Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of Energy in Southern Africa
versión On-line ISSN 2413-3051
versión impresa ISSN 1021-447X
J. energy South. Afr. vol.25 no.2 Cape Town may. 2014
General aspects of carbon dioxide as a refrigerant
J M Belman-FloresI; Vicente Pérez-GarcíaII; Jean Fulbert Ituna-YudonagoI; José Luis Rodríguez-MuñozII; José de Jesús Ramírez-MinguelaII
IDepartment of Mechanical Engineering, University of Guanajuato, Salamanca, Mexico
IIDepartment of Metal-Mechanical Engineering, University of Technological of Guanajuato Southwest, Valle-Huanímaro km 1.2, Valle de Santiago, GMexico
ABSTRACT
Carbon dioxide is an innocuous refrigerant for the environment. It is a substance of current interest in the refrigeration area. Its good thermodynamic and heat transfer properties have placed it in an excellent position for substituting refrigerants that contribute to global warming. This paper describes carbon dioxide as a refrigerant, the main characteristics that have made it a substance of current interest, its applications in subcritical and transcritical cycles, and a general vision of its usage at international level. Moreover, this paper presents the disadvantages of using this refrigerant and the upgrades made by the scientific community in order to improve the performance of those systems that work with this fluid. This paper is a reference for those interested in having a wider vision of frigorific technology based on carbon dioxide as a refrigerant.
Keywords: CO2, low GWP, COP, refrigeration, air conditioning
1. Introduction
In recent years, our planet has faced two major problems with refrigerants where ozone depletion potential (ODP) and global warming potential (GWP) are important factors to consider. The replacement of chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) for hydrofluorocarbons (HFCs), which have some potential for the destruction of stratospheric ozone (low ODP), was a step forward. However, this did not help global warming as a direct contribution by these refrigerants remains. It is for this reason that the scientific community is considering additional natural substances and other refrigerants with minimum GWP and zero ODP.
The use of CO2 as a refrigerant, concurrent with mechanical refrigeration began in the mid-century 18th. In 1744, Joseph Priestley dissolved carbon dioxide in water, which resulted in a decrease in the temperature of the liquid, suggesting suitable thermodynamic properties for refrigeration. Years later, William Cullen at the University of Glasgow studied the evaporation of liquids under vacuum. Under a number of experimental conditions, ice could be produced with evaporation.
Oliver Evans in the 19th century proposed that refrigeration could be achieved using mechanical vapour compression, and Alexander Twining proposed the use of CO2 as a refrigerant in an 1850 patent (Bodinus, 1999). While this work was ongoing in Europe, Thaddeous S.C. Lowe in America (circa 1867) described refrigeration using CO2 in his patent (Thévenot, 1979), and a subsequent machine was developed by Carl Linde (Aarlien, 1998). Five years later, Franz Wildhausen in Braunschweig patented a compressor using CO2, later licensed for development by J&E Hall that lead to the development of the first double-stage compressor (Cavallini and Steimle, 1998). The Sabroe company was first to develop a household refrigerator and at the end of the 19th century, CO2 as a refrigerant reached its peak being used in air conditioners and refrigerated displays. Although ammonia and sulphur dioxide were capable refrigerants, they were also toxic and flammable. This positioned carbon dioxide as the best alternative for the future.
Carbon dioxide dominated as a refrigerant worldwide in the early part of the 20th century until the development of the first synthetic refrigerants. The new synthetics were marketed globally offering cooling with smaller units with greater efficiency. This lead to the decline of carbon dioxide as a refrigerant in the 1930s. The use of CO2 as a refrigerant essentially ceased in the 1950's, since CFCs dominated the worldwide market.
For almost half a century, CFCs dominated as refrigerants. Following the work by Mario Molina and Sherwood Rowland in the 1970's which showed destruction of the stratospheric ozone and the production of holes over the Antarctic. The scientific community promoted the use of alternatives such as hydrofluorocarbons (HFCs) that were not reactive. Later these were judged as unfavourable alternatives that would contribute to global warming and industrialized countries within the European Union initiated processes to eliminate these refrigerants in compliance with the objectives established by the United Nations (Natarajan, 2008). With these events, a resurgence and interest in past refrigerants initiated with expectations of not harming the environment. Refrigerants less than 150 GWP are desirable, and carbon dioxide is one substance meeting these criteria (Calm, 2008).
Without the detrimental effects of CFCs and HCFs, Gustav Lorentzen in 1980 patented an application using carbon dioxide called transcritical cycle (Lorentzen, 1990). Figure 1 illustrates the refrigeration cycle with one enhancement: the inclusion of a heat exchanger (indicated with the number 12), known as an intermediate exchanger that produces an increase in energy performance.
