Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Institution of Civil Engineering
On-line version ISSN 2309-8775
Print version ISSN 1021-2019
J. S. Afr. Inst. Civ. Eng. vol.58 n.4 Midrand Dec. 2016
http://dx.doi.org/10.17159/2309-8775/2016/v58n4a5
TECHNICAL PAPER
The effect of type, concentration and volume of dispersing agent on the magnitude of the clay content determined by the hydrometer analysis
A Kaur; G C Fanourakis
ABSTRACT
Knowledge of the physical properties of soils, including the clay content, is of utmost importance in the field of geotechnical engineering. The hydrometer analysis is the most widely used technique for the analysis of the particle size distribution of the fine-grained fraction of a soil, calculated using sedimentation principles. The hydrometer analysis utilises a dispersing agent - Calgon 33:7 (comprising 33 grams of sodium hexametaphosphate and 7 grams of sodium carbonate when mixed in 1 litre of water) is universally considered as the most effective dispersing agent.
In this investigation, hydrometer analyses were conducted (according to the TMH1 1986 method) on two soils (alluvium and black clay), using five dispersing agents. The results show that the clay size fraction can vary significantly (from 1% to 32%) for the two soils, depending upon the dispersing agent used. From these initial results, the two most effective dispersing agents (Calgon and sodium pyrophosphate decahydrate - NaPP) were investigated further to establish the optimum concentration and volume. Calgon proved to be the most effective in the alluvial soil, increasing the clay content by 38%. The NaPP was most effective in the relatively active black soil, increasing the clay content by 25%.
Keywords: grain-size analysis, hydrometer tests, dispersing agents, concentration, volume
INTRODUCTION
The determination of the particle size distribution of a soil, including the clay content, is one of the most problematic areas in geo-technical engineering testing. The particle size analysis is a method of separating soils into different fractions based on the sizes of particles present in the soil. The particle size analysis is divided into two categories (coarse and fine) with associated laboratory test methods. A sieve analysis is used to separate the coarse-grained fraction of soil, i.e. the fraction of soil with particle sizes greater than 425 microns. On the other hand, sedimentation analysis, which is based on the principles of dispersion and sedimentation, is used for the analysis of the fine-grained fraction of the soil, such as silt and clay, of which the particle size is less than 75 microns. Analysis of these fine-grained soils is done either by the pipette method or by the hydrometer method (Arora 2003).
Since the clay content of a soil is used to determine its activity, which in turn is used for design purposes, it is very important to accurately determine the clay content of soils. Inaccurate clay content determinations have resulted in inappropriate design solutions, which have even led to unacceptable damage to the structures. In South Africa there is a problem with the accurate determination of the clay content of soils. This problem, which was formally expressed by Jacobsz and Day (2008), reinforces the need for research into all of the variables of the hydrometer test with a view to improving its accuracy and perhaps to standardise the test nationally, and possibly, in future, internationally.
In the hydrometer analysis, dispersing agents are used to disperse the fine-grained particles of the soil in the suspension medium (water). Dispersing agents can either act as a protective colloid on the solid particle or alter the electrical charge on the particle to prevent the formation of flocs (Sridharan et al 1991).
A variety of dispersing agents are used in different parts of the world to perform sedimentation analysis. These include sodium silicate and sodium oxalate (TMH1 1986), Calgon (BS 1990; IS 1985; ISRIC 2002), sodium pyrophosphate decahydrate (Schuurman & Goedewaagen 1971), sodium tetra pyrophosphate (Yoo & Boyd 1994), sodium hexametaphosphate (ASTM 1965; Lambe 1951; SANS 2014) and di-sodium di-hydrogen pyrophosphate (formerly used by the Soils Testing Laboratory of the South African government's Department of Water Affairs). The justification for the use of this latter dispersing agent (di-sodium di-hydro-gen pyrophosphate) could not be established.
Calgon, which is a combination of sodium hexametaphosphate and sodium carbonate, is one of the popular dispersing agents adopted by various countries for the sedimentation test analysis. The BS 1377 Part 2 (BS 1990) and IS 2720 Part IV (IS 1985) methods recommend 33 grams of sodium hexametaphosphate with 7 grams of sodium carbonate, while the (South African) Council for Scientific and Industrial Research (CSIR) recommends 35 grams of sodium hexameta-phosphate with 7 grams of sodium carbonate, and the International Soil Reference and Information Centre (ISRIC) recommends 40 grams of sodium hexametaphosphate with 10 grams of sodium carbonate. All the methods mentioned above use 125 ml of solution of the prescribed concentrations, except for ISRIC which uses only 20 ml of solution of the prescribed concentration.
Sridharan et al (1991) described a study on the effect of different types and quantities of dispersing agents on the grain-size distribution, particularly the percentage of clay-sized material. They concluded that the clay-size fraction can vary from 4% - 45% for marine clays, depending on the dispersing agent used, strictly following the IS (1985) method. It was further seen that 100 ml - 125 ml of Calgon (33 grams of sodium hexametaphosphate and 7 grams of sodium carbonate in 1 litre of distilled water) was found to be the most effective dispersing agent.
Bindu and Ramabhadran (2010) conducted a study to evaluate the effect of the concentration of the dispersing agent on the hydrometer analysis, and attempted to optimise the concentration of the dispersing agent to be added to obtain maximum dispersion. It was observed that the addition of sodium carbonate improved the dispersing capacity of sodium hexametaphosphate. The optimum volume and concentration was found to be 100 ml of 6% mixture of sodium hexameta-phosphate and sodium carbonate, and there was a significant decrease in dispersion with a further increase in the concentration as well as volume of the dispersing agent added.
The objective of the current study was to investigate the effect of different dispersing agents on two different soil samples with varying mineralogy. An effort was made to compare the results of the hydrometer test analyses by varying the concentration and volume of the best two dispersing agents on the two soil samples, following the THM1 (1986) test method.
EXPERIMENTAL DETAILS
Materials
Two soil samples were collected from various parts of South Africa. The first sample comprised an alluvial soil from the Sebokeng Township in the Gauteng Province, and the other was a black soil from the town of Brits in the North West Province. The Atterberg limits and activity of these soils were determined at the laboratories of the Department of Civil Engineering Technology of the University of Johannesburg, as shown in Table 1.

