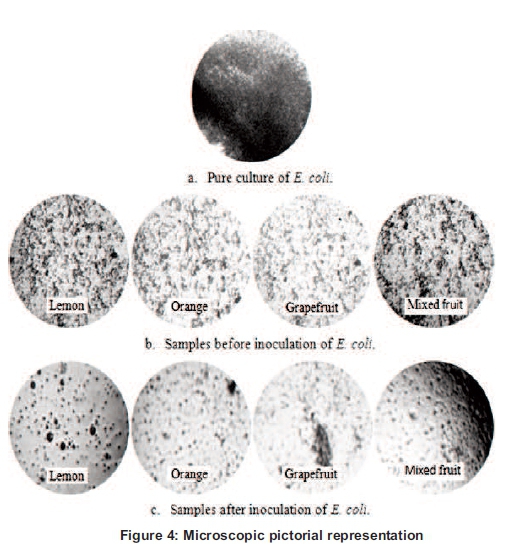
The reference slide was prepared by E. coli culture that displayed the rod shaped bacilli. The slide prepared by the fruit juice extract does not show the presence of E. coli instead random particles were observed. The slides of fruit juices prepared after the inoculation of E. coli showed few rod shaped E. coli (see Figure 4).
In a microbial fuel cell, microorganism uses carbohydrates of the citrus fruit juices as food and converts them to biogas, which is finally converted into H+, with the loss of electrons via a fermentation pathway in the absence of air oxygen in the sealed anodic chamber. The electrons move from the zinc electrode of the anodic chamber via copper wire and reach the copper electrode at the cathodic chamber in the form of current and the protons librated at the anodic chamber were transferred via the salt bridge to the cathodic chamber containing tap water where it reacted with O2 to produce extra ordinary pure water (Bennetto, 1990; Moawad, 2013; Delaney et al., 1984) (see Figure 5). The values obtained for voltage, current and power output by DGC, IDGC, CFC and MFC are displayed in Table 2.

3.5. Comparative study
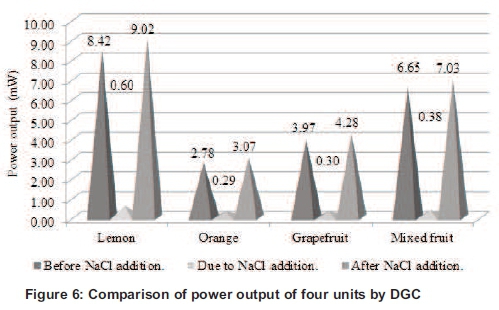
The voltage of the circuit was increased with the increase in the number of galvanic or fuel cells in series. Thus, the values of power output were also increased accordingly and the current of the circuit remains almost constant. The procedure was repeated with DGC and IDGC after the addition of NaCl (1ml of 1% solution) to lemon, orange, grapefruit and mixed fruit, which showed a slight increase in the values of the power output (see Figure 6).
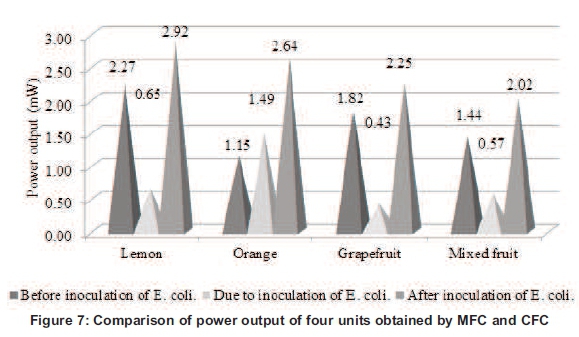
MFCs showed higher values of power output in all cases (lemon, orange, grapefruit and mixed fruit) as compared to their counterpart CFCs. Moreover, the MFC of lemon showed highest values of power output among the MFCs of citrus fruit (orange, grapefruit and mixed fruit series). However, the power outputs by MFCs are still lower as compared to their counterpart galvanic cells (DGC and IDGC). The difference in power output before and after the addition of microorganism is observed highest in the orange series. This is due to the fermentation of carbohydrates and acid contents (citric acid) present in oranges in the highest quantity as compared to other fruit and convert them into simpler compounds with the liberation of electrons and protons, which leads to surplus current (electrons) in the circuit and thus produced the highest power output difference. Therefore, it suggested that orange is a better choice for MFC in this experiment (see Figure 7).
