Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of Energy in Southern Africa
On-line version ISSN 2413-3051
Print version ISSN 1021-447X
J. energy South. Afr. vol.24 n.2 Cape Town May. 2013
RESEARCH ARTICLE
Reassessment of the environmental impacts of sulphur oxide emissions from power stations
Philip Lloyd
Cape Peninsula University of Technology, Cape Town, South Africa
ABSTRACT
It is a deeply entrenched belief that emissions of sulphur dioxide into the atmosphere are harmful to the environment, and that sulphur compounds should be removed from the gaseous wastes before discharge. The difficulties with this view are summarised. Extensive work in both North America and Europe has failed to demonstrate any of the early claims for impacts such as forest death. The claims for health effects seem unduly conservative and not supported by reliable data. There are even negative impacts from reducing sulphur emissions. Claims for high external costs associated with coal-fired power generation in South Africa are the result of arithmetic errors. The installation of flue-gas desulphurisation on the latest Eskom power station, Kusile, is shown to be completely unsustainable in the light of the minimal benefits that the considerable costs will bring.
Keywords: sulphur oxide, environmental impacts, power stations
1. Introduction
The combustion of fossil fuels is usually accompanied by the emission of sulphur oxides (SOx) into the atmosphere. The conventional wisdom is that these emissions are harmful to the environment, and that there is accordingly merit in taking steps to capture the SOx before its release. It is the purpose of this paper to challenge that view.
2. The conventional view
Figure 1 shows the origin of SOx in the atmosphere (Dentener et al., 2006). The total is approximately 144Tg/a. Natural emissions comprise those between approximately 12 and 3 o'clock. The anthropogenic emissions thus constitute about 75% of the total burden, and it is natural to assume that this must be harmful to the environment.

There was apparent confirmation of this view in the supposed formation of 'acid rain.' According to this hypothesis, the SOx is converted in the atmosphere to SO42- In the process, hydrogen ions are formed, and rain containing the SO42- becomes more acid as a result. The acid rain was reputed to be causing extensive damage to forests in both North-Western Europe and North America. In Germany and Austria, the phrase Waldsterben was coined to describe the phenomenon.
Moreover, SOx could affect health. The classic example was the great British smog of 1952, when several thousand lives were shortened. SOx concentrations of around 1ppmv and smoke of 1.8mg.m-3 were recorded over wide areas of London (Wilkens, 1952).
On both sides of the Atlantic, there were moves to understand the nature and extent of the problem. The greatest effort was in the US, where in 1982 a $600 million research programme was mounted called the National Acid Precipitation Assessment Program, (NAPAP). Before this had reported its findings, however, politics had moved ahead. In the US, the Clean Air Amendment Act of 1990 introduced a cap-and trade process for limiting SOx emissions. The Environmental Protection Agency (EPA) administers the Act, which was implemented in two phases. In Phase I, 5Mt of sulphur reduction were to be made by January 1, 1995, largely by requiring 110 electric power generating plants to cut sulphur dioxide emission rates to 1g/MJ. New generating units were required to limit sulphur dioxide to a 'lowest achievable emissions rate' of about 0.25g/MJ. In Phase II, all fossil-fired units over 75 MWe were required to limit emissions of sulphur dioxide to 0.5g/MJ by January 1, 2000. For any additional emissions, they were required to obtain an emissions allowance for each ton of sulphur dioxide emitted, subject to a mandatory fine of $2,000.00 for each ton emitted in excess of allowances held. The EPA distributes allowances equivalent to the emissions cap of 8.95 Mt each year.
In Europe, the Geneva Convention of Long-Range Transboundary Air Pollution [CLTRAP] came into force in 1983 (UNECE, 2009):
The history of the Convention can be traced back to the 1960s, when scientists demonstrated the interrelationship between sulphur emissions in continental Europe and the acidification of Scandinavian lakes. The 1972 United Nations Conference on the Human Environment in Stockholm signalled the start for active international cooperation to combat acidification. Between 1972 and 1977 several studies confirmed the hypothesis that air pollutants could travel several thousands of kilometres before deposition and damage occurred. (UNECE, 2009.
Note that acidification was claimed long before the issue had been the subject of serious study, and that damage was assumed, rather than being proven.
