Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of Energy in Southern Africa
versión On-line ISSN 2413-3051
versión impresa ISSN 1021-447X
J. energy South. Afr. vol.26 no.2 Cape Town may. 2015
Exhaust gas treatment for reducing cold start emissions of a motorcycle engine fuelled with gasoline-ethanol blends
A Samuel Raja; A Valan Arasu
Department of Mechanical Engineering, Thiagarajar College of Engineering, Madurai, Tamilnadu, India
ABSTRACT
In countries like India, transportation by a two wheeled motorcycle is very common owing to affordable cost, easy handling and traffic congestion. Most of these bikes use single cylinder air cooled four-stroke spark ignition (SI) engines of displacement volume ranging from 100 cm3 to 250 cm3. CO and HC emissions from such engines when started after a minimum stop-time of 12 hours or more (cold-start emissions) are higher than warmed-up emissions. In the present study, a 150 cm3 single cylinder air cooled SI engine was tested for cold start emissions and exhaust gas temperature. Different gasoline-ethanol blends (E0 to E20) were used as fuel expecting better oxidation of HC and CO emissions with additional oxygen present in ethanol. The effect of glow plug assisted exhaust gas ignition (EGI) and use of catalytic converter on cold start emissions were studied separately using the same blends. Results show that with gasoline-ethanol blends, cold start CO and HC emissions were less than that with neat gasoline. And at an ambient temperature of 30±1°C, highest emission reductions were observed with E10. EGI without a catalytic converter had no significant effect on emissions except increasing the exhaust gas temperature. The catalytic converter was found to be active only after 120 seconds in converting cold start CO, HC and NOx. Use of a catalytic converter proves to be a better option than EGI in controlling cold start emissions with neat gasoline or gasoline-ethanol blends.
Keywords: cold start, gasoline-ethanol blends, exhaust gas ignition, glow plug
1. Introduction
Emissions from an internal combustion engine, started after a minimum halt time of 12 hours or more, are known as cold start emissions (Favez et al., 2009). Carbon monoxide (CO) and unburnt hydrocarbon (HC) are emitted in larger quantities during the first few minutes of a spark ignition (SI) engine with cold start because of the inactive or less active catalytic converter (Iliyas et al., 2007). The catalytic conversion is more effective only after the catalyst reaches its light-off temperature which is above 150°C (Singer et al., 1999). The time period required for the catalyst to reach its light-off temperature is referred to as cold-start period or unstable engine running period. In water cooled engines, stable engine operation is achieved at coolant temperature of about 70°C and it may take 6 to 7 minutes of the engine running after cold start (Botsaris et al., 2003). In air cooled SI engines, stable engine operation is achieved earlier than water cooled SI engines due to higher exhaust temperatures resulting from less effective cooling by air. The test engine in the current work is a single cylinder air cooled engine and hence cold start emissions and exhaust temperature have been recorded for the first four minutes after a cold start.
Generally, engine-out emissions are controlled in any one or two or all of the three techniques namely fuel modification, engine modification and exhaust gas treatment. Fuel modification (gasoline-ethanol blends) and exhaust gas treatment (exhaust gas ignition and catalytic converter) are attempted in the current work. Considering fuel modification for SI engines, gasoline-ethanol blends have been reported as a suitable alternate fuel for neat gasoline by various researchers (Pourkhesalian et al., 2010; Bayraktar, 2005; Eyidogan et al., 2010) all over the world. The two factors that make gasoline-ethanol blends suitable for S.I. Engines are the presence of an oxygen atom in the fuel structure of ethanol and a higher octane number of ethanol than that of gasoline. According to different researchers (Balki et al., 2012; Masum et al., 2013; Canakci et al., 2013), the optimum blend may contain 5 percent ethanol to 20 percent ethanol. This variation is due to difference in the experimented parameters such as compression ratio, ignition timing, engine load and speed. In the current work, no modification was made in the compression ratio and ignition timing. Ethanol was blended with gasoline in 5, 10, 15 and 20 percent by volume and the blend is correspondingly named E5, E10, E15 and E20. The RVP (Reid Vapour Pressure) of ethanol is 17 kPa, far lower than 53.7 kPa for gasoline. But their mixture does not have an RVP value linearly proportional to the volume fraction. A volume fraction of 5 to 10% ethanol can achieve the maximal RVP and thus facilitate cold-start (Thring, 1983). On account of higher latent heat of vaporization of ethanol, poor cold start performance has been observed with such blends when used in a water cooled engine (Turner et al., 2011; Clairotte et al., 2013). But with electronic fuel injection, the optimum percentage of ethanol in the blend for least cold-start emissions is reported to be at least 20 but not exceeding 30 (Chen et al., 2011). It is also reported that E10 blend results in higher acetalde-hyde and ethanol emissions compared to neat gasoline (Poulopoulos & Philippopolous, 2003).
