Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of Energy in Southern Africa
versión On-line ISSN 2413-3051
versión impresa ISSN 1021-447X
J. energy South. Afr. vol.25 no.2 Cape Town may. 2014
Performance of a compression ignition engine operated with sunflower ethyl ester under different engine loads
Emin AçikkalpI; Hasan YamikI; Yakup IçingürII
IDepartment of Mechanical and Manufacturing Engineering, Bilecik SE University, Bilecik, Turkey
IIFaculty of Technical Education, Gazi University, Ankara, Turkey
ABSTRACT
This study investigated the performance of a compression ignition engine operating with sunflower ethyl ester. A thermodynamic analysis, including energy and exergy analysis at different engine loads (20%, 40%, 60%, 80%, 100%), was conducted. The study calculated the first and second law efficiency, effective work, heat exergy losses and exergy destruction values at 10 different engine speeds for 5 loads. Maximum work, maximum thermal efficiency, maximum exergy efficiency and maximum volumetric efficiency are determined to be 6.45 kW, 0.26, 0.24 and 0.71 respectively. Finally, optimum operating conditions are discussed and it was determined that the engine should be operated at a lower engine speed for partial loads.
Keywords: energy analysis, exergy analysis, exergy destruction, biodiesel, ethyl ester
1. Introduction
Diesel engines are widely used in a variety of vehicles due to their high fuel efficiency and low cost compared to other fuel engines. The resources of petroleum as fuel are dwindling day by day and the increasing demand for fuels, as well as increasingly stringent regulations, pose a challenge to science and technology. The commercialization of bioenergy has provided an effective way to fight the problem of petroleum scarcity and petroleum consumption's influence on the environment. All of these problems have motivated the scientific society to seek new, alternate energy sources that have lessened the effects of global warming and pollution. At this point, the scarcity of known petroleum reserves and increasing environmental consciousness has made renewable energy sources more attractive (Misra and Murthy, 2011; Moron and Villareyes, 2007). As a renewable, sustainable and alternative fuel for compression ignition engines, biodiesel instead of diesel has been increasingly used to study its effects on engine performance and emissions in the last 10 years. The advantages of using biodiesel as diesel fuel are minimal sulphur and aromatic content, and the higher flash point, lubricity and cetane number. It helps to reduce carbon dioxide emissions in the atmosphere; it is renewable in nature and safer to handle; it has no aromatic compounds, practically no sulphur content, and oxygen atoms in the molecules of the fuel may reduce the emissions of carbon monoxide (CO), total hydrocarbon (THC) and particulate matter (PM) (Scholl and Sorenson, 1993; Lapuerta et al., 2005; Lapuaerta, Armas and Ballesteros, 2002; Zang and Van Gerpen, 1996).
The combustion performance of the ethyl ester of used palm oil relative to baseline diesel fuel in a water-cooled furnace was investigated. The combustion efficiency was tested over a wide range of air/fuel ratios, ranging from very lean to very rich (10:1-20:1). The findings showed that at a lower energy rate, biodiesel burned more efficiently with higher combustion efficiency (66%) compared to the diesel fuel (56%). At higher energy inputs, the biodiesel combustion performance deteriorated, because of its high viscosity, density and low volatility (Tashtoush, Al-Widyan and Al- Shyoukh, 2003). Rakopoulos et al., (2011) conducted a study to evaluate the use of sunflower, cottonseed, corn and olive straight vegetable oils of Greek origin, in blends with diesel fuel at proportions of 10% and 20%. The study reported that the specific fuel consumption for all vegetable oil blends is a little higher than the corresponding one for the diesel fuel case. The engine brake thermal efficiency with all the vegetable oil blends was practically the same as that of the neat diesel fuel case.