CLTRAP has a number of on-going International Co-operative Programmes (ICP) studying effects of pollution on forests, water, materials, and vegetation. It also studies sampling and modelling. This work is reviewed in the next section
3. Problems with the conventional view
The first warning signs that something was amiss with the conventional view came when NAPAP published its preliminary findings in 1988. The unequivocal conclusion, after $600 million of research, was that there was no problem. The politicians who had funded the research were appalled. The programme director was relieved of his post, a tamer director installed, and two years later a much watered-down report issued. The New York Times (1990) said:
The report concluded that with the exception of damage to red spruce at high elevations in the East, forests in the United States are not suffering serious damage from acid rain. Dr. James R. Mahoney, director of the National Acid Precipitation Assessment Program, - said that he agreed with some of the reviewer comments about the forest problem and that the final report would probably be changed to reflect them. He said that there had been comments in ''both directions'' with some reviewers saying, ''We don't have evidence of damage and we shouldn't try to find something that isn't there.''
When the final report was issued, it concluded (NAPAP, 1991):
The literature reviewed in this report indicates that most forests in the U.S. and Canada are apparently healthy and growing without obvious symptoms of unusual or pollutant-related stress. . . . Acidic deposition has not been shown to be a significant factor contributing to current forest health problems in North America, with the possible exception of spruce die-back and mortality at high elevations in the northern Appalachians... .Acidic deposition appears to be beneficial to soil fertility, acting as a supplement to fertilizer by providing sulphur and nitrogen. Even when the cost of neutralizing by liming the acidity added by wet deposition is considered, it is likely that such deposition has a net benefit to the soil. (Emphasis added)
It is clear that, whatever some reviewers had felt, the balance of scientific evidence was that there was no problem - indeed, there were even benefits. However, by the time report was finalised, the US Congress had passed the Clean Air Amendment Act into law. The EPA was empowered to control SOx emissions whether or not they caused any damage.
In Germany, there was great concern about waldsterben, but on closer examination it transpired trees were being damaged by known pathologies. There was local damage, which was generally confined to a single species, but there was a long history of the specific pathologies for that species. In a careful survey of nearly 80 000 injured trees, only 0.1% were damaged by an unknown cause such as air pollution (Skelly & Innes, 1994). 'Classic Waldsterben as perceived by the general public does not exist. As time passes it is becoming increasingly clear that the forests of Europe are not dying.'
A typical ICP report from Denmark noted (UN Economic Commission for Europe, 2005):
A nation-wide systematic survey was set up in 1985 to detect signs of changing forest condition expressed as defoliation and discoloration. Defoliation results from 1989 to 2004 have shown that deterioration of forest health can usually be explained by specific events, such as drought, wind, deposition of salt, insects, fungi, fruiting or frost.
Fifteen years of research had failed to find any evidence of air pollution causing damage to forests. Indeed, much of the effort of CLTRAP regarding forests is still going into attempts to standardise methods for describing the health of trees.
The study of surface water has also been equivocal. Great success is claimed, yet the findings are hedged about with caveats (Skjelkvåle & de Wit, 2007):
This report contains surface water chemistry trend analyses for ICP Waters sites for the period 1994-2004 - and an assessment of biological recovery. [The] effects of environmental factors other than acid deposition - on chemical and biological recovery of surface waters are evaluated. Most regions in Europe and North America show significant decreases in sulphate whereas nitrate trends are more scattered. Indicators of chemical recovery show improvements, most clearly in Europe. Increases in organic acidity and sea salt deposition delay recovery in some regions. Climate change both delays and enhances chemical recovery depending on region and variable. International cooperative work to abate acidification has so far been very successful, but water chemistry and biology of many acidified systems is still far from any pre-industrial reference condition.
There is, of course, an implicit assumption that the waters have been 'acidified.' One of the key findings of NAPAP was that many of the North American waters studied were either naturally acidic or else were returning to a natural acidic state after having been turned alkaline by a century of slash-and-burn agriculture. The extensive papers of the ICP Waters programme give no indication of how the so-called pre-industrial reference condition was established.
Also, there is what can only be called excessive claiming. How is one to interpret, for instance, the following series of statements (Skjelkvåle & de Wit, 2008):
In many areas, water quality is now sufficient for the return of acid-sensitive species of fish, invertebrates and mussels. Biological monitoring in the ICP Waters programme focuses on acid-sensitive invertebrate species. In the most acidified regions in Europe signs of biological recovery are more difficult to find. This may be because the water quality here has not recovered sufficiently to permit widespread biological recovery. The lack of documented examples of biological recovery is related to both the dynamic nature of biological responses, but also to a lack of appropriate long-term monitoring data.
