Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of Energy in Southern Africa
On-line version ISSN 2413-3051
Print version ISSN 1021-447X
J. energy South. Afr. vol.25 n.4 Cape Town Nov. 2014
Investigations on the absorption spectrum of TiO2 nanofluid
A L SubramaniyanI; Sukumaran Lakshmi PriyaII; M KottaisamyIII; R IlangovanIV
IDepartment of Physics, Thiagarajar College of Engineering, Madurai, India
IIDepartment of Physics, Thiagarajar College of Engineering, India
IIIDepartment of Chemistry, Thiagarajar College of Engineering, Madurai, India
IVDepartment of Nanoscience and Technology, Alagappa University, Karaikudi, India
ABSTRACT
Nanofluids are tailored nano- colloidal suspensions of nanoparticles in a suitable base fluid. This present work investigates the absorption spectrum in TiO2-water nanofluids to identify the potential application of nanofluids in Direct Absorption Solar Collectors (DASC). Nanoparticles of Titanium dioxide (TiO2) are prepared by sol gel and characterized by X Ray Diffraction (XRD) and Scanning Electron Microscopy (SEM). TiO2-water nanofluids with weight fraction of 0.1% are prepared by a two-step process with sonication. The prepared nanofluids are investigated for their stability by a gravity sedimentation method and for their optical property by UV-Vis spectroscopy. Stability of nanofluid is essential for the applications of nanofluid in DASC. TiO2 nanoparticles with a crystallite size of 43nm are obtained .The SEM image reveals the agglomerated state of TiO2 nanoparticles and the stability of TiO2 nanofluid is reported as 9-10days. UV results indicate the decrease in absorption from 440-500nm, complete absorption from 500-700nm and increase in absorption from 700-900nm.TiO2 nanofluids are recommended as potential candidates for DASC in UV and IR regions.
Keywords: nanofluid, TiO2, sedimentation, absorption, DASC
1. Introduction
Increased consumption and growing demands of electrical appliances across the globe have posed a threat on the non- renewable energy resources and has made us exploit the maximum of renewable energy resources among which solar energy is the ultimate choice. Solar energy has been investigated for photovoltaic and thermal applications (Duffie, 1980). A major thrust in materials development is to identify new materials in order to increase the efficiency of Solar Thermal Collectors .The development of nanotechnology contributed to nano suspensions, which can increase the efficiency of DASC. Conventional solar collectors transfer the heat from the flat plate to the working fluid but have the drawback of high heat loss .The concept of Direct absorption solar collectors in which working fluid acts as absorber and carrier of heat started replacing the conventional solar collectors (Otanicar, 2009). Since thermal conductivity of pure liquids are much lesser in comparison to solids, suspension of solid particles in liquid could enhance the thermal conductivity and required optical properties. Micro colloidal suspensions suffered from drawbacks like abrasion and sedimentation for which nanofluids were substituted by Robert Taylor (2009). The term nanofluid was coined by Stephen Choi in 1995. Nanofluids are suspensions of nano-sized solid particles (1-100nm) in suitable base fluids. From 1995 till 2008, nanofluids have been explored as a heat transfer fluid and the best results have been reported by Eastman and Choi (2001). In the past five years, nanofluids have also been explored for their electrical (Ganguly, 2009) and magnetic properties (Philip, 2009).
Nanofluids can be prepared in two ways, a single step method (Zhu, 2004) or a two-step method (Eastman, 1997). One step method is based on simultaneous synthesis of nanoparticle and nanofluid and two step methods involve synthesis of nanoparticles in the first step followed by dispersion in a suitable base fluid. The success of nanofluid for DASC depends on suitable choice of base fluid and nanoparticles. The best nanofluids for energy harvesting demands high stability and high absorption of solar radiation. In this present work, an attempt has been made to identify stability and absorption property of 0.1wt% TiO2-water nanofluid.
2. Experimental
2.1 Preparation of TiO2 nanoparticle and TiO2 nanofluid
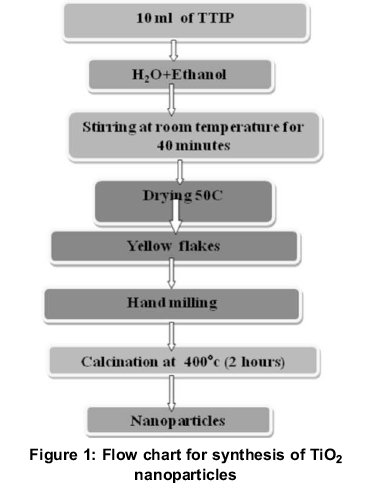
TiO2 nanoparticles were prepared via a sol-gel method using titanium tetraisopropoxide (TTIP), distilled water, and ethyl alcohol as the starting materials. All the reagents used were of Analytical grade. 10 ml of Titanium tetraisopropoxide was dissolved in absolute ethanol (20 ml) and distilled water was added (30 ml). The obtained solutions were kept under slow-speed constant stirring on a magnetic stirrer for 40 min at room temperature to obtain a thick solution. The thick solution was dried at 50°C for 1.5 hours to evaporate water and carbon to the maximum extent. After hand milling the dried powders obtained were calcined at 400°C for 2 hours to obtain desired TiO2nanoparticles, as reported by (Thangavelu, 2013). A flow chart for synthesis of TiO2is represented in Figure 1 and the nnaoparticles of TiO2before milling is shown in Figure 2.