In recent years, the exergy analysis method has been widely used in the design, simulation and performance assessment of various thermal systems. This analysis is based on the second law of thermodynamics. Exergy is defined as the maximum theoretical useful work obtained as a system interacts with the equilibrium state. Exergy is generally not conserved as energy but destroyed in the system. It is possible to determine the optimum speed of an auto cycle engine using combined energy and exergy analysis. Energy and exergy efficiencies are calculated for different engine speeds and compared. Determination of the optimum engine speed should not be based on energy analysis alone (Kopaç and Kokturk, 2005). Exergy destruction is a measure of irreversibility that is the source of performance loss.
Investigated is the effect of varying dead state temperatures on the exergy efficiency of a high-oleic methyl ester (HOME) fueled internal combustion engine (ICE). This engine is a 4.5L, four stroke, four-cylinder, turbocharged, 66.5 kW maximum power capacity John Deere 4045T diesel engine run with HOME, which is genetically modified with a high-oleic soybean oil methyl ester. The results obtained are discussed from the exergetic point of view. It was found that exergetic efficiency increased as the dead state temperature decreased. As a result, exergy efficiency values ranged from 29.78% to 34.93% based on dead state temperatures between 5 oC and 30 oC (Caliskan, Tat and Hep-basli, 2009). There has also been presented a comparative second law analysis of internal combustion engine operation for methane, methanol and dode¢ cane fuels (Rakopoulos and Kyritsis, 2001). Analyzed is a diesel cycle considering combustion and heat transfer effect on performance. The effects of the compression ratio and cut-off ratio on the heat transfer were analyzed. Exhaust temperature and work output increased (Parlak, 2005). Energy and exergy analyses were performed in a four-stroke turbocharged diesel engine fuelled with No. 2 diesel and two different biodiesel fuels. Exergy efficiencies are calculated between 37.46 % and 38.48 %, with no statistically significant difference. Exergy destruction of the engine is between 59.03 kW and 61.76 kW for three fuels (Caliskan et al., 2010).
Evaluated is the performance of an internal combustion engine at the steady-state condition through energy and exergy analysis by using experimental test results. The energy efficiency has a maximum point at the speed of 2500 rpm. The exergy analysis reveals that the engine's optimum speed is 300 rpm, as the exergy efficiency has a maximum magnitude at this speed (Ameri et al., 2010). The use of biodiesel and their blends results in a very similar exergetic performance with No. 2 diesel fuel in terms of fuel exergy input, exergetic efficiency, exergy destruction and exergy losses. Exergy losses due to the exhaust gas and heat transfer are other contributors in decreasing order (Canakci and Hosoz, 2006). Using exergy as a measure of quality, the petroleum diesel fuel is of greater quality than biodiesel because of the net calorific value of diesel that of biodiesel (Sekmen and Yilbasi, 2011).
The energy demands of the world increase day by day. That's why using and exploring different energy resources like biodiesels have gained importance. Sunflower ethyl ester is assumed to be a renewable energy source, and it can be used in internal combustion engines. In this study, a compression ignition engine operating with sunflower ethyl ester was investigated for different engine loads. This is because engines usually operate at less than full load. Energy and exergy analyses were performed and optimum operating conditions were determined.
2. Materials and methods
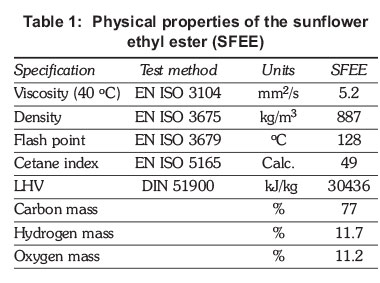
Sunflower ethyl ester was the test fuel. The physical properties of the fuels tested are presented in Table 1. The tests were conducted on a single cylinder, four stroke, naturally aspirated, air cooled diesel engine coupled with an electrical dynamometer. A schematic diagram of the systems can be seen in Figure 1. The detailed technical specifications of the engine are given in Table 2. The test fuels are 100 % ethyl ester; the biodiesel molar ratio of alcohol to oil used was 5:1, whereas the catalyst amount was 1% of the oil's weight.