In order to overcome the poor vaporization effect of ethanol, modifications in the engine such as heated intake air or/and heated intake fuel system have been attempted (Spegar et al., 2012; Sales & Sodré, 2012; Raja & Arasu, 2014). This modification has not only reduced cold-start CO and HC but also has increased exhaust gas temperature which enables the catalytic converter (if used) to reach its light-off temperature quicker.
Among various exhaust gas treatment techniques for SI engines, use of a catalytic converter is popular owing to its ability to convert the harmful CO,HC and NOx emissions into CO2, H2O, O2 and N2in stable engine running conditions (Twigg, 2007). The substrate used in a catalytic converter can be ceramic or metallic. At low speeds, conversion of HC emissions is higher with a ceramic substrate whereas a metallic substrate behaves better at higher engine speeds and higher exhaust temperatures. But the substrate type has a less significant effect on CO or NOx conversion (Santos & Costa, 2008). Among the various metals, Platinum (Pt), Palladium (Pd) and Rhodium (Rh) have been found to remain metallic and not to form volatile metallic oxides. Due to this reason, currently Pt or Pd is used for conversion of CO and HC emissions and Rh is used for conversion of NOx emissions (Twigg, 2006) in the present day catalytic converters. In the current work, a catalytic converter of metallic substrate loaded with Pd and Rh catalysts in the ratio of 37:3 g/ft3 has been used.
To increase the catalyst surface temperature quickly to its light-off temperature, glow plugs installed at the front and at the middle of converter have been used to heat the metal substrate and hence the catalyst (Horng & Chou, 2004). The results show that CO oxidation was higher with heating at inlet and at catalyst temperature of 180°C.
Though adequate literature is available on the effect of gasoline-ethanol blends in water cooled engines, only a few references (Leong et al., 2002; Yang et al., 2005) are available for air cooled engines. Moreover, the effect of EGI or the effect of a catalytic converter in such engines fuelled with gasoline-ethanol blends, on cold-start emissions has not been addressed. The aim of the present work is to fill this gap and hence it focuses on the experimental study on the effect of EGI or use of a catalytic converter on cold-start emissions of an air cooled motorcycle engine fuelled with gasoline-ethanol blends.
2. Experimental set-up and procedure
A single cylinder air cooled motorcycle SI engine with specifications shown in Table.1 was used to conduct experiments which were conducted in three phases. In the first phase, cold start emissions and exhaust temperature were recorded for neat gasoline and gasoline ethanol blends without a catalytic converter and without exhaust gas ignition. These experiments were carried out to study the effect of gasoline-ethanol blends alone in the cold start emissions. In the second phase, experiments were done with the same fuels with exhaust gas ignition (EGI) using a glow plug. The glow plug was mounted after the exhaust port. The objective of this phase is to study the effect of EGI alone on cold start emissions. As a considerable percentage of two wheelers (manufactured before 2010) are still running on roads without a catalytic converter, these two sets of experiments were performed to study the effect of gasoline-ethanol blends and EGI on motorcycle engines equipped with no catalytic converter. To meet the stringent emission norms, from 2010 onwards, a 4-stroke motorcycle S.I. Engine is fitted with a catalytic converter. Hence, third phase of experiments was completed with the same fuel blends with a catalytic converter and without EGI to study the effect and behaviour of a catalytic converter alone on cold start emissions.