The TMH1 (1986) method was used for the determination of the liquid limit and plastic limit. The clay content was determined by means of the hydrometer analysis (Method A6 of TMH1 1986), with a deviation in the prescribed dispersing agent type, quantity and adjustment in the readings by subtracting the hydrometer readings obtained on the 'blank' companion specimens to account for the effect of the dispersing agent. TMH1 (1986) Method A6 prescribes 5 ml of sodium silicate and 5 ml of sodium oxalate as the dispersing agent. The activities of the soils used for current study were computed by using the clay content obtained by the hydrometer analysis when 125 ml of Calgon 33:7 (a solution comprising 33 grams of sodium hexametaphosphate (NaHMP) and 7 grams of sodium carbonate (Na2CO3) in 1 litre of distilled water) was used.
Hydrometer tests
Hydrometer analyses were conducted on both samples according to the TMH1 (1986) method to determine their clay content.
These analyses were carried out in three stages. Stage I comprised two tests, one on each soil type, which excluded dispersing agents. These served as the control test results. Stage II testing entailed a total of ten hydrometer tests, five on each of the two soil types, using the dispersing agents shown in Table 2. Stage III comprised further testing on each of the two soil types, which entailed varying the concentration and volume of two of the dispersing agents (Calgon and sodium pyrophosphate decahydrate). These two dispersing agents were selected for further investigation, as the results of the Stage II testing indicated these to be the most effective of the five dispersing agents. The objective of this Stage III testing was to compute the optimum concentration and volume of the dispersing agent.

Internationally there are at least four methods - BS 1377 Part 2 (BS 1990), IS 2720 Part IV (IS 1985), South African CSIR, International Soil Reference and Information Centre (ISRIC 2002) - which recommend the use of Calgon as a dispersing agent.
However, all four of them use Calgon in different concentrations. BS 1377 Part 2 1990 (BS 1990) and IS 2720 Part IV (IS 1985) both recommend 125 ml of Calgon 33:7 (33 grams of sodium hexametaphosphate and 7 grams of sodium carbonate mixed with 1 litre of distilled water), CSIR recommends 125 ml of Calgon 35:7, while ISRIC recommends 20 ml of Calgon 40:10.
Amounts of 4%, 4.2%, 5%, 7%, 8% and 9% solution of Calgon, and 3%, 3.6%, 5%, 6% and 7% solution of sodium pyrophosphate decahydrate were prepared by mixing the required quantity in 1 litre of distilled water. The quantities (in grams) of chemicals added for the preparation of these stock solutions are given in Table 3.