Again, a twenty-year programme has failed to establish 'appropriate long-term monitoring data' or to document examples of biological recovery.
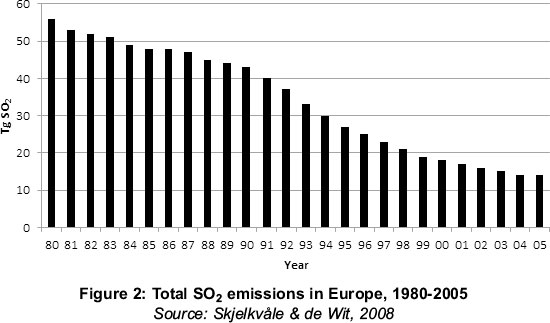
It is reasonably clear that emissions of SOx have declined, and that the sulphate content of surface waters has declined in sympathy, as Figures 2 and 3 illustrate. But a confounding issue has arisen - as the sulphate content has declined, so the dissolved organic carbon (DOC) has increased (Skjelkvåle & de Wit, 2008).
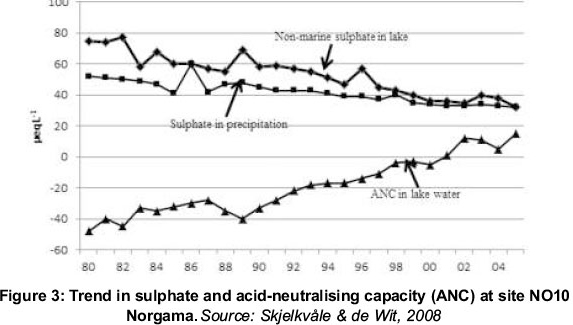
DOC is of great interest in analysis of surface water recovery because it is an indicator of organic (natural) acidity, which may partly counteract the positive effect of declining sulphate. In addition, increase in DOC also contributes to increased mobilisation and transport of heavy metals and organic pollutants from soils to water. The brown colour of DOC-rich water is important for aquatic biota because it reduces light penetration. Additionally, drinking water treatment involves removing colour from surface waters. Along with the rise in DOC, costs for treating drinking water may increase. So far, the mechanisms linking atmospheric deposition and surface water DOC remain poorly understood.
It is possible that SOx was actually beneficial to surface waters, in helping to suppress the DOC, and thus kept the water clear and enabled enough light to reach submerged plant life on which fish and other aquatic life feed.
It should also be noted that, while there has been a clear trend with time in the SOx, there is no such trend in acidity. The best that can be said is that, on average, the pH has risen (i.e. the acidity has become less), but it does not appear to have done so in a way that relates directly to the drop in SOx (Skjelkvåle & de Wit, 2008). The reason why pH is poorly linked to SOx is outlined in the next section.
The effects on materials provide further examples of equivocal results (Corr Institute, 2009):
Among the pollutants acting as corrosive agents, sulphur and nitrogen compounds including secondary pollutants, and particulates should be mentioned primarily. It has clearly been demonstrated that they enhance the natural corrosion process for several materials even if it in some cases may be difficult to quantify their contribution in the weathering process.
Yes, anthropogenic SOx can enhance corrosion, particularly in the presence of particulate matter, but it is difficult to determine the extent to which the anthropogenic SOx exacerbates corrosion above that which occurs naturally.
CLTRAP also focuses on pollution effects on vegetation, but SOx is not high on its agenda:
Attention is focused on ozone-induced damage to crops, as experiments have clearly indicated that crops are sensitive to ozone pollution. Less attention is paid to sulphur- and nitrogen-based pollutants, as these elements are components of fertilizers commonly used in agricultural systems. (UNECE, 2009)
The UN programme does not fare much better when it comes to modelling the effects of pollution (UNECE, 2009):
The objectives of the ICP Integrated Monitoring are firmly focused on long-term effects and on long-term monitoring. In the short term, however, mathematical models are being developed which can simulate ecosystem response to specific pollutant stress. The data collected in the programme are used to calibrate and test models that can then be used to predict ecosystem changes under a variety of bio-geophysical conditions and pollution scenarios.
Recall that this is a programme that has been operational for nearly 30 years, and while 'focused on long-term effects' it is reporting short-term rather than long-term results.
The health effects of SOx are by no means as unequivocal as the oft-quoted London smog effects would suggest. It is now quite clear that the smog effects arose from the effects of particulates (smoke) primarily, which strongly enhanced the impact of SOx.
