Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.95 n.1 Pretoria 2024
http://dx.doi.org/10.36303/JSAVA.580
REVIEW
Copper (Cu) metabolism in domestic herbivores as guide to criteria for predicting the Cu nutritional status of wild ruminants in southern Africa
JBJ van RyssenI; GF BathII
IDepartment of Animal Sciences, Faculty of Natural and Agricultural Sciences, University of Pretoria, South Africa
IIDepartment of Production Animal Studies, Faculty of Veterinary Science, University of Pretoria, South Africa
ABSTRACT
In southern Africa game farming has become an effective way of using underutilised resources and a valuable method of preserving and increasing wildlife numbers. However, little is known about the mineral requirements of wild animal species or the assessment of the mineral nutritional status of these species. To establish criteria for estimating the copper (Cu) nutritional status of wildlife, current knowledge about Cu metabolism and criteria for domestic animals has been used. Since the Cu metabolism of ruminants differs substantially from that of non-ruminants, Cu metabolism in domestic species such as cattle and sheep representing wild ruminants, and pigs and horses as non-ruminant species, has been scrutinised to propose criteria for wild bovids in southern Africa. In the adequate range of dietary Cu intakes, literature suggests that hepatic Cu concentrations in ruminants increase linearly with an increase in Cu intake, allowing a relatively reliable measure of sufficiency. In non-ruminants, hepatic Cu concentrations follow a lag phase during which hepatic Cu concentrations remain relatively constant with increasing dietary Cu intakes of more that 25 times their requirements. A consequence is that non-ruminants can tolerate much higher dietary levels of Cu compared to ruminants. It is proposed that at liver Cu concentrations of < 20 mg/kg dry matter (DM), a wild ruminant could benefit from Cu supplementation; liver Cu concentrations of between 20 and 300 mg Cu/kg DM suggest an adequate Cu intake; concentrations of 300 to 500 mg/kg DM indicate a potentially unhealthy accumulation of Cu, while liver Cu concentrations of > 500 mg/kg DM indicate that the animal probably consumed more Cu than required and might be at risk of developing Cu toxicosis.
Keywords: copper (Cu) deficiency, Cu poisoning, game, hepatic Cu, plasma/serum Cu, wildlife
Introduction
In southern Africa wildlife numbers on farms and the private wildlife ranching industry have expanded dramatically in recent years (Taylor et al. 2021). Taylor et al. (2021) estimated that herbivore numbers on private land in South Africa had increased more than ten-fold in the last 50 years. An important reason for this is that private landowners in South Africa were granted user rights over certain game species (Game Theft Act, No. 105 of 1991), leading to the commercial use of wildlife and thus financial gain through trophy hunting, trading in animals, ecotourism and game meat production. Phenotypic characteristics, such as horn size and form, fertility, coat colour and body size have become measured characteristics (Van Deventer 2019; Shepstone et al. 2022). Sustainability through sound management practices on ranches became an important aspect of wildlife ranching; for instance, based on a survey, Taylor et al. (2021) calculated that 85% of participants in the survey supplied mineral supplements at some stages of the year to their game. However, a serious limitation in providing mineral supplements to African game species is that at present no criteria exist to estimate the mineral nutritional status of African wildlife.
As a guide to interpreting mineral concentrations in animal tissues in terms of the nutritional status of domestic livestock, reference ranges of trace elements such as copper (Cu) have been established and are used widely. This information has been published in textbooks such as Puls (1994) and other publications (McDowell et al. 1984; Kincaid 1999). Reference ranges are analytical measurements of mineral or metabolite concentrations in animal tissue or fluid components that closely reflect the mineral nutritional status of the animal (Grace & Clark 1991), classified as either deficient, marginally deficient, adequate or potentially toxic. Specific mineral concentrations have been suggested, so-called threshold values, above or below, which toxicities or deficiency symptoms respectively, are likely to occur. However, Underwood and Suttle (1999) and Suttle (2022) prefer to express status as "marginal bands", i.e. ranges in which animals are likely to respond to supplementation. For a trace element assay to be an effective diagnostic tool, there needs to be a well-defined relationship between the concentration of a trace element (or related metabolite) in bodily tissues or fluid and the occurrence of deficiency or toxicity signs (Fraser 1982; Wilson & Grace 2001). In the case of Cu, cases of such relationships in wildlife have been reported in South Africa (Gummow et al. 1991; Penrith et al. 1996; Quan 2001).