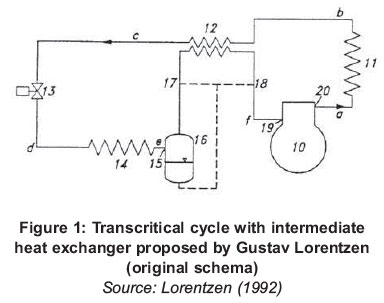
According to current world wide environmental awareness, natural refrigerants are one of the main focuses of attention for the scientific community. Hence, in view of the fact that carbon dioxide is one of the oldest natural fluids with the most recent re-utilization, this paper presents a modern situation and the tendencies in the use of this substance as a refrigerant, since nowadays it is becoming an interesting option in the areas of refrigeration and air conditioning again.
The following sections consider the advantages and disadvantages in the use of carbon dioxide as a refrigerant. Physical properties, technologies and subcritical and transcritical modes are also considered.
2. CO2 in subcritical and transcritical cycles
Cold generation, whether for refrigeration or air conditioning, is mostly achieved through vapour compression refrigeration. Cycles used to achieve efficient compression are classified in respect to their critical point: subcritical and transcritical. Subcritical cycles run below critical point of the working fluid in which transcritical is above. Carbon dioxide can be used in both cycles, however, when working as subcritical it does it as a secondary fluid and in this case, the primary fluid is another substance. A diagram of pressure enthalpy for both cycles is presented in Figure 2.
The COP defines the efficiency of a refrigeration cycle in subcritical and transcritical systems. The COP is the ratio between the energy absorbed in the evaporator and the work required by the compressor. In this context, when a carbon dioxide system operates in a subcritical cycle, the main constraint for the COP is sump temperature. According to the application, different performance values can be achieved in these cycles. Later, performance values of subcritical carbon dioxide systems will be described for each application. For a transcritical cycle, the limit of COP is the temperature of the refrigerant at the outlet of the evaporator. Lower evaporating temperature increases the COP of the system (Neksá, 2002). In this mode, several configurations have been developed in order to improve the COP, this includes the inclusion of an internal heat exchanger, a turbine, an ejector or a vortex tube, and expansion stages. Pérez-García et al., (2013) present some configurations where the carbon dioxide is used in transcritical cycle, in its work, the transcritical system has an energetic performance from 2.01 in a single cycle to 2.6 when the cycle is modified using a turbine. Sarkar (2010) also shows another configuration where the use of an ejector in a transcritical cycle causes an improvement in the performance of the system around 16% in respect to the single cycle. This enhancement can be obtained by increasing the refrigeration capacity.
Figure 3 shows how the COP is increased in the transcritical cycles by making use of different elements like a turbine (TC), and intermediate heat exchanger (IHXC) and an ejector (EC). All of them were compared with the simple cycle (SC) in order to compare the increase in the energy efficiency of the cycle.
Due to this characteristic, it is one of the applications of CO2 as a refrigerant in heat pumps, in which the energy performance is really competent compared with the use of synthetic and high GWP fluids. A description of the use of the CO2 heat pumps showing adequate results of performance, this type of facility can be found in the work of Neksá et al., (1998, 1999). The authors demonstrate an excellent performance of the heat pump in which a COP of 4.3 is reached for the analysed prototype. This is for cold weather like in Oslo. Another significant advantage of transcritical systems in heat pump mode compare to conventional systems is that hot water up to 90°C can be produced without operational difficulties.
When carbon dioxide is used in a subcritical cycle, pressure and operating temperature range is limited in which the evaporation of a liquid is at least -55°C and the condensation is at maximum 30°C. There are three common applications of CO2 in a subcritical cycle: as secondary fluid, cascade and mixed systems. When carbon dioxide is used as a secondary fluid, it is pumped and not compressed; while a cascade system uses a primary fluid that allows condensation at temperatures below 0°C. The main application of this system is to be cooled to low temperature evaporating carbon dioxide below -30°C.
Mixed systems combine the two previous applications, and are very useful in places such as supermarkets where two ranges of temperatures (cooling and freezing) are required. Here the medium temperature (cooling) services operate with CO2 as a secondary fluid and low temperature (freezing) services do it using direct expansion evaporators. In transcritical cycles, the fluid is not condensed where the pressures are varying from 7.31 MPa up to 12 MPa as the phase exceeds a critical point (31°C and 7.3 MPa) and the process becomes a sensible cooling of the gas. In cold weather, the energy consumption in transcritical systems is relatively low, while for hot climates efficiency decreases due to the thermodynamic properties and heat transfer which this fluid has.