In these tests the minimum volumes of Calgon and sodium pyrophosphate decahy-drate used were 100 ml and 20 ml respectively. These volumes were incrementally increased until the optimum volume for each concentration was established.
Testing procedure and calculations
The testing was in accordance with the procedure described in TMH1 (1986). For all the tests performed, 50 grams of soil sample passing through a 425 micron sieve were mixed with the desired quantity of dispersing agent and about 400 ml of distilled water in a canning jar. The soil-water mixture was allowed to stand overnight. After the mixture had been allowed to stand, it was dispersed for 15 minutes with a standard paddle. The paddle was washed clean with distilled water allowing the wash water to run into a container with the suspension. The suspension was then poured into the Bouyoucos cylinder, and the canning jar was rinsed with distilled water from the wash bottle. The cylinder was then filled with distilled water to the 1 130 ml mark with the Bouyoucos hydrometer (152H) inside. Then the hydrometer was removed and the cylinder was inverted a few times, using the palm of one hand as a stopper over the mouth of the cylinder to ensure that the temperature was uniform throughout. After bringing the cylinder to a vertical position, a stop watch was started. The hydrometer was inserted and readings were taken at 18 and 40 seconds without removing the hydrometer from the cylinder. The hydrometer was then taken out and rinsed with water and it was again inserted into suspension when the elapsed time was two minutes. This reading was noted and the hydrometer was removed and placed in distilled water. This procedure was repeated for readings at 5 minutes, 15 minutes, 30 minutes, 1 hour, 4 hours and 24 hours. After taking each hydrometer reading, the temperature of the liquid was recorded. Temperature corrections were applied to the readings.
A blank solution, comprising distilled water and dispersing agent, was also prepared in a second Bouyoucos cylinder in the same proportions as solutions prepared with the soil. The dispersing agent and water mixture was also soaked overnight and identical hydrometer tests were performed for the blank solutions.
The hydrometer readings taken on the samples which contained soil were appropriately adjusted by subtracting the hydrometer readings obtained on the "blank" companion specimens, at the relevant times. This accounted for the effect of the dispersing agent on the readings. It should be noted that TMH1 does not make any provisions for this correction.
The percentages finer than 0.075 mm, 0.05 mm, 0.04 mm, 0.026 mm, 0.015 mm, 0.01mm, 0.0074 mm, 0.0036 mm and 0.0015 mm were respectively calculated by the readings taken at 18 sec, 40 sec, 2 min, 5 min, 15 min, 30 min, 1 hour, 4 hours and 24 hours, by means of Equation 1.

Where
P = percentage finer than relevant size
Sm= mass of soil fines used in analysis (50 grams)
Sf= percentage soil fines in total sample (< 0.425 mm)
C = corrected hydrometer reading
The percentage clay content present in each sample (fraction finer than 0.002 mm) was obtained from the relevant particle size distribution curve. The tests which gave the best dispersing agent, optimum concentration and volume were repeated to check the consistency of the results.
RESULTS AND DISCUSSION
Stage I results
In this stage, two tests were performed, one on each soil. These were the control tests that excluded dispersing agents. Figure 1 shows the soil suspension after 24 hours of both the black soil and the alluvial soil. In both cases it can be seen that the supernatant water is almost clear of soil grains including colloids.

The particle size distribution curves for these two control tests are shown in Figure 2.