There are several approaches to the question of health effects. One body studying the question is the American Conference of Governmental Industrial Hygienists (ACGIH). They are concerned, as their name suggests, with industrial exposures. Threshold Limit Value (TLV) is a term used by the ACGIH to express the airborne concentration of material to which nearly all persons can be exposed day after day without adverse effects. The ACGIH recommends a TLV for SO2 of 2.0ppm (5.2 µg/m3) average concentration for a normal 8-hour workday or 40-hour workweek (ACGIH, 2009).
This is particularly applicable to adults working in industry. Children or those sensitive to SOx such as asthmatics would be expected to require a lower level. There has been extensive work by the World Health Organisation (WHO) on a possible lower level (WHO, 2009). After reviewing 65 recent studies, it was concluded that a 24h interim target of 50µg/m3 and a guideline of 20µg/m3 average over 24h was satisfactory. This is over a two-hundredth of the TLV, and seems very low in comparison.
Recent changes in the Kilauea volcano on Hawai'i have increased ambient sulphur dioxide levels to nearly 9ppm (20mg/m3), over 1000 times the WHO's guideline (Hawaii Gov, 2009).. The area has been declared a natural disaster area since June 2008, and schools have been equipped with shelters provided with special filters for use during high SO2 events. The State advises that 'For people with asthma, heart or lung disease, and older adults who are particularly vulnerable, you should remain indoors or relocate during heavy vog episodes.' ('vog' is a local term for volcanic smog). If the best advice to the most vulnerable is that they should stay inside or possibly relocate, at over 1000 times the WHO guideline, it is a clear signal that the guideline may indeed be overly conservative.
In support of this view, there is a claim that there is 'little evidence for the widely expressed view that atmospheric pollution is related to increased prevalence or morbidity of asthma or is related to the causation of asthma.'(Barnes, 1994). It may therefore be concluded that, while 2ppm SO2 may be satisfactory for industrial work, the 20µg/m3 (8ppb) guideline suggested by WHO is impractically low. Further, it seems likely that the risks associated with SOx pollution have been overstated, and that the benefits of achieving ever lower SOx emissions are probably illusory.
What this review has shown is that the assumption that anthropogenic SOx is necessarily bad for the environment is a poor one. The environment seems quite capable of dealing with a significant increase in SOx, over and above that found naturally in the environment today. That is not to say that there are no health effects to be associated with SOx; merely that the claims for significant health effects at very low levels seem overstated.
4. SOx in the environment
There is an unfortunate tendency to declare all emissions as 'pollution'. In Cape Town recently, the Chief Air Pollution Officer intoned 'March has been a terrible month for pollution. The veld fires have put so much smoke into the air that our instruments have been overwhelmed.' Smoke from veld fires is NOT 'pollution'. Indeed, many of the plants in the fynbos will not germinate unless the moisture in their vicinity carries smoke-derived chemicals. Some smoke is natural, and if it is natural, it isn't pollution.
This is particularly true of SOx. As Figure 1 shows, there are considerable quantities of SOx generated from natural sources. Sulphur is one of the elements essential for life (Solberg, et al, 2007). The amino acids cystine and methionine contain sulphur, and are incorporated in essential proteins, hence are critical for plant growth. The beautiful blue of the jacaranda flower comes from a sulphur compound present in large quantities, which you can easily prove for yourself if you store some petals under water for a few days. Anaerobic decay releases the sulphur, and you can smell it.
Plants obtain most of the sulphur they require from the air. Some originates from the soil, but much is deposited in the soil by rain, and then taken up by the plants. It is estimated that, in temperate climes, a SOx concentration in the air of about 60ppb SO2 is needed to maintain plant health. Of course, if the plants are harvested, the sulphur is then lost and the soil may become sulphur deficient. (Equally, if plants need 60ppb (125µg.m-3), it is difficult to see how the WHO can conclude that humans are at risk above 20µg/m3. If the WHO was correct, then plants would be stressed at levels deemed safe for humans, which seems unlikely.