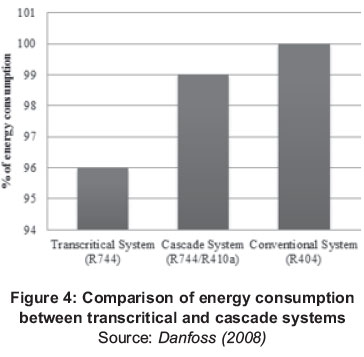
Figure 4 shows a comparison of the energy consumption during 37 weeks between a potential system and a cascade system using R-410 and R-744 (CO2) against the consumption of a system that uses R-404A, which is one of the conventional refrigerants used in supermarkets in Denmark (Danfoss, 2008). The transcritical systems energy is lower compared with other fluids for the same operating conditions, which is the reason why the use of transcritical systems is a highly competitive choice to replace conventional systems in Northern Europe.
In general terms, it is concluded that CO2 applications in the cold generation are many. Among these applications CO2 is found in frigorific facilities as secondary fluid, in automotive air conditioning, vending machines and supermarkets (Bensafi and Thonon, 2007).
3. CO2 features
One of the characteristics that CO2 has in comparison with other refrigerant fluids is the operational pressure which is superior to all other conventional refrigerants and those from a new generation (10 times higher than that of the ammonia, R-404A, R134a, R-22, R-12 and HFO-1234yf). This particularity makes it necessary to use especial equipment for its handling. However, at the same time, it offers advantages that no other refrigerant fluid has. The high pressure turns it in a high density gas according to its thermophysical properties. This causes it to obtain a major refrigerant effect with little mass circulating through the vapour compression system.
In a frigorific system, the ideal option is that oil and refrigerant fluids are completely miscible one in the other as this allows that the oil coming from the compressor circulates through all the circuit and returns back to the compressor 100%. In this context, the oil, type POE (polyolester), meets this characteristic. Notwithstanding, it has been found a notable diminishing in the coefficient of heat transference close to the pseudocritical temperature (Zingerli and Groll, 2000). This is why Chaobin Dang et al., (2007) have done research with oil, type PAG (polyalkaline), which is partially miscible, they found that the diminishing in the coefficient of heat transfer is, principally, due to parameters like: the temperature, mass flow rate, and the piping diameter, in which the major loss of heat transfer is when the diameter of the piping decreases.
3.1 CO2 advantages
Carbon dioxide has a benefit in being more economical. Take into consideration that it is less expensive to produce in comparison to synthetic refrigerants, and that most alternatives require additional safety and physical transport regulations.
Its thermodynamic and transport properties are excellent. In addition, it is environmentally friendly; it is not flammable and relatively chemically inert. Some of the important physical properties of CO2 are shown in the phase diagram in Figure 5.
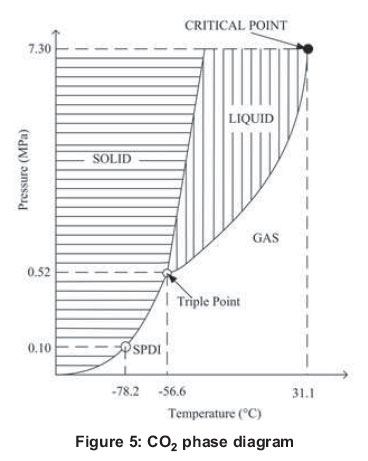
Two of them are indicated as the triple point and the critical point, a third is indicated as SPDI, the sublimation point of dry ice that occurs at atmospheric pressure and at a temperature of 78.2°C. The critical point which takes place at a temperature that is easily attainable in environmental conditions in warm places. The critical temperature and pressure conditions shown in Figure 5 cause the rejection of heat to the environment and does not involve a condensation of fluid work (Cavallini, 2004). In the cold generation by vapour compression, it is desirable to manage the working fluid with inlet pressure to the compressor that is equal to or greater than the atmospheric one, so that air does not infiltrate into the system. Carbon dioxide meets this basic characteristic for having more than 0.52 MPa inlet pressure. On the other hand, Table 1 presents the properties of several refrigerants compared to CO2.
Here is possible to see the notable difference of CO2 regarding the volumetric capability of heat, which implies using a less quantity of refrigerant mass than in any other system in order to achieve the desire refrigerant effect, and by this to overcome to all its competitors. It is also remarkable that in regard to the environmental aspect, it is the second best after ammonia. Nonetheless, ammonia is toxic for human beings and therefore, respecting toxicity - CO2 is a better option.
