Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the South African Institution of Civil Engineering
versão On-line ISSN 2309-8775
versão impressa ISSN 1021-2019
J. S. Afr. Inst. Civ. Eng. vol.56 no.3 Midrand Out. 2014
TECHNICAL PAPER
Effect of activator dosage, water-to-binder-solids ratio, temperature and duration of elevated temperature curing on the compressive strength of alkali-activated fly ash cement pastes
J Shekhovtsova; E P Kearsley; M Kovtun
ABSTRACT
In this paper the effect of sodium oxide concentration, the water-to-binder-solids ratio, temperature, and the duration of elevated temperature curing on the compressive strength of alkali-activated fly ash cement pastes was investigated. Alkali concentration varied between 3% and 15% Na2O of fly ash mass. An increase in Na2O from 3% to 9% greatly improved the compressive strength of the pastes from 26.1 MPa to 50.8 MPa at 28 days. A further increase in Na2O up to 15% did not provide an increase in the strength, but a decrease was observed, as well as higher strength variation. The paste activated with 9% Na2O had the highest strength at 28 days and a low standard deviation, and 9% Na2O was thus considered as the best value in the present study. The temperature and the duration of elevated temperature curing were found to be critical factors affecting the compressive strength at early age, but their effect decreased significantly in the long term. The water-to-binder-solids ratio affected the compressive strength considerably. An increase in the water-to-binder-solids ratio of the pastes from 0.18 to 0.29 resulted in a decrease in the compressive strength from 49.3 MPa to 21.3 MPa.
Keywords: fly ash, sodium hydroxide, alkali-activated cement, temperature, compressive strength
INTRODUCTION
It is known that concrete is one of the most widely used construction materials, and ordinary Portland cement has generally been used as a binder component. The production of cement requires high-energy efforts which have a significant impact on the global emission of greenhouse gases. During the production of 1 ton of cement, between 0.73 and 0.99 tons of are released into the atmosphere (Hasanbeigi et al 2012; Turner & Collins 2013). One of the major cement manufacturers in South Africa, Pretoria Portland Cement (PPC), reported that in 2011 its carbon footprint for cement was 892 kg CO2per ton of cement, which is an increase of 2.6% compared to 2010 (PPC Integrated Annual Report 2011).
Various ways of reducing CO2 emission by using alternative binders are being investigated all over the world. Partial replacement of plain Portland cement by fly ash or slag was found to reduce concrete CO2 emission by between 13% and 22% (Flower & Sanjayan 2007).
Alkali-activated binders and geopolymers are potential alternatives to plain Portland cement. Geopolymers are inorganic materials with three-dimensional silico-aluminate structures resulting from poly-condensation. Davidovits called the reaction which takes place as a result of alkali activation of alumi-nosilicates at low temperatures geopolymeri-sation (Davidovits 1988). According to data published in literature, carbon emissions from geopolymers can be 80% less than that from traditional cements (Van Deventer et al 2010), and greenhouse gas emissions can be reduced by between 44% and 64% (McLellan et al 2011). Rich sources of aluminosilicates, most widely used for alkali activation, are fly ashes, metakaolin and blast furnace slag (Shi et al 2006; Palomo et al 1999a, 1999b; Van Jaarsveld & Van Deventer 1999; Bakharev et al 1999; Duxson et al 2007a).
The process of alkali activation of fly ash starts from the dissolution of the alumino-silicate source in the alkaline solution during which the breakdown of the Si-O-Si and Al-O-Al covalent bonds in the amorphous phase of the initial material occurs, and ions (silicon and aluminium) pass into the solution with the formation of Si-OH and Al-OH groups, followed by the polymerisation and reorganisation of species. Thereafter the solidification of aluminosilicate gel occurs with the formation of three-dimensional low-ordered structures (Van Deventer et al 2007; Fernandez-Jiménez et al 2005b). This gel has been found to be responsible for the cementitious properties of the final material (Criado et al 2008), and the quantity affects the mechanical strength of the final product (Zhang et al 2013).
Utilisation of ash is a serious problem for South Africa, as energy production relies mostly on coal. Sasol (petrochemical) and Eskom (power utility) are the largest coal processing companies in South Africa. More than 30 million tons of coal are consumed annually by Sasol, and about 8 million tons of gasification ash are produced (Matjie et al 2005). Eskom consumes more than 100 million tons of coal per annum (Ash Management in Eskom 2013), and more than 35 million tons of ash (fly and bottom) were produced in 2011 (Eskom Integrated Report 2011). Almost 90% of the ash produced by Eskom is fly ash. The morphological features of fly ash result in improved workability of cement systems, while the pozzolanic activity makes fly ash suitable for use as a cement extender (Kruger & Krueger 2005). However, in South Africa only about 5% of all fly ash produced by Eskom is beneficially used (Ash Management in Eskom 2013; Bada & Potgieter-Vermaak 2008) and millions of tons of ash are being stored and disposed of in ash dams and landfills annually, creating the risk of toxic elements present in fly ash being released into the soil and groundwater (Carlson & Adriano 1993).