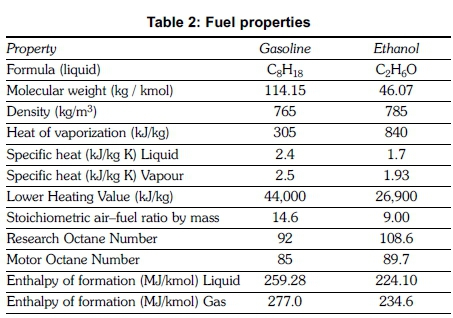
The exhaust pipe of the test engine was modified to accommodate a catalytic converter, glow plug, thermocouples and an exhaust gas sampling probe at the required locations and their positions are indicated in Figure 1. A time interval of 12 hours was given between each experiment to ensure a cold start. An idling speed of 1400 ± 50 rpm was maintained in all experiments. The fuel blends were prepared just before starting the experiment to ensure that the fuel mixture was homogenous and no water was formed by reaction of ethanol with water vapour in the atmosphere. Properties of neat gasoline and neat ethanol are given in Table 2.
As the multiple attempts to start the engine affected the cold start emissions considerably, adequate care was taken to start the engine in the first attempt using the starter motor. Emissions were recorded for every two seconds for 4 minutes from the engine start by 5-gas analyser with specifications shown in Table 3 and the values were stored in a personal computer interfaced with an exhaust gas analyser. In the third phase of experiments, a new catalytic converter with specifications shown in Table 4 was used. Exhaust gas temperatures before and after the catalytic converter were measured using K-type thermocouples.


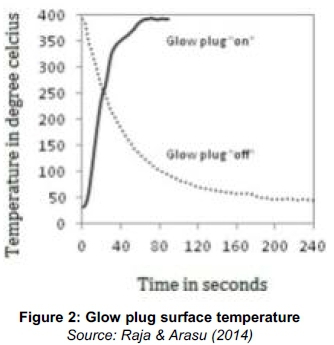
An extra 12 V, 80 Ah battery was used as power source to energize the glow plug for the exhaust gas ignition. The voltage and amperage for the glow plug were 11.9 V and 4.65 A respectively. To understand the characteristics of the glow plug, it was energized continuously by battery and its surface temperature was measured in still air condition after installing the glow plug in the exhaust pipe. At about 60 seconds, it reached the highest temperature (390°C) and remained constant. At this point of time, power supply was cut and the surface temperature was measured. At about 200 seconds, it reached almost a constant value of 50°C. The variation of glow plug surface temperature with time during on and off conditions is shown in Figure 2 (Raja & Arasu, 2014).
3. Results and discussion
3a) Phase 1 experiments - no modifications to the exhaust system
The variations of exhaust emissions (CO, HC, CO2, O2and NOx) and exhaust temperature with time in seconds are shown in Figure 3. It illustrates the sole effect of gasoline ethanol blends on cold start emissions in the absence of EGI and a catalytic converter. On comparison with neat gasoline, all blends showed reduction in CO, HC, NOx emissions and exhaust temperature. But there was an increase in O2 and CO2 emissions. The reduction in CO and HC emissions is due to the excess oxygen present in ethanol structure and this is confirmed by the increase in CO2 and O2 emissions. The reason for reduction in exhaust gas temperature is the higher latent heat of vaporization of blends compared to that of neat gasoline. Normally, NOx emissions are formed due to both oxygen availability and higher combustion temperature. Here, a drop in combustion temperature (inferred from exhaust temperature) plays a dominant role for reduction in NOx emissions though oxygen content is in excess. Despite the fact that NOx emissions are reported to be insignificant during cold-starts of gasoline and diesel fuelled engines (Weilenmann et al., 2005), its variation with ethanol percentage in the blend is presented here to understand the effect of fuel, EGI and the catalytic converter.

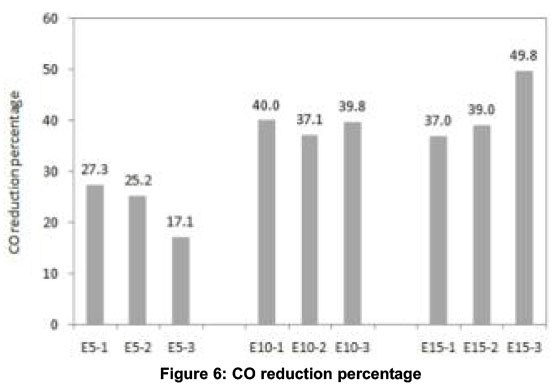
CO and HC emissions generally decrease with higher oxygen content and higher combustion temperature. With gasoline-ethanol blends, oxygen percentage in the exhaust is increased and combustion temperature is decreased. Therefore, the factor which reduces CO emissions with E5 and E10 is higher oxygen availability and the factor which increases CO emissions with E15 and E20 is lower combustion temperature. The same reasoning holds good for the increase in HC emissions with E20 compared to that of E5, E10 and E15. To confirm these observations, experiments were conducted with E25 which led to very high amounts of CO and HC emissions. The magnitudes were about 50 -75% higher (not shown in this paper) than those measured using neat gasoline and moreover unstable engine operation was noticed. This may be attributed to flame quenching and misfiring during combustion of E25 blend-air mixture. Hence, results are presented up to E20 in this paper. Taking average of CO and HC emissions for 4-minutes, highest reduction in CO (40%) was observed with E10 and highest reduction in HC (44.8%) was observed with E15, as shown in Figures 6 and 7 respectively.