The air and fuel flow rates entering the engine were measured using a laminar flow element and a digital scale, respectively. Temperature measurements at different locations of the experimental system were conducted using thermocouples. Energetic and exergetic values were calculated by a 300 1/min increase in fixed cycle variable speed experiments.
3. Thermodynamic analysis
Energy and exergy analyses were conducted under steady-state conditions for the control volume. An energy analysis for the control volume can be written by means of the first law of thermodynamics (Moran and Shapiro, 1995; Cengel and Boles, 2008).
_ESPACO_

Where in and out represent input and output states respectively. W and Q denote work rate and heat rate. Energy input to the system is the chemical energy of fuel, which is calculated as the following (Kopaç and Kokturk, 2005; Caliskan et al., 2010 and Ameri et ai, 2010):
_ESPACO_

Where m is the mass flow ratio and LHV is the lower heating value of the fuel. Effective work of the engine can be calculated as (Kopaç and Kokturk, 2005; Caliskan et al., 2010 and Ameri et al, 2010):
_ESPACO_

Here, n is the engine speed and t is the torque. Heat loss from the exhaust is (Caliskan et al., 2010):
_ESPACO_
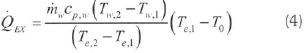
Heat loss with other processes (radiation, cooling water and lubrication oil) is calculated as follows:
_ESPACO_

The energy efficiency of the system can be described as the ratio of the work output of the engine to the fuel energy (Moran and Shapiro, 1995; Cengel and Boles, 2008):
_ESPACO_

In exergy analysis, the dead state was assumed as 298.15 K and 100 kPa. From the second law of thermodynamics, entropy and exergy analyses for the control volume can be described as the following, respectively (Moran and Shapiro, 1995; Cengel and Boles, 2008):
_ESPACO_

Where S gen and ExD represent the entropy and the exergy rate respectively. The exergy of a substance is (Moran and Shapiro, 1995; Cengel and Boles, 2008):
_ESPACO_

Exergy transferred with heat can be described as (Moran and Shapiro, 1995; Cengel and Boles. 2008):
_ESPACO_

where T0 is the environment temperature and Tk is the high temperature source. Exergy transferred with work is equal to work done by the engine (Moran and Shapiro, 1995; Cengel and Boles, 2008):
_ESPACO_

The exergy input of the system is equal to the chemical exergy of the fuel (Kopaç and Kokturk, 2005; Caliskan et al, 2010 and Ameri et al, 2010):
_ESPACO_

Where af is the chemical exergy factor, and it can be calculated as (Moran and Shapiro. 1995):
_ESPACO_

where o,c,h and s represent the mol weight of the elements. The physical exergy of any system is (Moran and Shapiro, 1995: Cengel and Boles, 2008):
_ESPACO_
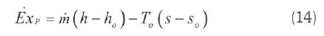
Where h is the specific enthalpy and is the specific entropy. The chemical exergy of a gas mixture can be calculated as (Kopaç and Kokturk, 2005; Ameri et ai, 2010):
_ESPACO_
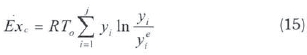
where R is ideal gas constant and y is mol ratio. Exergy is the rate of effective work to fuel exergy (Moran and Shapiro. 1995: Cengel and Boles. 2008):
_ESPACO_
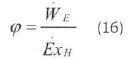
4. Results
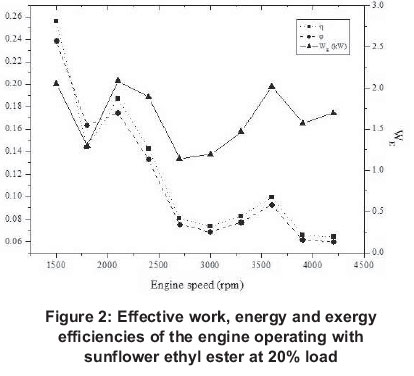
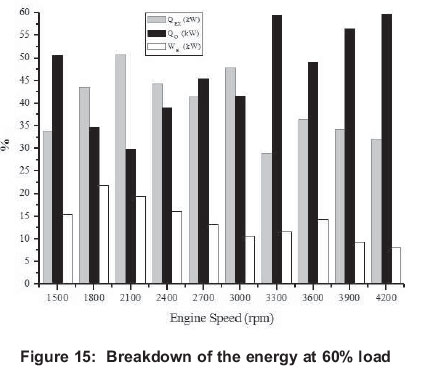
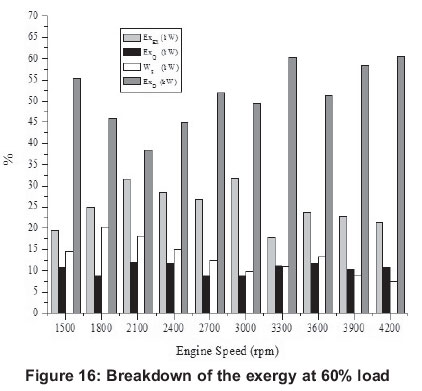
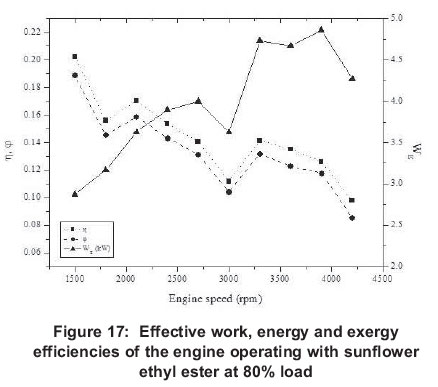
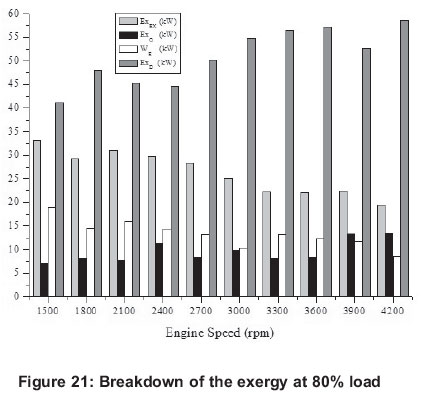
Experiments were conducted at different engine loads and at different engine speeds for a compression ignition engine operating with sunflower ethyl ester. Results are shown in Figure 2-26. After investigating these figures, results can be presented as follows.