Sulphur deficiency is a widely recognised problem in agriculture. It has risen in importance in recent years partly because the traditional superphosphate, which contained significant quantities of sulphate, has been purified so that it now is a high grade of phosphate with only traces of sulphate. Sulphur deficiency has also become more important because of the accent on removing sulphur from gaseous emissions, as the following makes clear (Zhao et al., 1999):
Industrial pollution as a result of coal combustion has also contributed substantial amounts of S to plant needs in industrialized countries. Over the last two decades, however, many regions of the world have seen a fundamental shift in the S balance towards deficit in agricultural systems for several reasons. The share of ammonium sulphate in the total N consumption dropped from 7.2% in 1973 to 3% in 1991. Total N consumption world-wide doubled between 1974 and 1990, whereas total S consumption remained static at about 10Mt. In addition, yields of agricultural crops have increased markedly, resulting in increasing removal of nutrients, including S, from the soil. One of the most important reasons behind the increasing S deficiency in Western Europe has been the massive decrease in the inputs of S from atmospheric deposition. In the UK, total emissions of sulphur dioxide decreased from 3.2 Mt of S in 1970 to 1.4Mt in 1995. A similar trend has occurred in North America. The Sulphur Institute in Washington has estimated that the annual S fertiliser deficit worldwide will increase from a current level of 7-5 million tonnes to over 11 million tonnes by 2010. (Emphasis added)
Yields with proper S application improve by up to 50% ((McGrath et al., 1996). The optimum yield requires 10-20kgS/ha (McGrath et al., 1996). Between 1984 and 2006, the average and maximum S flux from rainfall over North America fell from 4.0 to 2.4kgS/ha and from 13 to 10kgS/ha, respectively (Lloyd, 2009). It therefore appears that much of the American cornfields require supplementary sulphur to achieve their optimum yield, and that the reduction in 'free issue' S by restriction of emissions from coal-fired power stations and similar sources has increased the production costs of grain.
One environmental effect of SOx releases is an increase in haze levels. Indeed, atmospheric SOx appears to be strongly associated with haze (Schichtel et al., 2001). However, there are other species involved in haze formation, and the relationship between haze, as measured by the visibility extinction coefficient, and SOx is not direct. Nevertheless, during the 1990's Eskom regularly measured visibility on the Eastern Highveld, and was able to demonstrate significant improvement in clarity as ground-level sources of SOx (primarily burning coal waste dumps) were brought under control.
However, the give-and-take of impacts occurs here, too. Haze originating from SOx has a significant cooling effect, of the order of -0.4W.m-2, according to the IPCC. Global warming could take place significantly faster if SOx emissions were to be strongly curtailed.
5. The role of acid in the SOx saga
Sulphur after combustion reaches the atmosphere as sulphur dioxide, SO2. Sulphur dioxide is soluble in water (11g/l at 20ºC) and its solutions are slightly acid due to the formation of the weak sulphurous acid, HSO3. The salts of sulphurous acid are called sulphites.
When SO2 dissolves in atmospheric moisture, the sulphurous acid will be oxidised to sulphuric acid, which is a strong acid. The oxidation process is slow in pure water, but rain contains traces of many species, some of which can catalyse the oxidation process and so speed it up. Manganese is a particularly efficient catalyst, but even iron has a significant effect.
However, the sulphuric acid does not necessarily turn the rain into a corrosive mix, as is often assumed. All rain is acid, because it contains some CO2 dissolved from the atmosphere, and, just as a solution of SO2 forms a weak acid, so CO2 in water forms the weak acid carbonic acid whose salts are carbonates. In an unpublished study (Lloyd, 2009) of the National Acid Deposition Program (NADP) data (NADP 2008), it was found that there was an average rainfall pH across the US of 4.65 in 1984 which increased to 4.82 by 2006. Although there was a marked reduction in the SOx in rain due to the cut in emissions, the impact on rainfall acidity was small.
To understand this, it is necessary to recognise that a) rain contains both cations and anions, and b) there is an ionic balance, with equal quantities of equivalents of cations and anions. When sulphurous acid is oxidised to sulphuric acid:
HSO3 + H2O - H2SO4 + H+,
the spare H+ cation cannot exist without some kind of balance being struck. What in essence happens is that some of the dissolved carbonate reverts to CO2 and returns to the atmosphere:
CO3= + 2H+ - H2O + CO2.
Thus, the pH of the rain is NOT greatly affected by the oxidation of SO3- to SO4=, which is why a very significant drop in the S content of rain over the US over the period 1984-2006 had a minimal effect on the pH of the rain.