Developing an alternative application for fly ash as a raw material to produce an environmentally friendly construction material, contributes towards finding a solution for fly ash utilisation. This would expand the raw-material base of the building industry without using natural mineral resources. Although a considerable amount of literature exists on alkali-activation of fly ash, little is known about the activation of South African coal fly ash in particular.
The research of Swanepoel and Strydom (2002) on geopolymerisation of fly ash and kaolinite mixtures yielded conflicting results. They reported the potential suitability of South African fly ash for geopolymerisation despite achieving quite a low strength (8 MPa) at high-energy efforts (elevated temperature curing at 60°C for 48 hours).
The suitability of South African fly ash for use in alkali-activated binders was studied. This paper presents the results of an investigation into the effects of alkali content, water-to-binder-solids ratio, as well as temperature and duration of elevated temperature curing on the compressive strength of alkali-activated fly ash cement pastes containing low-calcium fly ash and sodium hydroxide solution as an aluminosili-cate source and activator, respectively. The study was performed on pastes in order to eliminate the effect of additional variables, such as aggregate, with the aim to obtain main trends which would also be relevant for alkali-activated fly ash cement concretes.
MATERIALS AND METHODS
Classified low-calcium fly ash (class F) from Lethabo power station in South Africa was used for the preparation of alkali-activated fly ash cement pastes. The chemical composition of the fly ash is given in Table 1. The mineralogical composition of the fly ash was studied by means of X-ray diffraction. The fly ash sample was analysed using a PANalytical X'Pert Pro powder diffractometer in θ-θ configuration with an X'Celerator detector and variable divergence-and receiving slits with Fe filtered Co-Ka radiation. The phases were identified using X'Pert Highscore plus software, and 20% Si (Aldrich 99% pure) was added to the fly ash to determine the amorphous (glass) content. The relative phase amounts were estimated by the Rietveld method using the Autoquan - BGMN Rietveld Program, employing the fundamental parameter approach. Fly ash consists mainly of the amorphous phase (59.7% wt), with crystalline inclusion of mullite (29.9% wt) and quartz (10.2% wt). The laser diffraction method analysis of particle size distribution (Malvern Mastersizer 2000) showed that more than 80% of the fly ash particles were smaller than 45 μm.
Commercially available sodium hydroxide flakes (98.5% purity) were used to prepare the activator solutions. The Na2O content was calculated as a percentage of the fly ash mass. It is essential to note that a noticeable decrease in the consistency of the pastes was observed when hot activator solution was mixed with the fly ash. Therefore, activator solutions were prepared in advance and cooled down to room temperature before mixing with the fly ash.
The experimental program was divided into three stages:
1. Investigation into the effect of Na2O content on the compressive strength and microstructure of alkali-activated fly ash cement pastes at constant water-to-binder-solids ratio, temperature and duration of elevated temperature curing.
2. Study of the effect of temperature and duration of elevated temperature curing on the compressive strength of the pastes at a constant activator content and water-to-binder-solids ratio.
3. Investigation into the effect of the water-to-binder-solids ratio ((mass of water from sodium hydroxide solution + mass of added water) / (mass of fly ash + sodium oxide in sodium hydroxide)) on the compressive strength of alkali-activated fly ash cement pastes at constant sodium oxide content, temperature and duration of elevated temperature curing.
The combined effect of the variables on the compressive strength of the alkali-activated fly ash cement pastes was not studied.
Alkali-activated fly ash cement pastes were prepared by mixing the fly ash with the activator solution for three minutes in a pan mixer. The pastes were cast into prismatic 40x40x160 mm moulds immediately after mixing, covered with a film, and placed without any delay into an oven pre-heated to the desired temperature. During the first stage of the study five levels of Na2O content, varying from 3% to 15%, at a constant water-to-binder-solids ratio of 0.2 were investigated. Samples were oven-cured at an elevated temperature of 60°C for 24 hours. After elevated temperature curing, samples were taken out of the oven without gradual cooling, de-moulded, cooled down to room temperature, and tested. Samples, to be tested at other ages, were kept in a room at constant temperature and humidity (25°C and 55% RH). Compressive strength testing was carried out on halves of broken prisms, and the test results of the experiment are expressed in this paper as the arithmetic mean of the six compressive strength values obtained in compliance with SANS 50196-1 on a set of three prisms. Flexural strength results are not presented in this paper, due to an extremely high strength variation and the absence of any significant trend.
During the second stage of the study, the Na2O content that provided the highest compressive strength at 28 days in the first stage was used to investigate the effect of temperature and duration of elevated temperature curing on the strength development of alkali-activated fly ash cement pastes. Four different temperatures (25°C, 40°C, 60°C and 80°C) and six durations of elevated temperature curing (ranging from 4 to 24 hours with 4-hour intervals) were studied in order to find efficient curing conditions. The water-to-binder-solids ratio was kept constant at 0.2.
The effect of the water-to-binder-solids ratio (in the range of 0.18 to 0.29) on the strength development of the alkali-activated fly ash cement pastes was investigated during the third stage of the study. The Na2O content, provided by the highest 28-days compressive strength, was used for paste preparation during the third stage. The pastes were cured at 60°C for 24 hours. At low water-to-binder-solids ratios (0.2 and below) the pastes were dry and lumpy, but the consistency of the pastes was improved significantly when external energy was applied. Vibration for an appropriate time resulted in proper compaction of the alkali-activated fly ash cement pastes.