In addition to above experimentation, a comparison of series and parallel sequence were made with lemon fruit and electrical parameters were used using DGC, IDGC and MFC methods. The results showed that, in parallel sequence, voltage remain constant nearly at a value obtained with a single unit however, the current increased with increasing the number of galvanic or fuel cells in contrast to the series circuit, where the voltage was increased with the increase in the number of galvanic or fuel cells and the current in the circuit remains almost constant (Table 3).
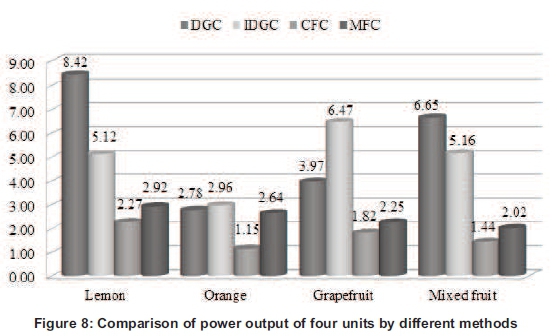
Furthermore, comparison of power output showed that the lemon has displayed the highest values of power output by DGC among the other citrus fruit, followed by mixed fruit, grapefruit and orange respectively. However, the values of power output found less in IDGC, CFC and MFC respectively. Orange has the lowest value of voltage in DGC and thus showed the lowest value of power output. However, the orange showed competitive value of power output by MFC as compared to other fruit. Grapefruit has the highest power out in IDGC and thus considered as most suitable for this method. Mixed fruit showed the average values of other fruits and thus no benefit or loss is considered with this series (see Figure 8). The merits and demerits of different fuel cells (DGC, IDGC, CFC and MFC) have been summarized in Table 4.
4. Conclusions
The physiochemical analysis of fruit juices showed that lemon has the lowest pH value (highest acid contents/ citric acid) which make this most preferable to utilized lemon for bioelectricity generation among the other fruit (orange, grapefruit and mixed fruit). The voltage of the circuit found to be increased with increasing the number of cells connected in series in all cases and the current remains almost constant. The current of the circuit was found to be increased with increasing the number of cells connected in parallel sequence in all cases and the voltage remains constant. The power output by DGC is found higher as compared to the IDGC, CFC and MFC method. The power output after the addition of the NaCl solution in all cases showed slightly higher values as compared to the values obtained before the addition of the NaCl solution in the galvanic cells. The power output by the MFC method is found higher due to the addition of microorganism as compared to their counterpart CFC method. Finally, the results showed that lemon generates the highest power output by the DGC method, and grapefruit showed the highest power output by IDGC. However, orange showed significant increase in power output by MFC as compared to CFC, which is considered due to highest sugar content in orange among the other fruit included in this article. The shortage of non-renewable fossil fuels will increase the cost of conventional electricity with time, and the advancement in the field of genetic engineering and bioelectricity technology will certainly result in the development of renewable, environmental friendly and a convenient source of energy around the globe.
Acknowledgements
The authors are greatly thankful to the Higher Education Commission of Pakistan for financial support. In addition, we are highly thankful to all those authors whose references were cited in this research article.