Stage II results
The particle size distribution curves obtained for the two soil types, with the use of each of the five dispersing agents used (detailed in Table 2), are shown in Figure 2. From Figure 2 it is evident that the control samples of both the alluvial and black soils yielded near-zero clay contents.
The analyses using the five dispersing agent types indicated that the clay content of the alluvial soil ranged from 7% to 21%, and that of the black soil ranged from 0.1% to 32%. Furthermore, it can be seen that in the case of both soil types, Calgon (combination of sodium hexametaphosphate plus sodium carbonate), which has been recommended by many methods and researchers internationally (BS 1990; IS 1985; ISRIC 2002; Bindu & Ramabhadran 2010; Sridharan et al 1991), yielded the maximum clay content. This was most effective in the case of the black soil.
The second best dispersing agent after Calgon was found to be sodium pyrophos-phate decahydrate (NaPP). Calgon yielded 21% and 32.1% clay content, while sodium pyrophosphate decahydrate yielded 20% and 20.5% clay content in alluvial and black soil respectively.
In addition, sodium silicate and sodium oxalate, which is prescribed in TMH1 (1986), was the least effective dispersing agent in the case of the black soil, yielding a clay content of 0%. The least effective dispersing agent in the case of the alluvial soil was sodium silicate and di-sodium di-hydrogen pyroph-osphate, which yielded a clay content of 6.2%.
Stage III results
Calgon
The effect of different concentrations and volumes of Calgon for the alluvial and black soils is shown in Figures 3 and 4 respectively. The clay contents determined by the different concentration and volume combinations are shown in Table 4. The best and worst results for each concentration are shown in green and red respectively.

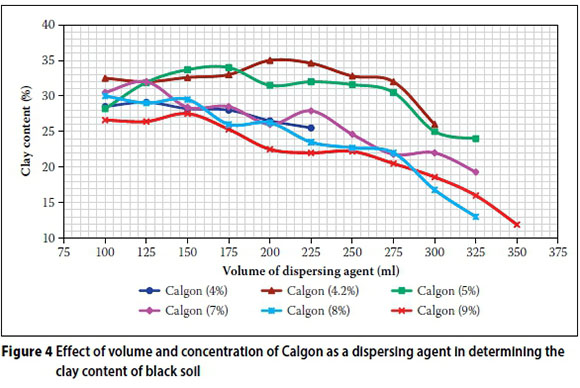

It is evident that at the volume of 125 ml (the volume recommended by BS 1377 Part 2 (1990) and IS 2720 Part IV (1985), 5% Calgon yielded the maximum clay content of 25.5% for the alluvial soil (Figure 3 and Table 4), while for black soil the 4.2%, 5% and 7% Calgon all yielded the maximum clay content of 32% (Figure 4 and Table 4).
Figures 3 and 4, and Table 4 indicate the following:
■ The 4.2% Calgon proved to be the best dispersing agent yielding maximum clay contents of 29.5% and 35% for the alluvial and black soils respectively. This is in close agreement with the concentration prescribed by the South African CSIR CA 17 method, but in disagreement with Bindu and Ramabhadran (2010) and Sridharan et al (1991). According to Bindu and Ramabhadran (2010), the optimum concentration of Calgon was found to be 53:7, while Sridharan et al (1991) found Calgon 33:7 to be the optimum concentration.
■ The optimum volumes of Calgon for the alluvial and black soil were 225 ml and 200 ml respectively. This is in disagreement with Bindu and Ramabhadran (2010) and Sridharan et al (1991). An optimum volume of 100 ml (53:7) was obtained by Bindu and Ramabhadran (2010), while Sridharan et al (1991) indicated that 100-125 ml of Calgon 33:7 yielded the optimum clay content. The results obtained by Sridharan et al (1991) were in close agreement with the concentrations and volumes prescribed by the British and Indian standards.
■ Low (4%) and high (9%) concentrations of Calgon proved to be less effective in the case of both soils.
■ In the case of the alluvial soil, 5% and 8% Calgon concentrations were more effective than 4.2% Calgon for volumes up to 175 ml.
■ In the case of the black soil, the 5% Calgon was more effective than 4.2% Calgon for volumes ranging from 125 ml to 175 ml.
■ In the case of the black soil, for all the concentrations of dispersing agent, any further increase in volume of chemical after attaining optimum volume generally resulted in a decrease in clay content. Similar results were obtained by Bindu and Ramabhadran (2010) and Sridharan et al (1991).
With regard to the concentrations of Calgon, no general trend was evident regarding the relative effect of the quantities (ratio of) sodium carbonate and sodium hexam-etaphosphate on the results obtained for both soils. However, in the black soils, with the exception of the 4% Calgon results, an increase in sodium hexametaphosphate resulted in a decrease in clay content. This decrease is due to saturation absorption of the dispersants onto the clay particles, after which aggregation of particles might occur (Bindu & Ramabhadran 2010).
Sodium pyrophosphate decahydrate (NaPP)
The effect of different concentrations and volumes of NaPP for the alluvial and black soils are shown in Figures 5 and 6 respectively. The clay content determined by the different concentration and volume combinations are shown in Table 5. The best and worst results for each concentration are shown in green and red respectively.