3.2 Thermodynamic properties
The thermodynamic properties of fluids are essential for the design of equipment, particularly in relation to the energy requirements, phase equilibria, and the size decision thereof (Kim et al., 2004). In the transcritical region, the enthalpy and the entropy decrease along with the temperature in sudden changes close to the critical point. Pressure influences the enthalpy and the entropy above the critical temperature whereas its effect is little under it. The main characteristic that will condition the design of a facility using CO2 is the high pressures to which the system operates. Density is another important factor in CO2; in high densities, the required displacement by the compressor and the diameter of the connection piping is reduced. This represents a small advantage in view of the fact that if the diameters of the piping in conventional systems are small, as a result, small system designs will be obtained.
Surface tension in refrigerants fluids influences nucleated boiling and two-phase flow characteristics. A low surface tension reduces the overheating required for nucleation and growth of bubbles of steam, which will improve heat transfer. The surface tension of the CO2 is low compared to other refrigerants. It has a coefficient of heat transfer between 60 and 70% higher than conventional refrigerants except for ammonia (Padalkar and Kadam, 2010). The volumetric capacity for CO2 is 3 or 4 times higher than for other refrigerants. Therefore, CO2 systems require less CO2 as well as a smaller compressor, heat exchangers and pipeline. The main energy characteristics of these refrigerants compared to CO2 are shown in the Table 2.
In Table 2, it can be see a low value of latent heat for CO2; this means that CO2 needs the least amount of energy to change the phase, this is directly linked to its thermodynamic properties and certainly its low latent heat represents a disadvantage, since for a refrigerant the greater is its latent heat of vaporization, the greater the heat absorbed per kilogram. Therefore, if there is a high value of latent heat, cold production is high and the mass flow will be less.
3.3 Environmental aspects
The GWP of a substance only quantifies the contribution to climate change from a greenhouse gas when it spreads directly into the atmosphere, which could be termed as direct greenhouse effect. Refrigerant fluids fume enters the atmosphere due to leakage in plants caused by losses during peacekeeping operations or by the non-recovery of the refrigerant when the facility is not used. In addition to the direct effect of the refrigerant in facilities, there is also the indirect effect linked to energy consumption. This indirect effect is not exactly a feature of the fluid, but that depends on the facility, amount and type of energy consumed. Thus, this occurs as a result of the energy consumption of the facility if the energy used comes, totally or partially, from the combustion of fossil fuels that emit CO2 into the atmosphere. The parameter that measures both effects as mentioned is the Total Equivalent Warming Impact (TEWI) and its definition by means of mathematical expressions is (ARIAH 2012):
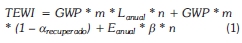
Being the first two terms of the equation the direct effect and the third indirect effect. Different authors have lectured on the data needed to obtain the value of the TEWI, and may conclude that this parameter is not calculated for a refrigerant, but for a given application (Sand et al., 1999; Fischer, 1997):
- Type of refrigerant used in the application, to get the value of the GWP
- The amount of refrigerant mass released to the atmosphere during the operation of the system at all its life. (Lanual, in kg).
- Useful life of the system (n, in years).
- Refrigerant charge (m, kg).
- Recovery factor ( from 0 to 1).
- Energy consumed annually in the operation of the installation (Eanual, in kWh per year).
- Indirect emission factor ( , in kg of CO2 per kWh).
Based on this definition, for a facility that operates 365 days a year and that spends 7 kWh/day with a leakage ratio of 0.25 and a recovery percentage of 20%, the TEWI for facilities that use different refrigerants is the one shown in Figure 6 (Australian Institute of Refrigeration Air Conditioning and Heating, 2012).
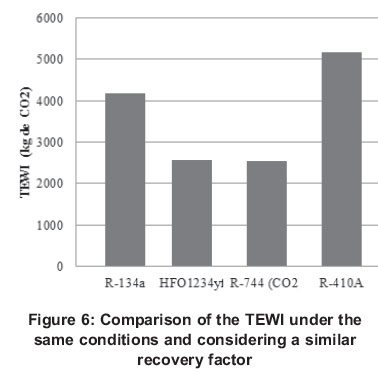
Therefore, facilities operating with CO2 under the conditions set out in Figure 7 emit less CO2 both evaluating the direct effect as indirect, proving to be the only drawback, the high pressures of work. Also, another important feature of these kinds of facilities is their poor efficiency in warm environments. Hinde et al., (2008) mentioned that the most practical application might be using CO2 in cascade systems or using it as secondary fluid. Aprea et al., (2012) researched on direct and indirect contribution of having CO2 as refrigerant, and they pointed out that this contributes indirectly to CO2 emissions in greater quantity than their own R-134a. Nevertheless, this does not happen in every case, the authors refer to the operation conditions in which CO2 is a viable alternative in a transcritical cycle, such conditions are at ambient temperature between 25 and 30°C and an evaporation temperature of 0 and 5°C.