The microstructure of fresh fractured surfaces of alkali-activated fly ash cement pastes, coated with carbon, was investigated with a scanning electron microscope (SEM) at 20 kV (JEOL JSM 5800).
RESULTS AND DISCUSSION
The effect of Na2O content on the compressive strength and microstructure
Compressive strength
Compressive strength and standard deviation values of alkali-activated fly ash cement pastes with different sodium oxide contents are shown in Table 2.
The compressive strength of alkali-activated fly ash is strongly affected by the concentration of alkaline solution (Fernandez-Jimenez & Palomo 2005a) and this is confirmed by the present study. An increase in Na2O from 3% to 9% of fly ash mass resulted in a significant increase in the compressive strength of the pastes tested after elevated temperature curing. A further increase in alkali content to 12% and 15%, however, resulted in a decrease in the compressive strength. This is in good agreement with the results published by Steveson and Sagoe-Crentsil (2005) and Gou et al (2010) who noticed an increase in compressive strength with increased alkali content up to 10%, but a decrease in strength at 12% and 15%. Compressive strengths from 23.0 MPa to 25.5 MPa were obtained by Somna et al (2011), with NaOH concentrations varying from 9.5 M (7.3% of Na2O) to 14 M (10.1%). They reported a decrease in compressive strength for a concentration of 16.5 M (11.3%) due to early precipitation of aluminosilicate products. Palomo et al (1999a) indicated that the strength decrease of the alkali cement was caused by an excess of OH- concentration in the system. Rattanasak and Chindaprasirt (2009) found that higher concentrations of Si4+ and Al3+ were obtained with a 10 M NaOH (7.7%) compared to 15 M (10.7%). A high concentration of OH- resulted in a decrease in the ability of Al and Si to participate in the geopolymerisation.
In this study, a gain in strength of up to 30% and more was observed after 28 days of curing for all samples (see Table 2). A noticeable change in compressive strength with time was observed by other researchers (De Vargas et al 2011; Somna et al 2011). This confirms that age has a significant effect on the strength of alkali-activated fly ash cement pastes, and the structural development does not stop with the discontinuation of elevated temperature curing. The strength increase probably relates to the transformation of aluminosilicate gel to more stable structures (Škvara et al 2009; Ravikumar et al 2010). At the age of 28 days the highest compressive strength value was again obtained for the 9% Na2O paste. The paste with 12% Na2O yielded a higher compressive strength value after 91 and 182 days, but after one year of curing, the strength of the Na12 paste was lower than that of Na9. The difference in compressive strength values between 9% and 12% at later ages is negligible, and therefore using lower alkali content is more economical, as the alkali is the most expensive component in the alkali-activated fly ash cement paste composition. Therefore, 9% of Na2O can be considered as the most suitable alkali concentration to produce alkali-activated fly ash cement paste containing the South African fly ash studied in this research.
It should be mentioned that the greatest gain in strength was observed for alkali-activated fly ash cement pastes with high alkali content. Similar findings were reported by De Vargas et al (2011). The authors explained that the gain in strength with time was caused by additional reaction products generated during the reaction between alkali activator and microspheres, which packed inside plerospheres. The alkaline solution first partially dissolved the external layer of the plerosphere and only then attacked the microspheres.
As can be seen from the standard deviation values in Table 2, the observed compres-sive strength of the 15% Na2O paste samples varied significantly. It can be assumed that the high concentration of alkali in the pastes results in inconsistent compressive strength. Additional tests were performed on larger sample populations for Na9, Na12 and Na15 pastes to establish the distribution of the compressive strength, and these results are shown in Table 3. Curing conditions were the same as in the first test. Samples Na3 and Na6 were not studied in the second test due to their relatively low compressive strength compared to samples with higher alkali dosage and satisfactory standard deviation. At least thirty-six compressive strength determinations at the age of 28 days were performed for each of these pastes. For the paste with 9% of Na2O, six results within 36 determinations of the compression strength varied by more than ± 10% from the mean, which was 16.7% of the total sample population. For the paste with 12% of Na2O, seven strength values out of 42 varied by more than ± 10% from the mean, which was also 16.7%. The paste with 15% of Na2O had 18 strength values out of 36 varying by more than ± 10% from the mean, which represented 50% of the total sample population. The strength deviation was thus greater for alkali-activated fly ash cement pastes containing 15% Na2O.
Results show that the coefficient of strength variation within the large batch of Na15 paste was much higher than that of Na9 and Na12 pastes (16.3%, 8.8% and 8.1% respectively). It is interesting to note that high alkali content affects not only the deviation of compressive strength within a batch, but also between two batches of the same composition. The average 28-days strength of the Na9 paste for the second (large) batch was almost the same as for the first batch (51.4 MPa and 50.8 MPa respectively) while the average 28-days strength of Na15 paste for the second batch was much lower than for the first batch (35.6 MPa and 49.8 MPa respectively). The same trend could be observed for Na12 paste where the average 28-days strength for the first batch was 48.6 MPa while it reached only 41.4 MPa for the second batch. Limited strength deviation should be expected because of the variability of the fly ash composition due to variations in coal composition and burning conditions. Nevertheless, the strength deviation between batches was much greater with higher alkali concentrations.