The mineral nutritional status of game has been a field of interest in South Africa for many years (Schulz et al. 1951; Boyazoglu 1973). Analyses have been conducted to determine the trace element concentrations in blood and liver samples of domestic and wildlife species to quantify the mineral nutritional status of an area or to establish if animals suffered from a deficiency or an excessive intake of specific elements. Interpretation is based on information from domestic animals. In this publication the focus will be on Cu as an essential micronutrient in animal nutrition, with the focus on possible criteria to be used to assess Cu status in African Bovidae.
Copper metabolism in ruminant versus non-ruminant species
Differences in Cu metabolism
López-Alonso et al. (2006) pointed out that ruminant species have a relatively unusual metabolism of Cu compared with non-ruminant species, leading to major inter- and intraspecies differences in Cu metabolism between non-ruminants and ruminants. Consequently, differences in requirements and especially maximum tolerance levels for Cu (EFSA Panel 2016). Therefore, when considering the Cu metabolism of wildlife in Africa, a clear distinction has to be made between the herbivorous ruminant species and the two non-ruminant (monogastric) groups, viz. the herbivorous hindgut (caudal) fermenters such as the elephant, rhinoceros species, equids such as the zebra, the warthog, leporids such as hare and rabbits, and the monogastric digesters such as wild pigs (Dehority 2002). Although there are distinct differences between ruminants and non-ruminants in the digestive processes, the proportion of dietary Cu becoming available for absorption would not affect criteria based on bodily tissue and fluid concentrations indicating Cu nutritional status of the animal, except for a possible systemic effect owing to thiomolybdates synthesised in the digestive tract (Van Ryssen & Stielau 1981; EFSA Panel 2016; Clarkson et al. 2020).
Although other indicators of Cu status in animals have been tested, such as erythrocyte superoxide dismutase (SOD) activity and plasma caeruloplasmin concentration (Suttle 1986; Mills 1987; López-Alonso et al. 2006; Hepburn et al. 2009), Cu concentrations in serum/plasma and liver tissues are presently considered the most feasible measures of Cu status of ruminants (López Alonso et al. 2006; Spears et al. 2022). Although probably not feasible, Arnhold et al. (1998) concluded that in goats the cerebrum is the best indicator organ of Cu status under conditions of Cu deficiency.
In ruminants the liver is the almost exclusive site of Cu accumulation in the body and represents a storage pool of Cu in the body, reflecting the long-term availability of absorbed Cu to the animal (Herdt & Hoff 2011; EFSA Panel 2016). Ruminants have a limited ability to modulate Cu excretion in the bile and, therefore, excessive hepatic Cu accumulation occurs (López-Alonso & Miranda 2020). Ruminants have poor homeostatic control over Cu absorption but have developed so-called systemic homeostasis by storing excess Cu in the liver (EFSA Panel, 2016; López-Alonso & Miranda 2020). Although the retention of dietary Cu in the liver is low, usually less than 5% of total Cu intake, at above minimum requirements, Cu accumulation in the liver follows a positive linear increase with an increase (and/or duration) in Cu intake (Dick 1954; Hemingway & MacPherson 1967; Van Ryssen & Stielau 1980; Suttle 1983; Woolliams et al. 1983). However, at low Cu intake hepatobiliary excretion is reduced and intestinal uptake and transfer are increased (EFSA Panel, 2016). The limited capacity in sheep to excrete/ reduce the Cu in the livers once it has accumulated (EFSA Panel 2016), is evident from reports that sheep died from Cu toxicosis several months after Cu supplementation had been withdrawn (Bracewell 1958; Kowalezyk et al. 1962). At higher Cu intakes, Woolliams et al. (1983) demonstrated that Cu concentrations in the liver start to follow a curvilinear pattern and suggested that it could reflect a saturation in the mechanism of absorption and an increased endogenous loss through the bile. López-Alonso et al. (2017) also reported that at high Cu intakes, the Cu concentrations in all four subcellular fractions of the liver (nuclei, large granules, microsomes and cytosol) followed a linear increase until a plateau phase was reached, indicating an approaching manifestation of toxicity symptoms.