In a study conducted by Kruse (2000), it is mentioned "in the same way" applications in which CO2 is an alternative as a refrigerant to minimize the TEWI. The authors also mention that the use of CO2 in a potential cycle is a good choice as a substitute for conventional systems. The drawback arising with this type of system is the high cost of the operating pressures. On the other hand, in Germany, there is a study relative to the direct and indirect emissions of greenhouse gases of the fluids used in supermarkets, (Rhiemeier et al., 2008) in this study showed different results, one of them is presented in Figure 7. The presented emissions are annual and measures per linear meter deeming 6.15% of loss of refrigerant.
Figure 7 can be seen as the direct contribution of CO2 which is minimal, while the indirect contribution is significant and strengthens the study done by Aprea et al., (2012). However, adding the direct and indirect effects, the TEWI of systems that use R-134a is slightly above the systems that use CO2 by using transcritical systems representing a viable alternative, since the negative contributions to the environment are lower than those of the refrigerant R-134a.
3.4 Energy aspects
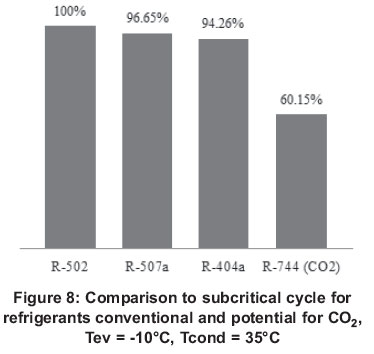
Although the energy performance of facilities operating with CO2 in cooling mode are below those that do so with HFC's, when CO2 is used in heat pump mode (Brian et al., 2011), performance competes adequately with respect to conventional refrigerants. It has been shown that during cold weather, facilities using CO2 such as refrigerant in heat pumps are even more efficient which actually make use of refrigerants, due to its excellent heat transfer properties. Figure 8 shows a comparison of the results of the simulation of a low-temperature commercial refrigeration cycle. It shows that the energy behaviour of the refrigerant fluids R-502 and its substitutes R-404a and R-507a is very similar, while using the CO2, the performance drops considerably when operating at the same conditions.
In automotive air conditioning CO2 is considered as an option and when comparing it with R-134a, it is reported as acceptable. However, the automotive industry has tilted for a mixture known as HFO-1234yf, although nowadays there are still some doubts about the safety of this fluid due to its flammability.
Germany, at the end of February 2013, announced that it will not use this refrigerant in their automotive brands. Companies such as Volkswagen, Audi, BMW and Daimler, have opted to conduct research to improve the transcritical cycles and apply them to their vehicles, arguing that the HFO is not safe enough for its vehicles using it (http://www.r744.com/news/view/3957). The energy aspect, on domestic air-conditioning, CO2 competes with the R-134a which is still used in Europe, while in the United States it is being displaced by the HFO-1234yf.
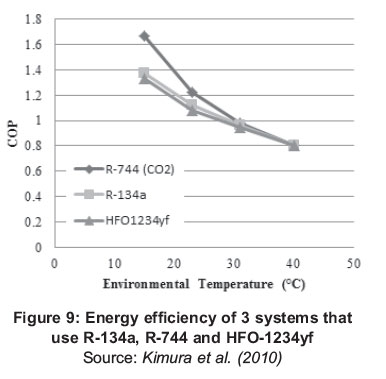
As part of a new technology that is being introduced in the developed countries CO2 is energetically compared with the two previously mentioned fluids (R-134a and HFO-1234yf) by way of exemplifying the potential of this refrigerant. Various laboratory tests were performed and Figure 9 shows these results, showing a comparison of the achieved yields of these 3 fluids under similar operating conditions.
On the other hand, Figure 10 shows the energy consumption of the compressor on each system for the different refrigerant fluids.
It is noted how the power consumption of a compressor that uses CO2 is lower in certain temperature ranges and certainly these values show a set of points at the end of which the consumption is similar.