Another important observation was the formation of efflorescence on the surfaces of the Na12 and Na15 paste samples after 28 days of hardening. The formation of efflorescence is an indirect indicator of excess Na2O content in the alkali-activated fly ash cement pastes with 12% and 15% of Na2O. The excess alkali migrates with moisture to the surface of the samples and produces salts that appear as white efflorescence. The presence of excess alkali could be one of the causes of the lower-strength, high-standard deviation, and variations in the strength between the batches of alkali-activated fly ash cement pastes.
Microstructure
SEM images of alkali-activated fly ash cement pastes with different Na2O content at the age of 40 days are presented in Figures 1 and 2.
Figure 1 shows the microstructure of the Na3, Na6 and Na9 pastes. The surface of the particles is covered by shell-shaped reaction products with smooth fly ash particles under the shell. It was found in previous research that the main reaction product of the alkali activation of fly ash was disordered aluminosilicate gel, also known as geopolymeric gel (Palomo et al 1999a; Duxson et al 2007b; Provis & Van Deventer 2009). It can be seen that fly ash particles are glued to one another by reaction products. Voids observed between fly ash particles indicate that not enough aluminosilicate gel has been formed to fill the space, resulting in the friable appearance of the microstructure. The amount of unreacted fly ash particles decreases with increasing concentration of the alkali, and the matrix appears more continuous, which indicates a formation of increased amounts of gel (see Figure 2). As it was assumed that the aluminosilicates gel was responsible for the mechanical properties of the final product (Zhang et al 2013), it was expected that the more continuous appearance of the matrix was related to the greater amount of gel, resulting in the higher compressive strength. This is in good correlation with the compressive strength results, with the exception of the Na15 paste. The matrix of the Na15 paste appears continuous and the microstructure looks the most solid, but the compressive strength tends to be less than that of the Na9 and Na12 pastes (see Table 2).
One of the reasons for the strength drop with increasing alkali content over 9% Na2O could possibly be micro-cracks which can be observed in images of the microstructure of alkali-activated fly ash cement pastes containing 12% and 15% Na2O (see Figure 2).
The nature of these cracks is not yet clear. De Vargas et al (2011) discovered similar cracks in alkali-activated fly-ash-based geopolymers. The authors reported that micro-cracks could be found easier in the samples with higher amounts of alkali (16.4% Na2O compared to 8.2% Na2O), but they did not observe a reduction in compressive strength. At the same time Fernandez-Jimenez and Palomo (2005a) linked cracks with elevated temperature curing during the activation process, or mechanical damage during sample preparation for SEM observation. However, all the samples presented in the micrographs in this study had the same regime of elevated temperature curing and sample preparation for the SEM study, but cracks were only observed in the Na12 and Na15 pastes. The appearance of the small cracks differs from long, wider cracks which most probably are the result of sample preparation. The small cracks appear within the continuous matrix and do not go out beyond its boundaries. A detailed study of these cracks and their origin should be conducted.
The effect of temperature and duration of elevated temperature curing on the compressive strength
Based on the results of the first stage of this study, the Na9 paste was used to investigate the effect of temperature and duration of elevated temperature curing on the com-pressive strength of alkali-activated fly ash cement pastes. Compressive strength results and standard deviation values for the second stage of the study are shown in Table 4.
It was expected that an elevation of curing temperature would accelerate the dissolution of the glass phase of the fly ash and, as a result, the strength development of the alkali-activated fly ash cement pastes. Previous studies showed that temperature accelerated the alkali activation of metaka-olin (Alonso & Palomo 2001), as well as slag pastes (Bakharev et al 1999). The accelerating effect of elevated temperature also applies to fly ash pastes (Katz 1998). The results presented in this paper confirm the trend (see Table 4).
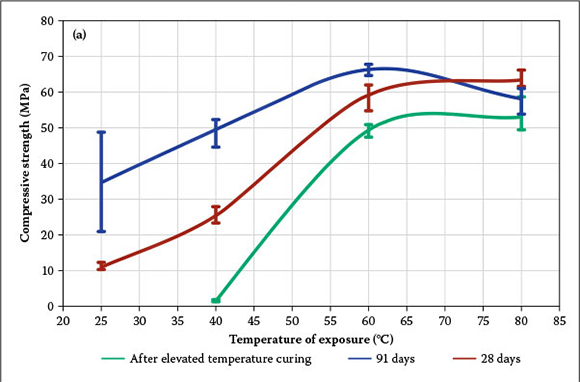
The importance of elevated temperature curing can clearly be seen in Figure 3a. The error bars on all figures represent deviation of the compressive strength from the mean for six values. Samples cured at 25°C did not set after 24 hours and could hardly be de-moulded after seven days, when the com-pressive strength was 1.1 MPa. Even such a low strength was an indicator of chemical reaction between the fly ash and the activator solution. The strength of the paste cured at 60°C for 24 hours, and tested immediately after the elevated temperature curing exceeded the strength of the paste, cured at 25°C for 91 days. Curing of the alkali-activated fly ash cement paste at 25°C is not practical, due to slow strength development, intensive efflorescence formation, relatively low strength and high standard deviation (see Table 4). Thus, elevated temperature curing is necessary to provide faster strength development and lower standard deviation of the strength. An increase in temperature from 25°C to 60°C produced significant acceleration in the strength development. Immediately after elevated temperature curing, the paste cured at 40°C, 60°C and 80°C for 24 hours had a compressive strength of 1.6 MPa, 49.4 MPa and 53.0 MPa respectively. The increase in the compressive strength of the paste was a result of an increase in the degree of polymerisation caused by the elevation of the curing temperature (Povnaník 2010). It is important to note that when the paste was cured at elevated temperature for 24 hours, an increase in the temperature to above 60°C did not result in a considerable gain in compressive strength. The use of 60°C instead of 80°C for the prolonged elevated temperature curing of alkali-activated fly ash cement pastes will require less energy, and will thus be more economical.