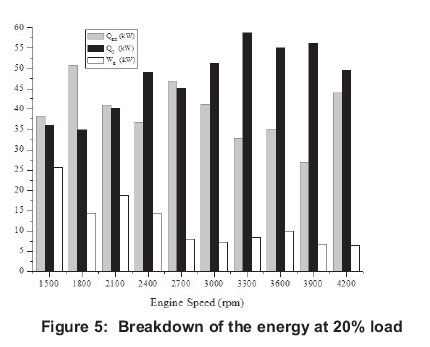




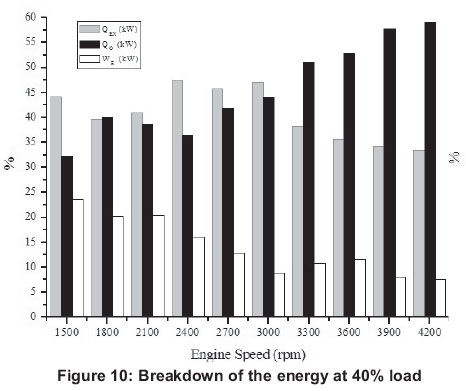







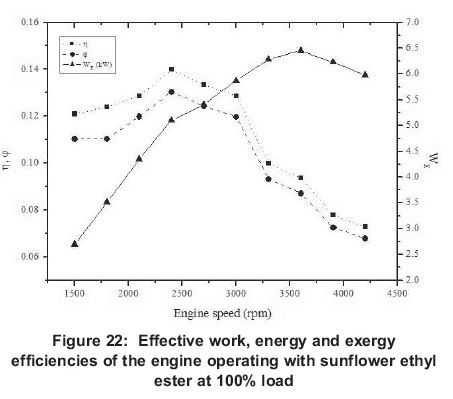
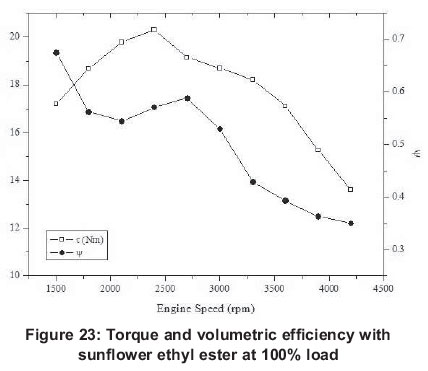