3.5 CO2 disadvantages
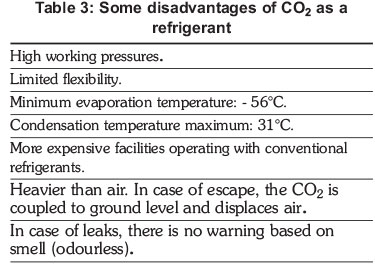
In the refrigerants used in the industry, only ammonia is lighter than air, which means that in case of leakage this moves above the air leaving people who are operating facilities vulnerable (only if the leak is higher and the amount of refrigerant is excessive). Nonetheless, industry has presented technological advances on security with refrigerants heavier than air and also toxic. It is clear that CO2 as any other refrigerant is not an ideal substance to be consider in refrigeration, however, from an environmental point of view it is a dormant option that can be consider for its use.
In air conditioning, the most commonly used fluids are currently R-407C, R-410A and R-290. CO2 can be used in this application and in fact, the use of this fluid has increased mostly in the countries of Northern Europe. Figures 11 and 12 show a comparison of the energy performance of these fluids for heat pump and refrigeration modes.
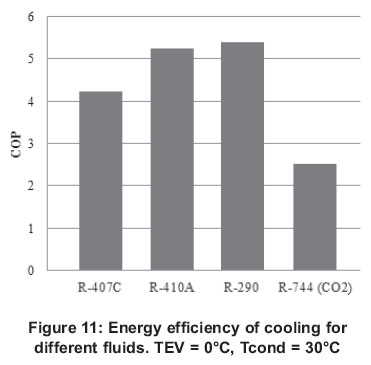
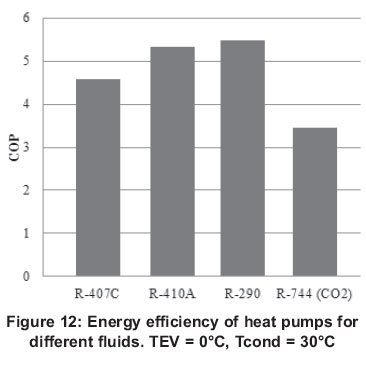
As it can be seen the energy performance of the cycle transcritical with CO2 is below the other systems, this is because of the condensation temperature which is approaching the critical point of the fluid, and the amount of energy rejected is increasing. This is reflected in a reduction of the heat transfer and requires a surface contact area larger to maintain a constant heat transfer, which without doubt directly affects the performance of the cycle.
4. CO2 applications worldwide
Refrigeration with CO2 covers a wide field of application ranging from the industrial sector, transport, to the commercial sector. In the industrial sector, CO2 refrigeration systems are used in the processes of extraction of heat in the dairy industry and soft drinks, amongst others (where companies such as Nestle, Coca-Cola, Mc Donalds and Unilever are present).
In the field of transportation, this refrigerant presents interesting results in automotive air conditioning (Jing-yang et al., 2003). Is worth mentioning that in air conditioning, the use of CO2 is still more extensive because in this area the fluid is highly competitive and even better than any of its competitors due to its excellent heat transfer properties. Heat pumps are mainly using CO2 technology. As technology advances, more compact facilities operating with CO2 are found than those used some decades ago.
Today, technology has advanced so much that it is possible to have refrigeration systems by vapour compression operating with CO2 in cars. Due to their thermodynamic properties, at the last Olympic Games of Beijing 2008, CO2 was replacement used for buses (http://conf.montrealprotocol.org). In Germany in 2009 the transport company Berliner Verkehrsbetriebe (BVG) set the operating of buses in the city of Berlin using CO2 as refrigerant in the air conditioning system (http://www.bvg.de).
In shopping malls, refrigeration equipment using CO2 as a primary and secondary refrigerant has shown good results to be implemented in refrigerators for the conservation of plant products (http://www.achrnews.com).
In 2009, Pick n' Pay with help of GIZ Proklima, installed two cascade systems with ammonia and carbon dioxide (Johannesburg and Cape Town) in two of its supermarkets. In 2011, Makro South Africa installed cascade systems of ammonia and carbon dioxide in three out of fifteen of its stores in South Africa (Gesellschaft für Internationale Zusammenarbeit, 2011). Another area of application is in the area of leisure and recreation where cold production equipment is used with CO2 for skating (http://www.danfoss.com).
The use of CO2 under transcritical cycle is also used in the food industry in the extraction of oils in bio-materials such as herbs, natural plants such as legumes, nuts and palms among others (Norhuda and Jussof, 2009). It is also used as a solvent for the processing of molten polymers, composites training and microcellular foam (Sameer, 2006). In the medical industry as a sterilizer in the transplantation of tissue (http://www.scientificamerican.com), as well as in the aeronautical industry for the cooling systems of rockets (Urbano et al., 2009).