After 28 days of curing, the compressive strength of samples T40D24, T60D24 and T80D24 increased from 1.6 MPa, 49.4 MPa and 53.0 MPa, to 25.5 MPa, 59.1 MPa and 63.5 MPa, respectively. Sample T25D24 could not be de-moulded at day one, but it still gained 10.9 MPa after 28 days of hardening. The compressive strength of the alkali-activated fly ash cement pastes increased with the reaction time, and the gain was greater in those cases where the pastes were cured at low temperatures (25°C and 40°C). At the age of 91 days, the paste that had been cured at 80°C had a lower compressive strength compared to the paste cured at 60°C. This strength decrease was probably caused by the contraction of aluminosilicate gel, due to dehydration and excessive shrinkage occurring during the curing at high temperatures for 24 hours (Van Jaarsveld et al 2002). Chindaprasirt et al (2007) drew conclusions regarding the effect of elevated temperature curing on strength, considering seven-day strength results, but the results of this study and others (De Vargas et al 2011; Arioz et al 2012) show that the difference in strength of alkali-activated fly ash cement pastes cured at low and high temperatures decreases with age, and even more, lower temperatures could lead to higher strength in the long term.
Another important observation is the long-term gain in compressive strength of the alkali-activated fly ash cement pastes cured at elevated temperatures for different periods of time. After 28 days of hardening, the compressive strength of sample T60D4 raised by 20 times (from 2.1 MPa to 41.5 MPa), while the compressive strength of sample T80D4 increased only by 1.4 times (from 36.0 MPa to 50.8 MPa) (see Table 4). For samples T60D24 and T80D24, the compressive strength increased from 49.4 MPa to 59.1 MPa, and from 53.0 MPa to 63.5 MPa respectively, showing a 1.2 times gain for both samples. These results show that the ageing of the alkali-activated fly ash cement pastes has a greater effect on the strength development of the pastes cured at the lower temperature for a short period of time.
It was shown that the speed of reaction between alkali and fly ash depends on curing temperature, especially in the first few hours (Palomo et al 1999a). A relatively small increase in the temperature for elevated temperature curing resulted in a significant gain in the compressive strength of the pastes cured for a short period of time (Figure 3b). Increasing the temperature from 65°C to 70°C produced a significant increase in the strength from 3.2 MPa to 15.5 MPa. A further increase (by 5°C) doubled the strength from 15.5 MPa to 30.5 MPa. Subsequently, increasing the temperature to 80°C did not lead to a significant increase in the strength in comparison to 75°C. After 28 and 91 days of hardening, the difference in the strength of the alkali-activated fly ash cement pastes, cured at elevated temperatures in the range of 60°C to 80°C, was not significant. Thus, despite the significant effect of the increase in the curing temperature (in the range from 60°C to 80°C) on the early compressive strength of the pastes, the difference in the curing temperature had a limited effect on the long-term strength of the pastes when elevated temperature curing was applied for a short period of time (four hours).
Figure 4 shows the effect of the duration of elevated temperature curing on the compressive strength of the alkali-activated fly ash cement pastes cured at 60°C and 80°C.
After four hours of elevated temperature curing at 60°C (see Figure 4a), the compressive strength of the paste tested immediately after the curing was 2.1 MPa. With other parameters being equal, the compressive strength of the paste cured at 80°C was 36 MPa (see Figure 4b). This once again confirms that temperature during the initial curing plays a key role in the strength development of alkali-activated fly ash cement pastes. For longer durations of elevated temperature curing, the difference in the strength of the pastes cured at 60°C and 80°C becomes less prominent. The compressive strength of sample T60D24 was 49.4 MPa, while that for sample T80D24 was 53.0 MPa. It is important to note that a significant increase in the compressive strength, tested immediately after elevated temperature curing, of the paste cured at 60°C was observed only when the duration of the curing was increased to 16 hours (see Figure 4a). Subsequent increases in the duration of elevated temperature curing did not result in a substantial increase in strength. A similar trend was observed when the paste was cured at 80°C (see Figure 4b). Insignificant strength gain took place when the duration of elevated temperature curing exceeded 16 hours. In the long term, curing at 80°C for more than 16 hours negatively affected the compressive strength. At 28 and 91 days, the pastes cured in an oven for 20 and 24 hours had lower strengths in comparison to the paste cured for 16 hours. Van Jaarsveld et al (2002) reported that curing for longer periods of time at elevated temperature appeared to weaken the microstructure, suggesting that small amounts of structural water needed to be retained in order to reduce cracking and maintain structural integrity. Despite the fact that a few researchers (Swanepoel & Strydom 2002; Chindaprasirt et al 2007) reported 48 hours as an optimal duration of elevated temperature curing, the results of this study confirm the findings made by Van Jaarsveld et al (2002), and indicate that the duration of elevated temperature curing should be limited to 16 hours.