3b) Phase 2 experiments - Impact of EGI
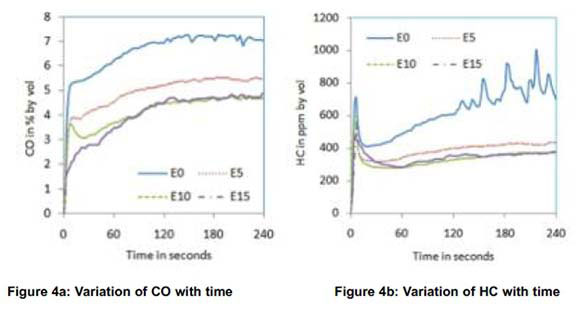
As E5, E10 and E15 showed better results compared to E20, these three blends were considered in the second and third phase of experiments. The first phase of experiments was repeated with EGI in the second phase for E0, E5, E10 and E15. Figure 4 shows the effect of gasoline ethanol blends and EGI on cold start emissions in the absence of a catalytic converter.
All emissions and exhaust gas temperature were almost similar for E10 and E15 with EGI. Comparing the 4-min average CO emissions for different blends to that of neat gasoline, highest CO reduction of 39% was observed for E15 and highest HC reduction of 46.5% was observed for E10 as shown in Figures 6 and 7 respectively. These reductions in CO and HC emissions reflect in the increase in CO2 and decrease in O2 as is shown in Figures.4c and 4d respectively. The decrease in CO and HC is due to the partial combustion with the support of EGI. NOx emissions reduced with all blends in the same pattern as that without EGI which indicates that the effect of EGI is not predominant. The drop in exhaust gas temperature observed in the first phase of experiments is overcome with EGI in the second phase. Exhaust gas temperature of all blends was almost the same as that with neat gasoline as shown in Figure 4f.
Comparing reductions in CO and HC emissions without EGI, a marginal improvement has been observed with EGI for almost all blends. The only positive change noticed was increase in exhaust temperature, which may help reduction of cold start HC and CO emissions by shortening the light off period of catalytic converter, if used.
3c) Phase 3 Experiments - Catalytic convertor installed
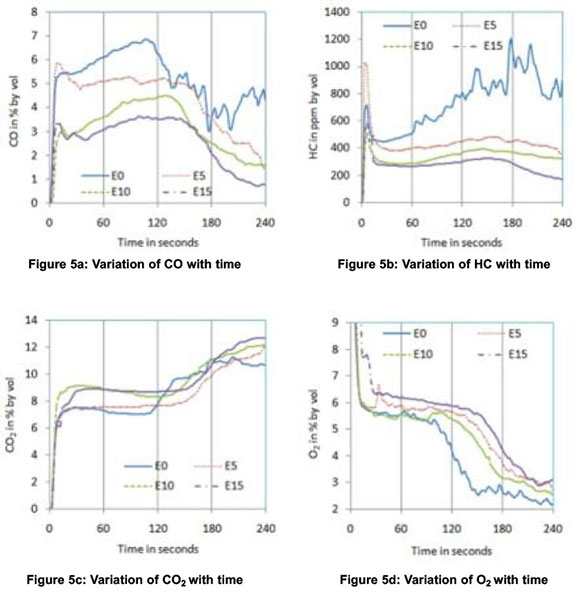
In order to understand the behaviour of a catalytic converter with gasoline-ethanol blends during the cold start period, a third phase of experiments was conducted and the results are shown in Figure.5. For all blends, CO emissions started to decrease after about 130 seconds whereas this cut-off time was about 105 seconds for neat gasoline. The delay in the activation of the catalysts is due to the lower exhaust gas temperatures with blends compared to neat gasoline. However, the highest percentage reduction in 4-min average CO and HC observed were 49.8 and 62.5 respectively for E15, as shown in Figures.6 and.7 respectively. No positive effect was noticed in HC emissions for neat gasoline which reveal that the light off period for HC is longer than that for CO (Heywood, 1998). But with the blends, a slight reduction in HC was noticed after 150 seconds. The sudden increase in CO2 and sudden decrease in O2 at about 120 seconds confirm the action of the catalytic converter on CO and HC emissions. The decrease in NOx after about 120 seconds further ascertains the redox nature of the catalysts used. The oxidation of CO and HC to CO2 are exothermic reactions and the heat released increases exhaust gas temperature. The increase in the slope of the after-converter temperature curve at about 140 seconds (with E10 blend) confirms this effect and is shown in Figure 5f.