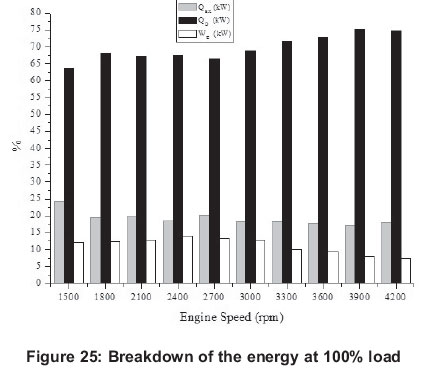

4.1 Energy
Energy related figures can be seen in Figures 5, 10, 15, 20 and 25. Other heat losses include radiation and cooling water losses. Other heat releases reached their maximum values between 3 300 rpm and 4 200 rpm for all engine loads. In addition, it can be seen that other heat losses generally increase with engine speed. Exhaust heat loss is greater at low engine speeds (1500-2400 rpm) for 20-80% engine loads, however, exhaust loss is nearly the same at all engine speeds for 100% engine loads. For all engine loads, except 100%, work efficiency is greater at low engine speeds (1500-2400 rpm) and it obtains the minimum value at 3000 rpm, however, with a 100% engine load it gets its maximum at 2400 rpm and its minimum is at 4200 rpm.
4.2. Exergy
Exergy related values are shown in Figures 6, 11, 16, 21 and 26. Exergy destruction is the highest value according to exergy analysis. The reasons for exergy destruction in the engine are friction, heat losses, and most importantly, the combustion process which, it can be seen, is getting bigger.
Investigating exergy destructions, it values, generally, at the high engine speeds for 20-80% engine loads. However, with 100% engine loads, all exergy destruction rates are nearly the same for all engine speeds. Exergy destruction can be decreased by increasing the air-mass ratio (Rakopoulos and Kyritsis, 2001).
In addition, exhaust heat loss exergy doesn't tend to follow any pattern for engine speeds at the partial engine loads, and heat release exergy reaches its minimum at 2700-3000 rpm, but at the full load, it is more balanced and has similar values. When results are investigated, it can be seen that maximum work (6.45 kW) is obtained at 3900 rpm for 100 % engine load; similarly minimum work is obtained (1.27 kW) at 300 rpm for 20 % load.
4.3 Effective work and torque
For 20 and 40% engine loads, maximum work is obtained at engine speeds (2100 and 2400 rpm), but, with 60, 80 and 100% engine loads work reaches it maximum at 3600 and 3900 rpm values. Work shows unbalanced changes at the partial loads, while it is balanced at full load. This is because the combustion process is more effective with the full load. The ratio of effective work to energy and exergy analysis decreases with engine speed.
Similar to effective work, torque values show unbalanced changes at partial loads, and it is balanced at 100% engine loads. With all engine loads, maximum torque values are obtained at a low engine speed (1500-2400 rpm) and generally, torque value is lower at high engine speeds. It reaches its minimum at 20% load and 3000 rpm (3.799 Nm) and its maximum at 100% load and 2400 rpm (20.303 Nm).
4.4 First law (energy or thermal), second law (exergy) and volumetric efficiencies
Results for energy efficiency show that maximum efficiency is obtained at 1500 rpm with 20% load, while minimum efficiency (0.064) is at 4200rpm with 20% load again.
Maximum exergy efficiency (0.24), as with energy efficiency, is reached at 1500 rpm for 20% load and minimum exergy efficiency (0.07) at 4200 rpm for 100%. Energy and exergy efficiencies are greater for low engine speeds than high engine speeds for all engine loads. For volumetric efficiencies, it reaches its maximum at 40% engine load and 2700 rpm (71%) and it reaches its minimum again at 40% load and 4200 rpm (0.31). It can be seen that at 20-60% engine loads the volumetric efficiency maximum is at 2700 rpm, but with 80100% engine loads its maximum is at 1500 rpm. Generally, it can be said that volumetric efficiency always decreases after 2700 rpm and it reaches bigger values at low engine speeds.
4.5. Fuel consumption and specific fuel consumption
Fuel consumption increases with engine speed for all engine loads. It ranges from 15 (g/m) for 20% load to 180 (g/m) for 100% load. Similarly, specific fuel consumption increases with engine speed for all loads generally, and it ranges from 7.5 (g/kWm) to 30 (g/kWm) approximately.
5. Conclusion
In this study, the effects of sunflower ethyl ester were investigated on the performance of the compression ignition engine at various engine loads.
• The maximum work (6.45 kW) is obtained at 3900 rpm for 100% engine load.
• The maximum efficiency (0.26) is obtained at 1500 rpm for 20% load.
• The maximum exergy efficiency (0.24) is at 1500 rpm for 20% load.