Rain pH values of the order of 4.5 cannot be very harmful to plant life. In fact, of the roughly 150 000 weekly samples taken by the NADP over the period 1984 to 2006, only one was below pH 3.5 and three below pH3.6, so very acid conditions do not persist. There are many reports of damage caused by 'acid rain', but these are all from laboratory studies which use artificial rain with a pH3 or below, for example, (Back & Huttunen, 1992). A thorough literature search has failed to reveal any evidence for an impact on plant physiology of rainfall above pH4. It has, however, shown that the pH of rain can change dramatically once the rain reaches the soil, and that soil pH is probably far more of a determinant of plant behaviour than rain pH.
The acceleration of corrosive effects of 'acid rain' on buildings is often regarded as a proven phenomenon. The effects on material were discussed earlier. It appears that the combination of carbon from incomplete combustion of fossil fuels, significant levels of ozone in cities due to high concentrations of unburned hydrocarbons, and SOx and NOx has a much more deleterious effect than SOx or NOx either individually or together.
5. Discussion and conclusions
In this paper, it has been shown that it should not be assumed that the emission of SOx by industrial operations is inherently bad. Any reasoned assessment of the impacts of the emissions shows that, at the level at which they occur, the negative impacts are very low, and in most cases are at least offset by positive benefits.
This is totally contrary to popular perception. Consider, for instance, a recent newspaper article:
There is no disputing that renewable and non-polluting energy sources are preferable to the country's dependence on finite and dirty fossil fuels. - - The true cost of so-called cheap coal-fired power stations is neither reflected nor accounted for by Eskom or the Government. - -The true and immediate but unacknowledged cost of continued coal mining is apparent in the catastrophic level of acidification from mining runoff of all the significant natural water resources in the country. Their waters have been rendered unfit for human consumption, unless treated in municipal works that are now in a state of collapse. Air quality is in a similar state, with research showing notable increases in pulmonary disease causing workforce absenteeism and compromised childhood development, among many other health problems recorded in areas polluted by coal mining. (EPA, 2009)
Investigation into the so-called 'true cost' reveals an unfortunate mistake. The basis is a report 'Counting the social costs' (von Horen, 1996), which attempts to calculate the impacts, in terms of human life, of emissions from power stations:
Emissions data was obtained from Eskom for each of its nine operational power stations for the main air pollutants: particulates, sulphur dioxide and nitrogen oxides (refer Table 4.5). Dose response data from North America were used, as described in Tables 4.6 and 4.7. (Von Horen, 1996: 77)
Table 4.6 refers to PM10 (particulates of <10 m in size) but only a small fraction of the particulate emissions quoted in Table 4.5 fall into this size range; and Table 4.7 refers to ozone, which is very much more damaging to life than either SOx or NOx. Ozone has a TLV of 0.05ppm under heavy work conditions.
So for all species that van Horen (1996) considered, the dose response was wildly (about a factor of at least 20) overstated. Not surprisingly, the costs he calculated were also about 20 times too high. Unfortunately, the error has not previously been noted, and his cost estimates have become widely believed - hence the surprising editorial comments from South Africa's premier business daily.
The misconception that the emission of SOx is harmful goes very deep. Recently, Eskom has decided to add flue-gas desulphurisation (FGD) to the latest announced coal-fired power station, Kusile. In the light of what has gone before, it is necessary to do a cost benefit analysis of this decision.
According to the USEPA (EPA, 2009), in 2001 capital costs for flue-gas desulphurisation were of the order of $100/kWe for new installations and $130/kWe for retrofits. Capital and operating costs for large (>400MW) boilers were $200-$500 per ton SO2 removed, assuming an 80% load factor for the installation. Recovery is typically >90%.
The likely present-day costs in South Africa can readily be calculated. Assume a 700MWe unit, 2 000MWth, fed with 22MJ/kg coal containing 1.9%S. The operating cost, at $350/tSO2, the median of the EPA's estimate, inflated at 5% and with $1=R10, is about R40 000/h at 90% recovery. The revenue, at a sent-out power cost without desulphurisation of, say, R0.28/kWh, would be about R160 000,/h at 80% load factor. So FGD adds about 25% to the cost of new-build electricity.
The capital cost today would be about $148/kWe, or, for the 4800MWe of Kusile, over R7 billion, to which must be added considerable infra-structural costs for limestone supply and waste handling, which goes some way to explaining why the expected cost of the station has risen from about R83bn to over R100bn.