3d) Consolidated comparison of 4-minute average CO and HC emissions
The percentage reduction in 4-minute average CO and HC emissions compared to that of neat gasoline in each of these three phases are presented in Figures.6 and.7 respectively. (1 - without EGI and without catalytic converter, 2 - with EGI and without catalytic converter, 3 - without EGI and with catalytic converter).
4. Conclusion
Experiments were conducted in three phases on an air cooled four-stroke motorcycle S.I engine to (1) study the effect of gasoline-ethanol blends; (2) study the effect of exhaust gas ignition; and (3) study the effect of a catalytic converter on cold start emissions.
The impact of gasoline-ethanol blends alone ranging between E5 to E20 on cold-start emissions was studied in first phase experiments. E10 showed highest CO reduction (40%) and E15 showed highest HC reduction (44.8%). The same experiments were repeated with exhaust gas ignition (EGI) in the second phase to study the combined effect of gaso-line-ethanol blends and EGI. Highest CO reduction of 39% was observed for E15 and highest HC reduction of 46.5% was observed for E10. From these two phases of experiments, it is concluded that gasoline-ethanol blends play a more important role in controlling cold start HC and CO emissions than the glow plug assisted exhaust gas ignition.
The combined effect of impact of a catalytic converter and gasoline-ethanol blends on cold-start emissions was studied through a third phase of experiments. It was found that a catalytic converter starts functioning after the light off period of 130 seconds or more for gasoline-ethanol blends where-as this light-off period was about 105 seconds for neat gasoline. However, the 4-min average CO and HC emissions decreased for all blends. The highest percentage reduction in CO and HC were observed to be 49.8 and 62.5 respectively for E15.
As most of the motorcycle engines manufactured before 2010 in India are running without a catalytic converter, use of E10/E15 blend and/or use of a catalytic converter are recommended for reducing cold start emissions.
References
Balki, MK., Sayin, C., and Canakci, M., (2012). The effect of different alcohol fuels on the performance, emission and combustion characteristics of a gasoline engine, Fuel, 115, pp. 901-906. [ Links ]
Bayraktar, H., (2005). Experimental and theoretical investigation of using gasoline-ethanol blends in spark-ignition engines, Renewable Energy, 30, pp. 1733-1747. [ Links ]
Botsaris, PN., Bechrakis, D., and Sparis, PD., (2003). An estimation of three-way catalyst performance using artificial neural networks during cold start, Applied Catalysis A: General, 243, pp. 285-292. [ Links ]
Canakci, M., Ozsezen, AN., Alptekin, E., and Eyidogan, M., (2013). Impact of alcohol-gasoline fuel blends on the exhaust emission of an SI engine, Renewable Energy, 52, pp. 111-117. [ Links ]
Chen, R.H., Chiang, L.B., Chen, C.N., and Lin, T.H., (2011). Cold-start emissions of an SI engine using ethanol-gasoline blended fuel, Applied Thermal Engineering, 31, pp. 1463-1467. [ Links ]
Clairotte, M., Adam, TW., Zardini, A.A., Manfredi, U., Martini, G., Krasenbrink, A., Vicet, A., Tournié, E., and Astorga, C., (2013). Effects of low temperature on the cold start gaseous emissions from light duty vehicles fuelled by ethanol-blended gasoline, Applied Energy, 102, pp. 44-54. [ Links ]
Eyidogan, M., Ozsezen, A.N., Canakci, M., and Turkcan, A., (2010). Impact of alcohol-gasoline fuel blends on the performance and combustion characteristics of an SI engine, Fuel, 89, pp. 2713-2720. [ Links ]