5. Conclusions
One of the main challenges when using CO2 as a refrigerant is to obtain significant increments in the performance of transcritical systems in comparison with the conventional systems inasmuch as the performances are energetically insufficient, in reference to the refrigeration mode. As the investigation advances, without question, the use of CO2 will be wider around the world, if there is no refrigerant in the market that can replace it as was done long ago with CFCs, of course.
The use of natural refrigerants like CO2 is a practice that humankind should always do whereas this guarantees a low negative contribution to the environment. Still, an important part is the energy efficiency that the systems present and in this sense, the transcritical systems are not the best. For this reason, configurations that promote the increasing of the energy performance must be used.
CO2 purposes are many and every day there are more of them. This is because this fluid is better positioned in the market day after day, and it is integrated into the range of strong options in refrigeration systems. One of the most latent applications is found without question in the area of automotive air-conditioning, in which, nowadays, there is no certainty about what refrigerant must be used. Given that, it is known that R-134a should be eliminated from automotive units in the years to come. Hence, it is here where we think there is an open area of opportunity for CO2 to become the ideal fluid.
The disadvantages of handling this fluid must be also considered. However, currently, material science and facility safety have caused that the risk for high pressures pass the background, and because of this, the best is to continue with the investigation of upgrades, especially in the transcritical mode.
Considering that the tendency is the use of refrigerants with a low GWP CO2 looms as a good alternative to replace synthetic fluids, which main disadvantage is their great contribution to global warming.
References
Aarlien R. (1998). Kohlendioxid- Besonderheiten und Einsatzachancen als Kältemittel/Statusbericht des Deutschen Kälte- und Klimatechnischen Vereins, Nr 20. DKV Sttutgart: DKV. [ Links ]
Aprea C., Greco A., & Maiorino A. (2012). An experimental evaluation of the greenhouse effect in the substitution of R134a with CO2, Journal of Energy, Vol. 45, 753-761. [ Links ]
Austin, B.T. & Sumathy, K. (2011). Transcritical carbon dioxide heat pump systems: A review, Renewable and Sustainable Energy Reviews, Volume 15, Issue 8, 4013-4029. [ Links ]
Australian Institute of Refrigeration, Air Conditioning and Heating (ARIAH) 2012. Methods of Calculating Total Equivalent Warming IMPACT (TEWI), Best Practice Guidelines. [ Links ]
Bensafi A. & Thonon B. (2007). Transcritical R744 (CO2) heat pumps, Report 2414173. [ Links ]
Bodinus W.S. (1999). In: H. M. Will (Ed.), The rise and fall of carbon dioxide systems. In: Will HM, editor. The first century of air conditioning. Atlanta, GA; ASHRAE, 29-34. [ Links ]
Calm M.J., (2008). The next generation of refrigerants, ACR Latinoamérica, 12 (1) 18-25. (in Spanish). [ Links ]
Cavallini A. & Steimle F 1(998). Natural Working Fluids in a Historic Perspective, Natural Working Fluids '98, IIR-Gustav Lorentzen Conference, Oslo (Norwey), June, Proceedings, 37-42. [ Links ]
Cavallini A. (2004). European Seminar CO2 as a refrigerant, Milan, Italy, (November). [ Links ]
Chaobin Dang, Koji Iino, Ken Fukuoka, Eiji Hihara (2007). Effect of lubricating oil on cooling heat transfer of supercritical carbon dioxide, International Journal of Refrigeration, 30, 724-731. [ Links ]
Chen Y., Jepson W.P (1999). EIS Measurement for corrosion monitoring under multiphase flow conditions, Electrochimica Acta, 44, 4453-4464. [ Links ]
Danfoss (2008). Refrigeration & Air Conditioning Division, DKRCE.PZ.000.G1.02/520H3090. [ Links ]
Fischer S.K., and Sand J.R. (1997). Total Environmental Warming Impact (TEWI) Calculations for Alternative Automotive Air-Conditioning Systems, SAE PaperMNo. 970526, February. [ Links ]
Gesellschaft für Internationale Zusammenarbeit (GIZ) (2011). Conversion of Supermarket Refrigeration Systems from F-Gases to Natural Refrigerants, Programme Proklima. [ Links ]