From Figures 5 and 6 and Table 5 the following is evident:
■ In the case of the alluvial soil, 40 ml of NaPP 5% gave the maximum clay content of 26%, while 40 ml of NaPP 3.6% yielded a clay content of 25.5% (which is a little less than the former). Hence, NaPP 3.6% may be considered as the optimum concentration for the alluvial soil.
■ When the test was conducted on the black clay, the clay fraction obtained was at a maximum (35.2%) when using 80 ml with a 3.6% concentration. Hence, 80 ml of NaPP 3.6% is considered as the optimum concentration and volume for the black soil.
■ In general, the 3.6% NaPP appears to be the most effective concentration in the case of both soils.
■ Low (3%) and high (7%) concentrations of NaPP generally proved to be less effective in the case of both soils.
■ NaPP concentrations of 5% and 6% yielded similar results in the case of both soils.
■ The volume of dispersing agent generally appeared to have an insignificant effect in the volume range of 40 ml to 120 ml in the alluvial soil, and for the volumes in excess of 40 ml in the case of the black soil.
Cn the basis of the above, the optimum NaPP volume for the alluvial and black soil appears to be 40 ml and 80 ml respectively.
Summary of results
Table 6 shows the optimum concentrations and volumes of Calgon and NaPP for the alluvial and black soil, and the clay contents yielded by these dispersing agents.
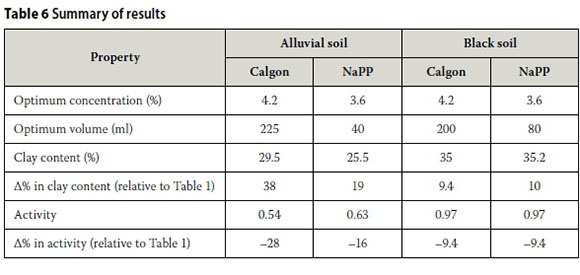
With reference to Table 6, it is evident that the Calgon and NaPP yielded the highest clay content for the alluvial and black soils respectively.
Furthermore, Figures 3 to 6 and Tables 4 and 5 indicate that increase in volume beyond the optimum volume resulted in a decrease in the percentage clay content in most cases. However, a contradictory trend was noticed where, with an increase in volume, there was an increase in percentage of clay content. The reason for this is that, with an increase in the volume of dispersing agent in the companion 'blank' solutions, the hydrometer readings increased, but the increase was not constant.
It was also found that, in the case of both soils and both dispersing agents, there was no correlation between the mass of dispersing agent granules in solution and the clay content yielded.
Furthermore, when comparing the maximum clay content of the two soils determined as part of this investigation (Table 6) with those in Table 1, the maximum clay content for the alluvial soil increased by 38% (from 21.4% to 29.5%). The maximum clay content for the black soil increased by 10% (from 32% to 35.2%). These increases in clay content resulted in decreases in the activity of the alluvial and black soils of 28% (from 0.75 to 0.54) and 10% (from 1.07 to 0.97) respectively.
CONCLUSIONS
The following conclusions were drawn from the study conducted:
■ Tests with different dispersing agents clearly indicated that the percentage of clay-sized material can vary significantly, depending on the type of dispersing agent. In this investigation, which included ten dispersing agent types, Calgon and sodium pyrophosphate decahydrate (NaPP) were the most effective dispersing agent types.
■ In the case of both soils, the optimum concentrations of Calgon and NaPP were found to be 4.2% and 3.6% respectively. Furthermore, 225 ml of Calgon yielded the highest clay content in the case of the alluvial soil, whereas 80 ml of NaPP yielded the highest clay content in the case of the black soil.
■ Relatively high and low concentrations of Calgon and NaPP yielded low clay content in the case of both soil types.
■ No correlation exists between the mass of the dispersing agent granules in solution and the clay content yielded.
The results of this investigation confirm the findings of Means and Parcher (1963) that different dispersing agent types are more effective with certain clay types.