Herdt and Hoff (2011) pointed out that in ruminants, hepatic Cu concentrations within the adequate range can act as a reliable indicator that the animal received sufficient dietary Cu in available forms. According to Herdt and Hoff (2011) Cu concentration in the liver is not a functional measure of status because a ruminant with a liver Cu concentration of 300 mg/kg dry matter DM is not healthier than one with a liver Cu concentration of 100 mg/kg DM. They pointed out that an animal with hepatic Cu concentration of 100 mg/kg would not benefit from additional dietary Cu, because it has adequate Cu nutrition (Herdt & Hoff 2011). Therefore, the conclusion of Boyazoglu (1973), indicating a typical or normal liver Cu concentration for ruminant species, is not valid when considering Cu metabolism in ruminants.
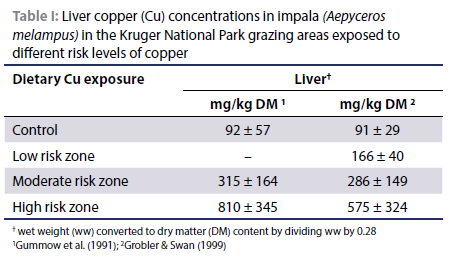
Evidence that liver Cu concentrations increase with increases in dietary CU levels can be deduced from available literature on the impala (Aepyceros melampus) (a mixed feeder consuming grass and browse) exposed to Cu pollution due to open-cast Cu mining activities at Phalaborwa next to the Kruger National Park in South Africa (Table I) (Gummow et al. 1991; Grobler & Swan 1999). Likewise, Ackerman et al. (1999) recorded liver Cu concentrations of impala consuming diets differing in Cu concentration: control (free range) (n = 6) = 52.6 ± 17.9 mg/kg (probably wet weight [ww]); exposed to Cu fall-out (n = 32) = 126.5 ± 125.7 mg/kg and a Cu treated group (n = 12) = 111.8 ± 41.0 mg/kg.
In the case of the African buffalo (Syncerus caffer), a bulk grazer, Grobler and Swan (1999) recorded liver Cu concentrations of 193 mg/kg DM in buffalo grazing a Cu-contaminated area vs. 72 mg Cu/kg DM in an uncontaminated area of the Kruger National Park. Gummow et al. (1991) previously reported that the livers of buffalo shot in the Cu-polluted area contained 287 ± 128 mg Cu/kg DM and in non-polluted areas 68.6 ± 36 mg/kg DM. These results support the principle that in these two ruminant species, liver Cu concentrations increase with increasing Cu consumption.
Compared with cattle and sheep where hepatic Cu concentrations increase linearly at lower dietary Cu concentrations, in non-ruminant species there is a lag phase in which limited changes in liver Cu concentration occur with increasing Cu intakes (EFSA Panel 2016; Hill & Shannon 2019). In monogastric species Cu only begins to accumulate in the liver after intake of high amounts of Cu, more than 25 to 50 times their requirements (NRC 2005; López-Alonso & Miranda 2020). At this critical stage liver Cu concentrations increase dramatically, e.g. in pigs at a dietary Cu concentration of approximately 150 mg/kg and in hens at 220 mg/kg DM (López-Alonso & Miranda 2020). Using results from 12 studies on the deposition of Cu in the liver of pigs, the EFSA Panel (2016) fitted a polynomial regression curve (adjusted R2 = 0.67) between dietary Cu levels (above requirements) and liver Cu concentrations. At low dietary Cu levels the concentration of Cu in the liver did not increase appreciably but started to increase at between 100 to 150 mg Cu/kg diet. This is utilised in the practice of using high levels of dietary Cu (> 150 mg Cu/kg feed) as a growth stimulant in growing pigs and broilers (NRC 2005).
It can be concluded that in domestic ruminants, liver Cu concentration would give the most reliable indication of Cu nutritional status of the animal, at least in the adequate range, while in non-ruminants liver Cu concentration would remain relatively constant over a wide range, covering Cu intakes from adequacy to more than 25 times requirements.