Favez, J.Y, Weilenmann, M., and Stilli, J., (2009). Cold start extra emissions as a function of engine stop time: Evolution over the last 10 years, Atmospheric Environment, 43, pp. 996-1007. [ Links ]
Heywood, J.B., (1998). Internal Combustion Engine Fundamentals, McGraw-Hill Publications, New York, USA. [ Links ]
Horng, RF, and Chou, H.M., (2004). 'Effect of input energy on the emission of a motorcycle engine with an electrically heated catalyst in cold-start conditions', Applied Thermal Engineering, 24, pp. 2017-2028. [ Links ]
Iliyas, A., Zahedi-Niaki, M.H., Eic', M., and Kaliaguine, S., (2007). Control of hydrocarbon cold-start emissions: A search for potential adsorbents, Microporous and Mesoporous Materials, 102, pp. 171-177. [ Links ]
Leong, S.T., Muttamara, S., and Laortanakul, P, (2002). Influence of benzene emission from motorcycles on Bangkok air quality, Atmospheric Environment, 36, pp. 651-661. [ Links ]
Masum, B.M., Masjuki, H.H., Kalam, M.A., Fattah, I.M.R., Palash, S.M., and Abedin, MJ., (2013). Effect of ethanol-gasoline blend on NOx emission in SI engine, Renewable and Sustainable Energy Reviews, 24, pp. 209-222. [ Links ]
Poulopoulos, S.G., and Philippopolous, C.J., (2003). The effect of adding oxygenated compounds to gasoline on automotive exhaust emissions', Eng. Gas Turbine power, 125, pp. 344-350. [ Links ]
Pourkhesalian, AM., Shamekhi, A.H., and Salimi, F., (2010). Alternative fuel and gasoline in an SI engine: A comparative study of performance and emissions characteristics, Fuel, 89, pp. 1056-1063. [ Links ]
Raja, A.S., and Arasu, A.V., (2014). Control of cold start hydrocarbon emissions of motor bike engine by gasoline-ethanol blends and intake air heating, Journal of Mechanical Science and Technology, 28, (4), pp. 1567-1573. [ Links ]
Sales, L.C.M., and Sodré, J.S., (2012). Cold start emissions of an ethanol-fuelled engine with heated intake air and fuel, Fuel, 95, pp. 122-125. [ Links ]
Santos, H., and Costa, M., (2008). Evaluation of the conversion efficiency of ceramic and metallic three way catalytic converters, Energy Conversion and Management, 49, pp. 291-300. [ Links ]
Singer, B.C., Kirchstetter, T.W., Harley, R.A., Kendall, G.R., and Hesson, J.M., (1999). A Fuel-Based Approach to Estimating Motor Vehicle Cold-Start Emissions, J. Air & Waste Manage. Assoc., 49, pp. 125-135. [ Links ]
Spegar, T.D., Burke, D., and Lavan, L., (2012). Delphi's Heated Injector Technology: The Efficient Solution for Fast Ethanol Cold Starts and Reduced Emissions, SAE Technical paper 2012-01-0418. [ Links ]
Thring, R.H., (1983), Alternative fuel for spark-ignition engines', SAE Paper 831685. [ Links ]
Turner, D., Xu, H., Cracknell, R.F, Natarajan, V, Chen, X., (2011), Combustion performance of bio-ethanol at various blend ratios in a gasoline direct injection engine, Fuel, 90, pp. 1999-2006. [ Links ]
Twigg, M.V., (2006). Roles of catalytic oxidation in control of vehicle exhaust emissions, Catalysis Today, 117, pp. 407-418. [ Links ]
Twigg, M.V., (2007). Progress and future challenges in controlling automotive exhaust gas emissions, Applied Catalysis B: Environmental, 70, pp. 2-15. [ Links ]
Weilenmann, M., Soltic, P, Saxer, C., Forss, A.M., and Heeb, N., (2005). Regulated and non-regulated diesel and gasoline cold start emissions at different temperatures, Atmospheric Environment, 39, pp. 2433-2441. [ Links ]
Yang, H.H., Hsieh, LT., Liu, H.C., Mi, H.H., (2005). Polycyclic aromatic hydrocarbon emissions from motorcycles, Atmospheric Environment, 39, pp. 17-25. [ Links ]
Received 18 August 2014
Revised 23 March 2015