The effect of the water-to-binder-solids ratio on compressive strength
Na9 paste cured at 60°C for 24 hours was studied during the third stage. The water-to-binder-solids ratio varied in the range from 0.18 to 0.29. The effect of the water-to-binder-solids ratio on the compressive strength of the alkali-activated fly ash cement pastes is shown in Figure 5.
Increasing the water-to-binder-solids ratio of the pastes from 0.18 to 0.29 resulted in a decrease in the compressive strength from 49.3 MPa to 21.3 MPa, but the consistency of the pastes improved. Fernandez-Jimenez and Palomo (2005a) admitted the importance of the water-to-binder ratio. According to Davidovits (1988), no water combined within the geopolymer. Water acts as a carrier of alkalis (Skvara et al 2009) and provides consistency to the fresh geopolymer mixture (Hardjito & Rangan 2005). Skvara et al (2009) reported that about 65% of all water in geopolymers was in "free" condition as it was evoporable at 180°C, with 30% presumed to come from nano-pores in the geoplymer gel. The water introduced to the paste could evaporate from unsealed samples during elevated temperature curing, thus negatively affecting the final structure of material. Samples should therefore preferably be sealed to prevent extensive moisture evaporation.
During the design of alkali-activated fly ash cement concretes, a required workability should be achieved at the lowest possible water-to-binder-solids ratio.
CONCLUSIONS
The trends observed for the alkali-activated fly ash cement pastes should be relevant for concretes containing alkali-activated fly ash cement.
The alkali content plays an important role in the development of the compressive strength of alkali-activated fly ash cement pastes and their microstructure. The recommended alkali concentration is 9% Na2O of fly ash mass, which provides the highest compressive strength and low standard deviation at 28 days. Excessive alkali content (> 9% Na2O) results in a decrease in strength, high standard deviation and high coefficient of strength variation between different batches. Alkali content higher than 9% Na2O also causes efflorescence formation and, possibly, the formation of micro-cracks in the micro-structure of the pastes. Therefore, the amount of alkali must be strictly controlled during the production of alkali-activated fly ash cement.
The temperature and duration of curing of alkali-activated fly ash cement pastes affect the compressive strength significantly. Curing at 25°C is possible, but it is not practical, due to delayed setting, intensive efflorescence formation, very slow strength development, relatively low strength at 28 days and large strength deviation. Therefore, it is important to provide elevated temperature curing, thus accelerating the strength development of the alkali-activated fly ash cement pastes.
Elevated curing temperature has a greater effect on the early strength than the long-term strength, especially of pastes cured for a short period of time. An increase in temperature over 60°C did not noticeably affect the 28-day and 91-day compressive strength of alkali-activated fly ash cement pastes cured for four and 24 hours. A decrease in the 91-day compressive strength of the paste cured at 80°C for 24 hours was observed, in comparison to the paste cured at 60°C for the same period of time. The duration of elevated temperature curing has a more prominent effect on the early strength. The 28-day and 91-day compressive strengths are less affected by the duration of elevated temperature curing. There is no significant increase in the early compressive strength when the duration of elevated temperature curing exceeds 16 hours. Elevated temperature curing at 60°C for 16 hours is recommended for curing of alkali-activated fly ash cement pastes.
The compressive strength of alkali-activated fly ash cement pastes is significantly affected by the water-to-binder-solids ratio. The compressive strength decreases with an increase in water-to-binder-solids ratio. Therefore, alkali-activated fly ash cement concretes should be designed to achieve a required workability at the lowest possible water-to-binder-solids ratio.
Alkali-activated fly ash cement pastes were produced with a compressive strength between 10 MPa and 60 MPa at 28 days. These results indicate that the Lethabo fly ash can be used as a source of alumino-silicates in alkali-activated fly ash cement formulation to produce a concrete with good mechanical properties. The short-term and long-term properties of alkali-activated fly ash cement concrete are currently under investigation at the University of Pretoria.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Dr R A Kruger and Ash resources (Pty) Ltd for supplying the fly ash for these experiments. The authors would like to thank Wiebke Grote and Jeanette Dykstra for XRD and XRF analyses, and the staff of the Microscopy and Microanalysis Laboratory of the University of Pretoria for providing access to the microscope, and assistance during the investigation.