References
Badwal, S. P S., Giddey, S., Kulkarni, A., Goel, J., and Basu, S. (2015). Direct ethanol fuel cells for transport and stationary applications - A comprehensive review. Applied Energy 145. pp. 80-103. doi:10.1016/j.apenergy.2015.02.002. [ Links ]
Bennetto, H. P (1990). Electricity generation by microorganism, Biotechnology Education, 1 (4), pp. 163-168. [ Links ]
Carvalho, J., Ribeiro, A., Castro, J., Vilarinho, C. and Castro, F (2011). Biodiesel production by microalgae and macroalgae from north littoral Portuguese coast. In: 1st International Conference held by Centre for Waste Valorisation (CVR) - Wastes: Solutions, Treatments and Opportunities, Guimarpes, Portugal, CVR, September 12th - 14th, 2011. [ Links ]
Christi, Y. (2007). Biodiesel from microalgae, Biotechnology Advances, 25, pp. 294-296. [ Links ]
Delaney, G. M., Bennetto, H. P, Mason, J, R, Roller, S. D., Stirling, J. L. and Thurstan, C. F (1984). Electron transfer coupling in microbial fuel cells, 2 performance of fuel cells containing selected microorganism-mediator-substrate combination, Journal of Chemical Technology and Biotechnology, 34B, pp. 13-27. [ Links ]
Dincer, I. (2000). Renewable energy and sustainable development, Renewable and Sustainable Energy Reviews, 4, pp. 157-175. [ Links ]
Franco, D. (2005). "Volta and the 'Pile'". Electrochemistry Encyclopaedia. Case Western Reserve University. [ Links ]
Goff, M., Nicholas, S. B., Shailesh, L., William, R. S. and Galen, J. S. (2004). Acid-catalysed alcoholysis of soybean oil, Journal of the American Oil Chemist's Society, 81, pp. 415-420. [ Links ]
Goodisman, J. (2001). Observations on lemon cells, Journal of Chemical Education, 75 (4), pp. 516-518. [ Links ]
Ha, J. H., Shah, N., Islam, M. and Park, J. (2010). Production of bioethanol by simultaneous saccharification and fermentation process using waste from beer fermentation broth, Journal of Biotechnology, 150 (1), pp. 147-148. [ Links ]
Hossain, A. B. M.S. and Mekhled, M. A. (2010). Biodiesel fuel production from waste canola cooking oil as sustainable energy and environmental recycling process, Australian Journal of Crop Science, 4 (7), pp. 543-549. [ Links ]
John A. A. (1989). Fuel Cell Handbook. New York: Van Reinhold Co. [ Links ]
Kelter, P B., Carr, J. D., Johnson, T. and Castro-Acuna, C. M. (1996). Citrus spp.: orange, mandarin, tangerine, clementine, grapefruit, pomelo, lemon and lime, Journal of Chemical Education, 73 (12), pp. 1123-1127. [ Links ]
Khan, A. M. and Naz, S. (2014). Biopower generation from kitchen wastewater using a bioreactor, Water Environment Research, 86(1), pp. 3-12. [ Links ]
Khan, A. M., Attaullah, Shaheen, A., Ahmed, I., Malik, F and Shahid, H. A. (2011). Correlation of COD and BOD of domestic wastewater with the power output of bioreactor, Journal of the Chemical Society of Pakistan, 32 (2), pp. 269-274. [ Links ]
Khan, A. M. (2009). Electricity generation by microbial fuel cells, Advances in Natural and Applied Sciences, 3(2), pp. 279-286. [ Links ]
Khan, A. M., Ali M. M., Naz S and Sohail M. (2010). Generation of electricity by the aerobic fermentation of domestic wastewater, Journal of the Chemical Society of Pakistan, 32(2), pp. 209-214. [ Links ]
Larhum, A. W. D. (2010). Limitations and prospects of natural photosynthesis for bioenergy production, Current Opinion in Biotechnology, 21, pp. 271-276. [ Links ]
Leo, B and Mugerwa, M. (1993). Fuel Cell Systems. New York: Plenum Press. [ Links ]
Moawad, E. Y. (2013). Growth Energy of Bacteria and the Associated Electricity, Generation in Fuel Cells, Bioengineering and Bioscience 1(1), pp. 5-10. [ Links ]
Mata, T. M., Martins, A. A. and Caetano, N. S. (2010). Microalgae for biodiesel production and other applications, Renewable and Sustainable Energy Reviews, 14, pp. 217-232. [ Links ]
Naidu, M. S.; Kamakshiaih, S. (1995). Introduction to Electrical Engineering. Tata McGraw-Hill Education. p. 50. ISBN 9780074622926. [ Links ]
Oon, H. L. (2007). A simple electric cell, Chemistry Expression: An Inquiry Approach. Panpac Education Pte Ltd: Singapore, pp. 236. ISBN 978-981-271-162-5. [ Links ]
Randhawa, M. A., Rashid, A., Saeed, M., Javed, M. S., Khan, A. A. and Sajid, M. W. (2014). Characterization of organic acids in juices in some of Pakistani citrus species and their retention during refrigerated storage, Journal of Animal and Plant Science, 24 (1), pp. 211-215. [ Links ]
Swartling, D. J. and Morgan, C. (1998) Lemon cells revisited-The lemon-powered calculator, Journal of Chemical Education, 75 (2), pp. 181-182. [ Links ]
Received 26 July 2014
Revised 16 March 2015