0.1g of prepared TiO2 nanoparticles are dispersed in 1 litre of water and sonicated as shown in Figure 3 to obtain TiO2nanofluid. Sonication is done at a frequency of 42 KHz for 15 minutes to obtain a uniform dispersion of TiO2in water. The prepared TiO2nanofluid is shown in Figure 4.


2.2 Sedimentation test
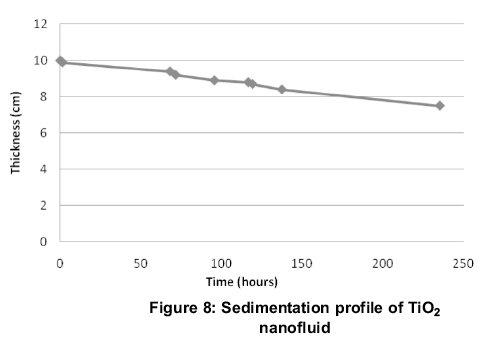
Ideal nanofluids have high stability and poor suspensions can alter the thermal and solar absorption properties of nanofluid drastically. The importance of stability of nanofluids and different methods of sedimentation analysis and mechanisms is discussed by (Mukherjee, 2013).In the present work, sedimentation is evaluated by gravitational sedimentation column method for TiO2nanofluid as shown in Figure 5. As time progresses, the nanoparticles tend to settle at the bottom .The thickness of the sediment is observed as a function of time to plot the sedimentation profile of TiO2nanofluid.

3. Results and discussion
3.1 Results of preparation TiO2 nanoparticle and nanofluid
Constant stirring on a magnetic stirrer after 30 minutes indicates a milky white solution which confirms the formation TiO2nanoparticles from TTIP. After drying at 50°C, yellow colour TiO2 flakes are obtained as shown in Figure 2.
3.2 Structural characterization
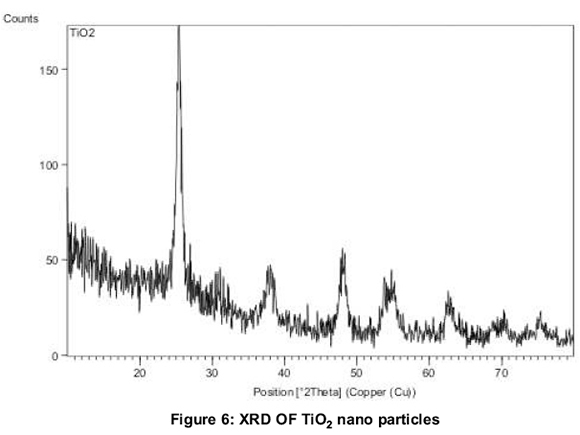
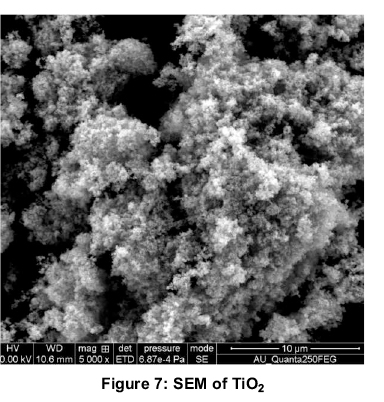
Figure 6 shows the X-Ray Diffraction pattern of final calcined TiO2nanoparticles. The X-ray diffraction peaks of prepared nanoparticle is in confirmation with JCPDS-89.4203. (Joint Committee for Powder Diffraction Society). This shows the broad peaks at 25.36, 37.91, 48.04 and 55.05 (anatase phase of TiO2. The broad peaks are obtained due to randomly oriented crystals in a nano crystalline material as indicated by (Akbari, 2011). Average crystalline size of samples TiO2are determined by the Debye-Scherer formula, D = 0.91λ, (ßcos θ) and were found to be 42.77nm.The SEM image (Figure 7) of nanoparticles reveals the high agglomeration. This may be due to lack of usage of surfactants during preparation of TiO2. Surfactants were not used purposely because they would alter the optical absorption of nanofluids. The average diameter of SEM can also correspond to 50-100nm thus supporting the results from XRD.
3.3 Sedimentation characterization of TiO2
A plot of thickness of the sediment and the time (Figure 8) reveals the sedimentation profile of TiO2. The time taken for the 25% formation of pure base fluid or the sediment to decrease to 75% of the original nanofluid column is 9.79 days.