• The maximum is at 40% engine load and 2700 rpm (0.71).
In conclusion, according to the results, it can be recommended that the engine should be operated at low engine speeds at partial loads, because at these engine speeds, energy and exergy values have the greatest values, while exergy destruction values are lower.
Nomenclature
CP specific heat capacity at constant pressure (kJ/kg)
E energy rate (kW)
Ex exergy rate (kW)
h enthalpy (kJ/kgK)
LHV lower heating value of fuel (kJ/kg)
m mass rate (kg/s)
n engine speed (rpm)
Q heat (kW)
s entropy (kJ/kgK)
T temperature (K)
W work (kW)
Subscripts
D destruction
Ex exhaust
f fuel
H high
E effective work
in inputs
k system boundary
o others, environment
out outputs
p physical
Q heat
T temperature
W work
Greek letters
a fuel exergy (kJ/kg)
φ second law (exergy) efficiency (%)
η first law (energy or thermal ) efficiency (%) r torque (Nm)
Ψ volumetric efficiency (%)
References
Ameri M., Kiaahmadi F, Khanaki M., and Nazoktabar M., (2010). Energy and exergy analyses of a spark-ignition engine, Int. J. Exergy, 7, 547-563. [ Links ]
Caliskan H., Tat M..E., Hepbasli A., and Van Gerpen J...H., (2010) Exergy analysis of engines fuelled with biodiesel from high oleic soybeans based on experimental values, Int. J. Exergy, 7, 20-36. [ Links ]
Caliskan H., Tat M.E., and Hepbasli A. (2009). Performance assessment of an internal combustion engine at varying dead (reference) state temperatures, Appl Therm Eng, 29, 3431-36. [ Links ]
Canakci M., and Hosoz M., (2006). Energy and exergy analysis of a diesel engine fuelled with various biodiesels, Energy sources, Part B, 1, 379-94. [ Links ]
Cengel Y and, Boles M., (2008). Thermodynamics: an engineering approach. 6th ed. New York: McGraw-Hill Inc. [ Links ]
Kopac M., and Kokturk L., (2005). Exergy Determination of optimum speed of an internal combustion engine by exergy analysis, Int. J. Exergy, 2, 40-54. [ Links ]
Lapuerta M., Armas O., Ballesteros R., and Rodriguez-Fernandez J., (2005). Diesel emissions from biofuels derived from Spanish potential vegetable oils, Fuel, 84,773-80. [ Links ]
Lapuerta M., Armas O., and Ballesteros R., (2002). Diesel particulate emissions from biofuels derived from Spanish vegetable oils, SAE Paper No. 200201-1657. [ Links ]
Misra R.D., and Murthy M.S., (2011). Performance, emission and combustion evaluation of soapnut oil-diesel blends in a compression ignition engine, Fuel, 9: 2514-8. [ Links ]
Moran M.J., and Shapiro H.N., (1995). Fundamentals of engineering thermodynamics. New York: John Wiley. [ Links ]
Moron-Villarreyes J.A., Soldi C., de Amorim A.M., Pizzolatti M.G., de Mendonca Jr A.P, and D'Oca M.G.M., (2007). Diesel/biodiesel proportion for by-compression ignition engines, Fuel, 86, 1977-82. [ Links ]
Parlak A., (2005). The effect of the heat transfer on performance of the diesel cycle and exergy of the exhaust gas stream in LHR diesel engine at the optimum injection timing, Energ Convers Manage, 46,167-79. [ Links ]
Rakopoulos C.D, and Kyritsis D.C., (2001). Comparative second law analysis of internal combustion engine operation for methane methanol and dodecane fuels, Energy, 26, 705-722. [ Links ]
Rakopoulos D. C., Rakopoulos C. D., Giakoumis A.M., Dimaratos M. A. Founti.,(2011). Comparative environmental behaviour of bus engine operating on blends of diesel fuel with four straight vegetable oils of Greek origin: Sunflower, cottonseed, corn and olive, Fuel, 90, 3439-46. [ Links ]
Rakopoulos D.C., (2013). Combustion and emissions of cottonseed oil and its bio-diesel in blends with either n-buranol or diethyl ether in HSDI diesel engine, Fuel, 105, 603-13. [ Links ]
Scholl K.W., and Sonrenson S.C., (1993). Combustion of soybean oil methyl ester in a direct injection diesel engine, SAE paper 930934. [ Links ]
Sekmen P, and Yilbasi Z., (2011). Application of energy and exergy analyses to a CI engine using biodiesel fuel, Mathematical and Computational Applications, 16,797-808. [ Links ]
Tashtoush G., Al-Widyan M.I., and Al-Shyoukh A.O., (2003). Combustion performance and emissions of ethyl ester of a waste vegetable oil in a water-cooled furnace, Appl Therm Eng, 23, 285-93. [ Links ]
Zhang Y., and Van Gerpen J.H., (1996). Combustion analysis of esters of soybean oil in a diesel engine, SAE Paper No. 960765. [ Links ]
Received 4 October 2013
Revised 16 April 2014