Differences in metabolic processes
According to López-Alonso and Miranda (2020) differences in the intracellular distribution of Cu in sheep, cattle and other ruminant species that are less susceptible to Cu toxicity may be explained by differences in metallothionein-synthesising capacity in ruminant species. This capacity is affected by the Zn status of the animal (Lopez-Alonso et al. 2005b; 2017). Metallothionein is suggested to play a major role in Cu excretion through the bile. It is well established that cattle and sheep differ in metabolising high levels of Cu in the liver, and that sheep are much more susceptible to Cu toxicity than cattle because cattle have a much better ability than sheep to transfer Cu from the liver through the bile to the digestive system, resulting in a decrease in elevated liver Cu concentrations (López-Alonso & Miranda 2020). According to Clarkson et al. (2020) the metallothionein transcription in liver lysosomes of cattle and sheep does not effectively respond to rapid increases in Cu in comparison with single-stomached species such as pigs. In monogastric species most of the hepatic Cu is bound to metallothionein, allowing for biliary excretion. Clarkson et al. (2020) demonstrated this by referring to results on the metallothionein concentrations in the livers of different animal species, published by Henry et al. (1994). Henry et al. (1994) reported that the livers of pigs and dogs contain from 500 to 600 mg metallothionein/kg and the livers of cattle and sheep about 200 mg metallothionein/ kg. According to Clarkson et al. (2020) this difference could be a possible reason why pigs and dogs have a much better ability than cattle and sheep to tolerate high Cu intakes. However, Henry et al. (1994) also recorded a hepatic metallothionein concentration in goats of 600 mg/kg, similar to that of the Cu-tolerant monogastric species. It is well-documented that goats can tolerate high dietary levels of Cu (Huang et al. 2013) though Cu accumulation in the liver in goats does not seem to have a lag phase with increasing Cu intakes to the extent observed in monogastric species (Zervas et al. 1990; Luginbuhl et al. 2000; Solaiman et al. 2001; 2006; Huang et al. 2013). A possible reason for this difference could be that the metallothionein synthesised in pigs differs from that in Cu-loaded sheep, as reported by Mehra and Bremner (1984).
Copper deficiency signs in ruminants
Suttle (2022) pointed out that a clinical diagnosis of hypocuprosis depends on the presence of clinical signs or substantial loss of production, biochemical evidence of subnormal liver and/or blood Cu and improvement after treatment with Cu compared with a control.
Typical clinical signs of Cu deficiency are enzootic ataxia (swayback) in newborn animals, defective keratinisation of wool (steely wool) and hair, and depigmentation (achromotrichia) starting around the eyes (goggled or spectacled face) (Underwood & Suttle 1999). According to the EFSA Panel (2016) in cattle and sheep breeds with highly pigmented coats, loss of coat colour is often the earliest and sometimes the only clinical sign of Cu deprivation (Hill & Shannon 2019). Changes in hair colour occurred 167 days after induced Cu deficiency. However, Suttle (2022) pointed out that loss of coat colour has also been recorded in cattle deficient in some vitamins as well as a cobalt deficiency.
More general and non-specific signs are increased susceptibility to infection (Woolliams et al. 1986) and signs of poor growth and health resulting in morbidity and mortality, signs related to the antioxidant function of Cu in the form of superoxide dismutase in the body. Other clinical signs are anaemia, reduced reproductive capacity and scouring and diarrhoea, some of which are associated with Cu deficiency owing to Mo toxicity (Underwood & Suttle 1999). Osteochondrosis (bone deformities with a frequent occurrence of the symptoms of "bunny-hopping gait" and "cow-hocked" stance) is well recognised as a Cu deficiency syndrome in deer and horses (Thompson et al. 1994; Wilson & Grace 2001).
In southern Africa the depigmentation of coat colour has been recorded in game species with brown coats, resulting in yellow-coated individuals. With a high demand for different coloured individuals within species, yellow individuals have been sold at exorbitant prices only to discover that after a short period of time on a different farm, the coats returned to their normal colours. The nutrition laboratory at the University of Pretoria (UP Nutrilab) analysed the serum from six yellow roan antelopes (Hippotragus equinus). The serum of five of the group contained 0.23 μg Cu/ml and one 0.78 μg Cu/ml (unpublished data). Since a concentration of < 0.6 mg/ml serum is considered Cu deficient (Suttle 1983), these results suggested that, except for one individual, the antelope were probably Cu deficient and not genetically yellow-coated animals.