3 .4 Optical characterization
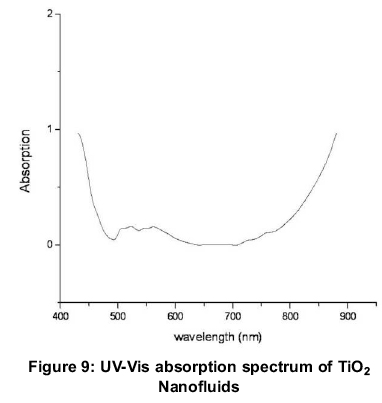
The prepared TiO2 nanoparticles were investigated for their optical properties by UV Visible absorption spectrophotometer at room temperature. The spectrum is recorded from 400 nm to 900 nm as shown in Figure 9. The absorption of TiO2 nanofluids decreases from 440-500 nm and increases from 700-900 nm, which proves the absorption and attenuation of TiO2 nanofluids in the visible range and IR regions. The results in IR region are in slight contradiction to the latest results of TiO2, 0.1vol% - water nanofluid reported by (Said, 2014). The variation in results are attributed to the nanoparticle size (43 nm), lack of surfactant and vol%. (Said, 2014) has investigated optical properties of TiO2 nanofluid with 21nm, surfactant and 0.1 and 0.3vol%.The prepared nanoparticles have a range of particle size as revealed by SEM which are also responsible for variation in previously reported results. Thus, TiO2 water nanofluids are good for direct absorption in UV and IR pectrum in the absence of surfactants.
4. Conclusions
Our conclusions are as follows:
1. TiO2 nanoparticles were prepared by the sol-gel method. XRD results show that TiO2 particles have an anatase phase and is crystalline in nature with crystallite size of 42.77 nm.
2. From SEM images, it is evident that TiO2 has an inhomogeneous size with high agglomeration and the average particle diameter is in the range of 50 -100 nm.
3. From sedimentation profile, the sedimentation time of TiO2 is 9-10 days. Lesser weight fraction of TiO2 nanofluids are recommended for higher stability.
4. TiO2 nanofluids without surfactants are better absorbers of ultraviolet and infrared wavelengths in comparison to TiO2 nanofluids with surfactants Nanofluids without surfactants may be less stable but better absorbers of solar radiation and may aid in efficient DASC.
5. Higher absorption can be obtained with increasing weight fraction of naoparticles in base fluid but a low weight fraction of naoparticle can give a more stable nanofluid.
Acknowledgements
The authors would like to thank Dir. V Abhai Kumar, Principle, TCE, Madurai, for the constant support and encouragement and the facilities provided by Dr R. Vasuki, TCE, Madurai. Special thanks are conveyed to the Department of Industrial Chemistry, Alagappa University Karaikudi for providing SEM facilities. The authors are thankful to the reviewers and editor for their constructive comments which has added quality.
References
Akbari, B. Tavandashti, M.P and Zandrahim, M. (2011). Iranian Journal of Materials Science & Engineering, 8(2), 48-56. [ Links ]
Choi, S.U.S (1995). FED-Vol.231/MD-Vol-66, 99-105. [ Links ]
Duffie, J.A., and Beckman, W.A. (1980). Solar Engineering of Thermal Processes, New York, John Wiley & Sons. [ Links ]
Eastman, J.A., Choi, S.U.S, Thompson, L.J., and Lee S. (1997). Materials Research Societ, Symposium-Proceedings, 457, 3-11. [ Links ]
Eastman, J.A., Choi, S.U.S., Li, S. Yu, W. and Thompson, J. (2001). , Applied Physics Letters, 78(6), 718-720. [ Links ]
Ganguly, S., Sikdar, S. and Basu, S. (2009). Powder Technology, 196, 326-330. [ Links ]
Mukherjee, S. and Paria S. (2013). IOSR JMCE, 9(2), 63-69. [ Links ]
Otanicar, T.P, and Golden J.S., (2009). Comparative Environmental and Economic analysis of conventional and nanofluid solar hot water technologies, Environ Sci Technol, 43, 6082-6087. [ Links ]
Said, Z., Saidur, R., and Rahim, N.A, (2014). International Communications in Heat and Mass Transfer, 59, 46-54. [ Links ]
Shima, P.D. Philip, J. and Baldev R. (2009)., Applied Physics Letters, 95,133112. [ Links ]
Taylor, R.A., Phelan, PE., Otanicar, T.P, Arvind, R., and Prasher, R. (2011). Nanoscale Research Letters 6:255. [ Links ]
Thangavelu, K., Annamalai, R., and Arulnandhi, D. (2013). IJETAE, Vol. 3, 636-639. [ Links ]
Zhu, H., Lin, Y. and Yin Y (2004). Journal of Colloidal & Interfacial Science, 227, 100-103 [ Links ]
Received 17 May 2014
Revised 6 November 2014