Finally, the effect of the dispersing agent on the hydrometer readings, particularly in the case of relatively high volumes, was considerable and hence should be accounted for by accordingly correcting (reducing) the hydrometer readings. The current South African method, SANS 3001 (SANS 2014), which utilises sodium hexametaphosphate as a dispersing agent, makes provision for such a correction to the hydrometer readings.
REFERENCES
Arora, K R 2003. Soil Mechanics and Foundation Engineering, 6th ed. New Delhi: Standard Publishers Distributors. [ Links ]
ASTM 1965. ASTM D422-63 1965. Particle Size Analysis of Soils. Philadelphia: ASTM. International. [ Links ]
BS (British Standard) 1990. BS 1377-2:1990. Methods of Test for Soils for Civil Engineering Purposes, Part 2, Classification Tests. London. British Standards Institution. [ Links ]
Bindu, J & Ramabhadran, A 2010. Effect of concentration of dispersing agent on the grain size distribution of fine grained soil. Proceedings, Annual Conference of the Indian Geotechnical Society, Mumbai, 16-18 December, pp 275-278. [ Links ]
IS (Indian Standard) 1985. IS 2720:1985 Methods of Tests for Soils, Part IV. New Delhi: Bureau of Indian Standards (BIS). [ Links ]
ISRIC (International Soil Reference and Information Centre) 20 02. Procedures for Soil Analysis. Technical paper 9. Wageningen, Netherlands: ISRIC. [ Links ]
Jacobsz, S W & Day, P 2008. Are we getting what we pay for from geotechnical laboratories? Journal of the South African Institution of Civil Engineering, 16(4):8-11. [ Links ]
Lambe, T W 1951. Soil Testingfor Engineers, New York: Wiley. [ Links ]
Means, R E & Parcher, J N 1963. Physical Properties of Soils. Columbus, OH: Charles E. Merrill Book Co. [ Links ]
SANS (South African National Standard) 2014. SANS 3001-14. Civil Engineering Test Methods, Part 3, Guidance for Design. Pretoria: SABS Standards Division. [ Links ]
Schuurman, J J & Goedewaagen, M A J 1971. Methods for the Examination of Root Systems and Roots. Wageningen, Netherlands: PUDOC. [ Links ]
Sridharan, A, Jose, B T & Abraham, B M 1991. Determination of clay size fraction of marine clays. Geotechnical Testing Journal, 14(1):103-107. [ Links ]
TMH1 1986. Standard Methods of Testing Road Construction Materials, Method A6. Pretoria: CSIR, National Institute for Transport and Road Research. [ Links ]
Yoo, K H & Boyd, C E 1994. Hydrology and Water Supply for Pond Aquaculture. New York; Chapman & Hall. [ Links ]
 Correspondence:
Correspondence:
A Kaur
Department of Civil Engineering Technology
University of Johannesburg
P O Box 17011
Doornfontein, 2028
South Africa
T: +27 11 559 6898
E: akaur@uj.ac.za
G C Fanourakis
Department of Civil Engineering Technology
University of Johannesburg
P O Box 17011
Doornfontein, 2028
South Africa
T: +27 11 559 6416
E: georgef@uj.ac.za

DR ARSHDEEP KAUR is a Postdoctoral Research Fellow in the Department of Civil Engineering at the University of Johannesburg. Her current research focuses on the accurate determination of the clay content of various South African soils. She holds the degrees BTech, MTech and PhD, all received in India. Her other research interests in geotechnical engineering include the improvement of soil strength. She consulted for her own structural designing firm in India from 2008 to 2010.

PROF GEORGE C FANOURAKIS joined the Department of Civil Engineering Technology at the (now) University of Johannesburg over 22 years ago, after leaving the employ of Jones and Wagener Consulting Engineers. He holds the degrees of MSc (Eng) and DTech (Eng). He is a Chartered Civil Engineer and Member of the Institution of Civil Engineers (UK). He is a Fellow of the South African Institution of Civil Engineering (SAICE), an Honorary Fellow (and Past-President) of the Institute of Professional Engineering Technologists (I PET) and Member of the Soil Science Society of Southern Africa. He received the 2006 Best Journal Paper Award from SAICE.