REFERENCES
Ash Management at Eskom 2013. Available at: http:// www.eskom.co.za/c/25/facts-figures/ [Accessed 4 June 2013]. [ Links ]
Alonso, S & Palomo, A 2001. Alkaline activation of metakaoline and calcium hydroxide mixtures: Influence of temperature, activator concentration and solids ratio. Material Letters, 47: 55-62. [ Links ]
Arioz, E, Arioz, O & Mete Kockar, O 2012. Leaching of F-type fly-ash based geopolymers. Paper presented at the 20th International Congress of Chemical and Process Engineering, Prague, Czech Republic, Procedia Engineering, 42: 1114-1120. [ Links ]
Bada, S O & Potgieter-Vermaak, S 2008. Evaluation and treatment of coal fly ash for adsorption application. Leonardo Electronic Journal of Practices and Technologies, 12: 37-48. [ Links ]
Bakharev, T Sanjayan, J & Cheng, Y B 1999. Alkali activation of Australian slag cements. Cement and Concrete Research, 29: 113-120. [ Links ]
Carlson, C L & Adriano, D C 1993. Environmental impacts of coal combustion residues. Journal of Environmental Quality, 22(2): 227-247. [ Links ]
Chindaprasir t, P, Chareerat, T & Sirivivatnanon, V 2007. Workability and strength of coarse high calcium fly ash geopolymer. Cement and Concrete Composites, 29: 224-229. [ Links ]
Criado, M, Fernandez-Jimenez, A, Palomo, A, Sorbrados, I & Sanz, J 2008. Effect of the SiO2/Na2O ratio on the alkali activation of fly ash. Part II: Si MAS-NMR survey. Microporous and Mesoporous Materials, 109: 525-534. [ Links ]
Davidovits, J 1988. Geopolymer chemistry and properties. Proceedings, Geopolymer 1988, 1st European Conference on Soft Mineralogy, Compiegne, France, Vol 1, pp 25-48. [ Links ]
De Vargas, A S, Dal Molin, D C C, Vilela, A C F, da Silva, F J, Pavão, B & Veit, H 2011. The effects of Na2O/SiO2 molar ratio, curing temperature and age on compressive strength, morphology and microstructure of alkali-activated fly ash-based geopolymers. Cement and Concrete Composites, 33: 653-660. [ Links ]
Duxson, P, Lukey, G C & Van Deventer, J S J 2007a. The thermal evolution of metakaolin geopolymers. Part 2: Phase stability and structural development. Journal of Non-crystalline Solids, 353: 2186-2200. [ Links ]
Duxson, P, Fernandez-Jimenez, A, Provis, J L, Lukey, G C, Palomo, A & Van Deventer, J S J 2007b. Geopolymer technology: The current state of the art. Journal of Materials Science, 42: 2917-2933. [ Links ]
Eskom Integrated Report 2011. Available at: http://financialresults.co.za/2011/eskom_ar2011/profile_ key_facts.php. [ Links ] [Accessed 1 October 2012].
Ferrnandez-Jimenez, A & Palomo, A 2005a. Composition and microstructure of alkali-activated fly ash binder: Effect of the activator. Cement and Concrete Research, 35: 1984-1992. [ Links ]
Fernandez-Jimenez, A, Palomo & A Criado, M 2005b. Microstructure development of alkali-activated fly ash cement: a descriptive model. Cement and Concrete Reseach, 35: 1204-1209. [ Links ]
Flower, D J M & Sanjayan, J G 2007. Green house gas emissions due to concrete manufacture. International Journal of Life Cycle Assessment, 12(5): 282-288. [ Links ]
Guo, X, Shi, H & Dick, W A 2010. Compressive strength and microstructural characteristics of class C fly ash geopolymer. Cement and Concrete Composites, 32: 142-147. [ Links ]
Hardjito, D & Rangan, B V 2005. Development and properties of low-calcium fly ash-based geopolymer concrete. Research Report GC 1. Peth, Australia, Curtin University of Technology, Faculty of Engineering. [ Links ]
Hasanbeigi, A, Price, L & Lin, E 2012. Emerging energy-efficiency and CO2 emission-reduction technologies for cement and concrete production: A technical review. Renewable and Sustainable Energy Reviews, 16: 6220-6238. [ Links ]
Katz, A. 1998. Microscopic study of alkali activation fly ash. Cement and Concrete Research, 28(2): 197-208. [ Links ]
Kruger, R A & Krueger, J E 2005. Historical development of coal ash utilization in South Africa. Proceedings, World of Coal Ash (WOCA) Conference, Lexington, Kentucky, USA. [ Links ]
Matjie, R H, Ginster, M, Van Alphen, C & Sobiecki, A 2005. Detailed characterisation of Sasol ashes. Proceedings, World of Coal Ash (WOCA) Conference, Lexington, Kentucky, USA. [ Links ]
McLellan, B C, Williams, R P, Lay, J, Van Riessen, A & Corder, G D 2011. Costs and carbon emissions for geopolymer pastes in comparison to ordinary Portland cement. Journal of Cleaner Production, 19: 1080-1090. [ Links ]
Palomo, A, Grutzeck, M W & Blanco, M T 1999a. Alkali-activated fly ashes. A cement for the future. Cement and Concrete Research, 29: 1323-1329. [ Links ]
Palomo, A, Blanko-Varela, M T, Granizo, M L, Puertas, F, Vazquez, T & Crutzeck, M W 1999b. Chemical stability of cementitious materials based on metakaolin. Cement and Concrete Research, 29: 997-1004. [ Links ]