Heuer J.K. & Stubbins J.F (1999). An XPS characterization of FeCO3 films from CO2 corrosion Corros, Sci. 41, 1231-1243. [ Links ]
Hinde D., Zha S., & Lan L. (2008). CO2 Experiences in North American Supermarkets, 8th IIR Gustav Lorentzen Conference on Natural Working Fluids, Copenhagen. [ Links ]
Jing-yang M.U., Jian-ping CHEN, Zhi-jiu CHEN (2003). System design and analysis of the trans-critical carbon dioxide automotive air-conditioning system, Journal of Zhejiang University SCIENCE VA. No. 3, 305-308. [ Links ]
Kim Man-Hoe, Pettersen Jostein, & Bullard Clark W. (2004). Fundamental process and system design issues in CO2 vapour compression systems, Progress in Energy and Combustion Science 30, 119-174. [ Links ]
Kimura Makoto, Yamaguchi Yukio and Sunao Suzuki (2010). Examination of LGWP Refrigerant for Cooling Show Case, SANDEN Corporation. [ Links ]
Kruse H. (2000). Refrigerant use in Europe, ASHRAE Journals September, 16-24. [ Links ]
Lorentzen G. (1990). Transcritical vapour compression cycle device. WO 90/07 683. [ Links ]
Lorentzen G. (1992). Method for regulating the capacity of a vapour compression cycle device and automotive air conditioner, ES 2 025 443 (in Spanish). [ Links ]
Natarajan Balaji (2008). PNUMA-DTIE-ORAP, Elimination of HCFC's: A convenient order to safeguard the ozone layer and climate opportunity, ACCION OZONO, September (in Spanish). [ Links ]
Neksá P (2002). CO2 heat pump systems, International Journal of Refrigeration, 25,421-427. [ Links ]
Neksá P, Rekstad H., Zakeri G.R., & Schiefloe P.A. (1998). CO2 heat pump water heater: characteristics, system design and experimental results, International Journal of Refrigeration, 21,172-9. [ Links ]
Neksá P, Rekstad H., Zakeri G.R., Schiefloe P.A., & Svensson M.C. (1999). In: CO2 technology in refrigeration, heat pump and air conditioning systems, Mainz, Germany. [ Links ]
Norhuda I., & Jussof K. (2009). Supercritical carbon dioxide (SC-CO2) as a clean technology for palm kernel oil extraction, Journal of Biochem Technology 1, 75-78. [ Links ]
Padalkar A.S., & Kadam A.D. (2010). Carbon Dioxide as Natural Refrigerant, International Journal of Applied Engineering Research, Volume 1, No. 2. [ Links ]
Pérez-García V, Belman-Flores J.M., Navarro-Esbrí J., & Rubio-Maya C. (2013). Comparative study of trans-critical vapour compression configurations using CO2 as refrigeration mode base on simulation, Applied Thermal Engineering, 51, 1038-1046. [ Links ]
Rhiemeier Jan-Martin, Harnsch Jochen, & Kauffeld Michael (2008). Kältemittelemissionen im Lebensmitteleinzelhandel, TEWI-Analysen und Betrachtung von Kosten und Unsicherheiten, Kaltetechnik-Supermarktkäle, Oktober, 31-35. [ Links ]
Sameer P., Nalawade, Francesco Picchioni, & L.P.B.M. Janssen, (2006). Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications, Progress in Polymer Science, 31, 19-43. [ Links ]
Sand J.R., Fischer S.K. & Baxter VD. (1999). TEWI Analysis: Its Utility, Its Shortcomings, and Its Results, International Conference on Atmospheric Protection, Taipei, Taiwan, September. [ Links ]
Thévenot R. (1979). A history of refrigeration throughout the world, Paris, International Institute of Refrigeration; [Fidler JC, Trans. [ Links ]].
Urbano A., Pizzarelli M., & Nasuti F. (2009). Numerical Analysis of Transcritical Fluids Heating in Liquid Rocket Engine Cooling Channels, Aerotecnica Missili & Spazio , The Journal of Aerospace Science, Technology and Systems. Vol. 88, No.1 / 2, 20-30. [ Links ]
US Department of Energy (US DOE) (2003). Energy Efficiency and Renewable Energy. Improving Compressed Air System Performance. DEO/GO- 102003-1822. [ Links ]
Wu S.L., Cui Z.D., Zhao G.X., Yan M.L., Zhu S.L., & Yang X.J. (2004). EIS study of the surface film on the surface of carbon steel from supercritical carbon dioxide corrosion, Applied Surface Science, 228, 17-25. [ Links ]
Zingerli A. & Groll E.A. (2000). Influence of refrigeration oil on the heat transfer and pressure drop of supercritical CO2 during in tube cooling In: Proceedings of the Fourth IIR-Gustav Lorentzen Conference on Natural Working Fluids, IIR, USA, 269-278. [ Links ]
Received 15 October 2013
Revised 2 April 2014