Cu concentrations in hair and fleece samples could be used to diagnose Cu deficiency in a grossly decomposed carcass. According to Underwood and Suttle (1999) Cu concentrations of 2-4 mg/kg DM in sheep wool; 4-8 mg/kg DM in cattle hair; 6-9 mg/kg DM in deer hair and 3-5 mg/kg DM in goat hair would suggest a Cu deficiency.
Chronic copper poisoning (CCP) signs in ruminants
At high intakes, Cu is gradually deposited primarily in the liver during the prehaemolytic phase without producing any significant clinical signs, that is, the animal appears clinically normal. This phase is characterised by Cu accumulation in liver (up to 1 500 mg/kg DM), though at an approaching crisis increased liver enzyme (glutamate dehydrogenase [GLDH], gamma-glutamyltransferase [GGT] and aspartate aminotransferase [AST]) activities are measured in serum (López-Alonso & Miranda 2020). Only when the liver's capacity to accumulate CU is overloaded may the haemolytic phase occur, expressed as the haemolytic crisis. This crisis is usually triggered by a stressful event of any nature, such as starvation, changes in the environment, transport or extensive handling. This results in hepatocellular necrosis and liberation of Cu into the bloodstream, producing intense haemolysis, jaundice and substantial liver pathology and nephrosis (Underwood & Suttle 1999; Minervino et al. 2009). Usually only a proportion of a herd or flock dies during or shortly after a haemolytic crisis (Howell & Gooneratne 1987). Some animals may survive into a posthaemolytic period. These animals may develop further haemolytic crises even when the source of Cu has been removed (Howell & Gooneratne 1987; Herdt & Hoff 2011). Wide variation in liver Cu concentration at the same Cu intake may explain the low proportion of deaths.
During the prehaemolytic stage, the Cu concentrations of the liver and kidney are usually elevated (Howell & Gooneratne 1987). Ross (1964) stated that while the liver gives a reliable indication of Cu status of a sheep, Cu concentration in the kidney cortex is a good criterion for diagnosing Cu poisoning. The Fe concentration in the kidney during this period stays normal or slightly elevated, and markedly elevated during and subsequent to haemolysis, and according to Howell and Gooneratne (1987) is the best indicator of a haemolytic crisis and previous haemolyses. In a Cu toxicity study on sheep, Howell and Gooneratne (1987) recorded kidney Fe concentrations of 102 mg Fe/kg DM in the control, 150 mg Fe/kg DM in the prehaemolytic stage, but 1 488 mg Fe/kg DM during the haemolytic stage and 1 957 mg Fe/kg DM at the posthaemolytic stage. Similar elevated Fe concentrations have been reported by Ishmael et al. (1972), Gopinath and Howell (1975), Bath (1979) and Van Ryssen and Barrowman (1988) in sheep, and Jenkins and Hidiroglou (1988) in a study on calves receiving a milk substitute high in Cu. Elevated concentrations of Fe in the kidneys and liver can be used as confirmation that the animal has experienced a haemolytic crisis. Normal Fe concentrations in the liver are between 161 and 1 070 mg/kg DM (Puls 1994).
Increased Zn concentrations have been recorded in the livers and kidneys of sheep (Ishmael et al. 1972) and calves (Jenkins & Hidiroglou 1988) that died from CCP. In a study on Cu toxicosis in cattle and buffalo, Minervino et al. (2009) recorded high positive relationships in both species between hepatic Cu and hepatic Zn concentrations, evident at > 1 000 mg Cu/kg DM. This might be associated with the redistribution of Zn in the body under conditions of stress which causes a transient reduction of the Zn concentration in serum and an increase in the concentration of Zn in the liver (Bremner & Beattie 1995), inducing hepatic metallothionein synthesis (Cousins 1996).
CU metabolism in ruminants: breed differences
Orkney sheep from the Scottish island of North Ronaldsay are particularly susceptible to Cu poisoning and suffer from Cu toxicity when consuming a normal sheep diet. In their native habitat, the breed subsists predominantly on a diet of seaweed low in Cu (Wiener et al. 1978). Extensive research has been conducted on genetic differences among sheep breeds in absorbing Cu, and thus in proneness to Cu poisoning. Woolliams et al. (1982) demonstrated how five British sheep breeds (Scottish Blackface ewes crossed with rams from different breeds) differed in capacity to accumulate Cu in the liver in a Cu toxicity evaluation study; after 13 weeks on a diet containing 20 mg Cu/kg, liver Cu concentrations (mg Cu/kg DM) were: Blackface, 567; East Friesland, 754; Finish Landrace, 767; Suffolk, 1 116; Texel, 1 492. It was concluded that the breeds differed in efficiency of absorbing Cu from the intestines. Breeds with a low efficiency of retention would prove the most resistant to Cu poisoning on a given diet. However, from the perspective of threshold values indicating Cu toxicity, Woolliams et al. (1982) emphasised there is no evidence that these sheep breeds differ in the threshold of liver and kidney cortex Cu concentrations at which the onset of signs of Cu poisoning occurred.