Povnaník, P 2010. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Construction and Building Materials, 24: 1176-1183. [ Links ]
PPC Integrated Annual Report 2011. Available at: http://www.ppc.co.za/pdf/ir/2011/PPC_integrated_annual_report_2011.pdf [Accessed 3 June 2013]. [ Links ]
Provis, J L. Yong, C Z. Duxson, P & Van Deventer, J S J 2009. Correlating mechanical and thermal properties of sodium silicate-fly ash geopolymers. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 336: 57-63. [ Links ]
Rattanasak, U & Chindaprasirt, P 2009. Influence of NaOH solution on the synthesis of fly ash geopolymer. Minerals Engineering, 22: 1073-1078. [ Links ]
Ravikumar, D, Peethamparan, S & Neithalath, N 2010. Structure and strength of NaOH activated concretes containing fly ash or GGBFS as the sole binder. Cement and Concrete Composites, 32: 399-410. [ Links ]
Shi, C, Krivenko, P V & Roy, D 2006. Alkali-activated cements and concretes. Abingdon, Oxford, UK: Taylor & Francis. [ Links ]
Skvara, F, Kopecký, L, Smilauer, V & Bittnar, Z 2009. Material and structural characterization of alkali-activated low-calcium brown coal fly ash. Journal of Hazardous Materials, 168: 711-720. [ Links ]
Somna, K, Jaturapitakkul, C, Kajitvichyanukul, P & Chindaprasirt, P 2011. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel, 90: 2118-2124. [ Links ]
Steveson, M & Sagoe-Crentsil, K 2005. Relationships between composition, structure and strength of inorganic polymers. Part 2: Fly ash-derived inorganic polymers. Journal of Materials Science, 40: 4247-4259. [ Links ]
Swanepoel, J C & Strydom, C A 2002. Utilisation of fly ash in a geopolymeric material. Applied Geochemistry, 17: 1143-1148. [ Links ]
Turner, L K & Collins, F G 2013. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Construction and Building Materials, 43: 125-130. [ Links ]
Van Deventer, J S J, Provis, J L, Duxson, P & Brice, D G 2010. Chemical research and climate change as drivers in the commercial adoption of alkali-activated materials. Waste and Biomass Valorization, 1: 145-155. [ Links ]
Van Deventer, J S J, Provis, J L, Duxson, P & Lukey, G C 2007. Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. Journal of Hazardous Materials, A139: 506-513. [ Links ]
Van Jaarsveld, J G S & Van Deventer, J S J 1999. Effect of the alkali metal activator on the properties of fly ash-based geopolymers. Industrial and Engineering Chemistry Research, 38: 3932-3941. [ Links ]
Van Jaarsveld, J G S, Van Deventer, J S J & Lukey, G C 2002. The effect of composition and temperature on the properties of fly ash- and kaolinite-based geopolymers. Chemical Engineering, 89: 63-73. [ Links ]
Zhang, Z, Provis, J L, Wang, H, Bullen, F & Reid A 2013. Quantitative kinetic and structural analysis of geopolymers. Part 2. Thermodynamics of sodium silicate activation of metakaolin. Thermochimica Acta, 565: 163-171. [ Links ]
 Correspondence:
Correspondence:
Julia Shekhovtsova
Department of Civil Engineering University of Pretoria
Pretoria 0002
South Africa
T: +27 12 420 4179
F: +27 12 362 5218
E: j.shekhovtsova@gmail.com
Elsabé Kearsley
Department of Civil Engineering University of Pretoria
Pretoria 0002
South Africa
T: +27 12 420 2179
F: +27 12 362 5218
E: elsabe.kearsley@up.ac.za
Maxim Kovtun
Department of Civil Engineering University of Pretoria
Pretoria 0002
South Africa
T: +27 12 420 6953
F: +27 12 362 5218
E: max.kovtun@up.ac.za

JULIA SHEKHOVTSOVA received her B Eng degree from the Belgorod State Technological University in Russia. Currently she is busy with her PhD at the University of Pretoria, South Africa. Her fields of interest include the utilisation of fly ash in alkali-activated non-Portland cement binders.

PROF ELSABÉ KEARSLEY graduated with a degree in Civil Engineering from the University of Pretoria In 1984. She holds a PhDfrom the University of Leeds. She worked as a Structural Design Engineer in both South Africa and the UKbefore becoming a staff member atthe University of Pretoria. She was the 2009 President ofthe South African Institution of Civil Engineering (SAICE) and she is currently the Head of the Department of Civil Engineering at the University of Pretoria. For the last 22 years she has been involved with cement and concrete materials research.

DR MAXIM KOVTUN completed his PhD at the Belgorod State Technological University in Russia in 2008. Currently he is appointed as a researcher at the University of Pretoria, South Africa, specialising in building and construction materials. His fields of interest include alkali-activated cements and concretes, and foamed concretes.