There are some other features about this decision that should be questioned. The station is to be dry-cooled, which will reduce its efficiency relative to an equivalent wet-cooled station (and also increase its capital cost). However, because of the water demand for FGD the total water demand for the station will be approximately the doubled. In addition, the lime needed for desulphurisation will have to be transported over at least 500km, and its production will consume significant quantities of electricity while also emitting considerable quantities of carbon dioxide.
So those are the costs, and they are clearly considerable. What are the benefits likely to be? Less SOx in the environment? If the environment were overloaded, such an argument might be acceptable. But the environment is in perfectly good shape. Figure 4 shows the SOx levels in the atmosphere in the vicinity of the new station. DEAT sets a limit of 19ppb, which has never been exceeded.1 As indicated earlier, a limit of 19ppb is very low and has no rational basis. So there is no benefit in enforcing further reductions against what is already a low limit.
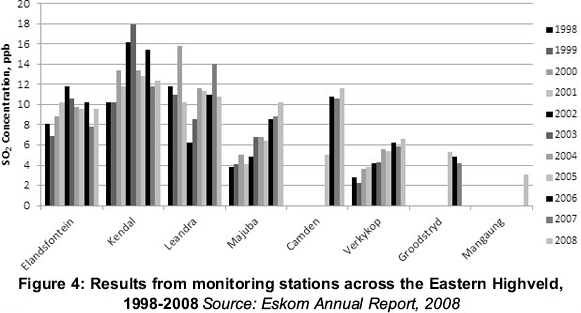
As this example shows, the misconception about the possible impacts of SOx emissions can have near-disastrous consequences. The problems facing our national energy supply are too well known to be repeated here. But at this juncture to:
- significantly increase the capital cost of a new station, when capital is already in short supply;
- reduce the output of the station by requiring dry cooling, when power is in short supply
- more than double the water consumption, when water is a valuable commodity in our dry land;
- increase the cost of producing electricity, when it is stated policy to produce power at the lowest cost to help alleviate poverty; and
- undertake FGD when there is no demonstrable benefit.
It is an unparalleled example of truly unsustainable development. Sustainable development requires taking into account, and balancing the conflicting needs of, the environment, the economy and the people. The decision to require FGD for Kusile puts 'the environment' in opposition to the interests of the people and their livelihood.
Two threads of this discussion should be brought out in closing. The first is that summarised by the NAPAP worker 'We don't have evidence of damage and we shouldn't try to find something that isn't there.' It is singularly difficult to prove the negative. Yet that is what the Precautionary Principle calls for, when it suggests that mankind should not undertake anything new until it can be shown not to cause serious harm. There are frequent calls on scientists to prove the negative. All those who are involved need to be aware of the threat, and be steadfast in their refusal to respond to the challenge.
The second thread is that of the flaws inherent in developing too simple a model of the world. 'Acid rain' could not capture anything of the complexity of Nature. It had the merit that it could be taught to the youngest child, so that all could grow up aware of the risks posed by 'acid rain'. It is, however, a highly flawed model, and it demonstrably overstates the impact of SOx on the environment and human health.
One of the problems is that some epidemiology employs a version of the linear no-threshold model of environmental impacts. This supposes that all additions of potential pollutants above natural background will be harmful. It originated in the field of radiation protection, and, in spite of a reasonable level of debate, continues to be the international position on radiation protection. When employed in other epidemiological fields, however, it is of extremely doubtful validity. It provides regulators with an excuse for seeking ever lower limits on emissions, so that the absurd position can be reached where what is necessary for plant life is deemed dangerous for human life.
The overall result is that society can be coerced into actions that have no rational basis other than that they assuage the feelings of guilt brought about by exaggerated risks. This also happens to be a useful political ploy, so support can be won for actions that have no strong foundation. The effects of such wrong decisions can be almost as devastating as war itself.
Note
1. This is the limit for the annual average concentration. It may be exceeded on an hourly or daily basis, but it is highly questionable whether a brief overexposure poses any significant health risks, given that populations living near active volcanoes are regularly exposed to short-term concentrations in excess of 500ppm
References
ACGIH (2009). TLVs and BEIs. Publ#0109, ACGIH Cincinnati. [ Links ]
Back, J. & Huttunen, S. (1992). Structural responses of needles of conifer seedlings to acid rain treatment. New Phytol. Vol.120, pp. 77-8. [ Links ]
Barnes, PJ. (1994). Air pollution and asthma.- no link. Postgraduate Medical J. Vol.70, pp319-325; doi:10.1136/pgmj.70.823.319. [ Links ]
Business Day (2009). Editorial, January 19, 2009. Johannesburg. [ Links ]
Corr Institute (2009). www.corr-institute.se/ICP-materials/html/Introduction.html. Accessed April 2009. [ Links ]
Dentener, F et al., (2006). Emissions of primary aerosol and precursor gases in the years 2000 and 1750. Atmos. Chem. Phys. Discuss., Vol.6, pp2703-2763. [ Links ]
Eskom (2008). Eskom Annual Report, Johannesburg. [ Links ]
Hawaii Government (2009). www.hawaii.gov/gov/vog. Accessed April 2009. [ Links ]
ICRP (2007). The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103, Ann. ICRP 37 (24). [ Links ]
Lloyd, PJ.D. (2009). Unpublished study. McGrath, S.P, Zhao, FJ. & Withers, PJ.A. (1996). Development of sulphur deficiency in crops and its treatment. Proc. Fertiliser Socy, No. 379.Peterborough, The Fertiliser Society. [ Links ]
National Acid Precipitation Assessment Program (NAPAP) (1991). The U.S. National Acid Precipitation Assessment Program 1990 Integrated Assessment Report. NAPAP Office of the Director, Washington, D.C. [ Links ]
National Atmospheric Deposition Programme (2008). http://nadp.sws.uiuc.edu/ Accessed Nov 2007-Jun 2008. [ Links ]
New York Times (1990). 'Acid Rain Report Unleashes a Torrent of Criticism' March 20, 1990. [ Links ]
Schichtel, B.A.,Husar, R.B., Falke, S.R. & Wilson, W.E. (2001). Haze trends over the United States, 1980-1995. Atmospheric Environment Vol.35, pp. 5205-5210. [ Links ]
Skelly J.M. & Innes J.L. (1994). Waldsterben in the Forests of Central Europe and North America: Fantasy or Reality? Plant Disease Vol. 78(11) pp. 1021-1032. [ Links ]
Skjelkvále, B.L & de Wit, H. (2007). Trends in surface water chemistry and biota; the importance of confounding factors. NIVA Report No. OR-5385, Norwegian Natl. Water Res. Inst., Oslo. [ Links ]
Skjelkvále, B.L. & de Wit, H. (2008). 20 year with monitoring effects of long-range trans boundary air pollution on surface waters in Europe and North-America. Brit Lisa Skjelkvále and Heleen de Wit, Eds., Norwegian Natl. Water Res. Inst., Oslo. [ Links ]
Solberg E. D., Malhi S. S., Nyborg M., Henriquez B. & Gill K. S. (2007). Crop response to elemental S and sulphate-S sources on S-deficient soils in the parkland region of Alberta and Saskatchewan. J. Plant nutrition ISSN 0190-4167, Vol.30, Nos.1-3, pp321-333. [ Links ]
UN Economic Commission for Europe (2005). Europe's forests in a changing environment - twenty years of monitoring forest condition by ICP Forests. UN Economic Commission for Europe, Federal Research Centre for Forestry and Forest Products, Geneva. [ Links ]
USEPA (2009). Air pollution control technology fact- sheet, USEPA Report EPA-452/F-03-034, www.epa.gov/ttn/catc/dir1/ffdg.pdf. Accessed Feb 2009. [ Links ]
UN Economic Commission for Europe. (2009). www.unece.osrg/env/lrtap/. Accessed April 2009. [ Links ]
UN Economic Commission for Europe (2009). www.unece.org/env/lrtap/WorkingGroups/-wge/vegetation.htm. Accessed April 2009. [ Links ]
UN Economic Commission for Europe (2009). www.unece.org/env/lrtap/WorkingGroups/-wge/im.htm. Accessed April 2009. [ Links ]
van Horen, C. (1996). Counting the social costs. Electricity and externalities in South Africa. Elan Press and UCT Press. [ Links ]
Wilkens, E.T. (2006). Air pollution aspects of the London fog of December 1952. Quarterly J. Royal Meteor. Socy. Vol. 80 (344), pp. 267-271. [ Links ]
WHO (2005). Air quality guidelines: Global update 2005. WHO, Copenhagen. [ Links ]
Zhao, FJ., Hawkesford, M.J. & McGrath, S.P (1999). Sulphur assimilation and effects on yield and quality of wheat. J. Cereal Sci Vol.30, pp. 1-17. [ Links ]
Received 31 July 2009
Revised 12 May 2013