Breed differences among cattle breeds have become more apparent lately, for instance the Jersey breed is more susceptible to Cu toxicosis than the Friesland (Spears et al. 2022). According to López-Alonso and Miranda (2020) there seem to be differences among cattle breeds in efficiency of absorbing Cu and biliary excretion of endogenous Cu, and possibly differences in amount of feed taken in.
Although wildlife is not classified into breeds, subspecies or lines have been identified, e.g. four recognised subspecies of sable antelopes (Hippotragus niger) in southern Africa (Shepstone et al. 2022). Furthermore, groups within species are frequently isolated on game farms and reserves where a degree of inbreeding is possible (Shepstone et al. 2022), or where the "founder effect" might have introduced high frequencies of specific genes. It is therefore quite likely that different groups within a species would differ in ability to metabolise Cu. Since stress is a factor triggering a haemolytic crisis, differences in proneness to stress in wildlife species could be reflected in species differences in susceptibility to Cu toxicosis.
Cu metabolism in ruminants: species differences
In an overview on the widespread occurrence of Cu poisoning among sheep in the Karoo region of South Africa, Bath (1979) noted that Cu poisoning apparently did not occur among goats grazing the same pasture as sheep. This observation is supported by Zervas et al. (1990) who demonstrated that at the same level of Cu intake, Cu concentrations in the livers of a Greek sheep breed were six to nine times higher than that in the livers of a native Greek goat breed. Likewise, Huang et al. (2013) recorded that the liver Cu concentration of a Chinese goat breed was 839 mg/kg DM on a diet containing 640 mg Cu/kg DM. However, in New Zealand, cases of Cu poisoning have been reported among Angora goats (Humphries et al. 1987).
In comparing the Cu status of goats, cattle, sheep and different species of deer, Arnhold et al. (1998) recorded that goats displayed Cu deficiency signs at a much lower liver Cu concentration than deer species followed by cattle and sheep. Bartoskewitz et al. (2007) concluded that the white-tailed deer is less susceptible to Cu poisoning than sheep, while Wilson and Grace (2001) suggested that differences in Cu metabolism exist within deer species and that it is desirable to establish reference ranges specific to different deer species and even sub-species. A problem with such comparisons in game is that sources of dietary Cu are not necessarily comparable between species, because free-ranging species differ substantially in plants they select, e.g. between grazers and browsers, and thus in their dietary exposure to Cu (Quan 2001). Minervino et al. (2009) compared the development of CCP in a mixed breed of cattle with the Murrah buffalo breed (assuming the water buffalo) in Brazil. They concluded that buffaloes and cattle might be equally susceptible to CCP, but that although the buffalo accumulated less liver Cu than cattle, they have a lower threshold of hepatic Cu accumulation that leads to the clinical manifestation of CCP. Van Saun (2009; 2012) reported that two camelid species, the llama and alpaca (pseudo-ruminants with three-chambered stomachs [Dehority 2002]), are prone to Cu poisoning when fed diets containing more than 20 mg total Cu/kg and at a high Cu : Mo ratio of 16 : 1. In the dietary guidelines for animals in zoos, it is also stated that sheep and llamas are extremely susceptible to Cu toxicosis (Lintzenich & Ward 1997).
Based on evidence from domestic ruminants, it can be deduced that, regarding tolerance to Cu toxicity in wild ruminants, differences among species are likely to be substantial considering that there are 78 species of extant Bovidae (excluding sheep and cattle) in Africa (Gagnon & Chew 2000).
Cu metabolism in different non-ruminant species
According to NRC (2005) horses and rabbits are more resistant to Cu toxicosis than pigs or poultry, and rats can tolerant high levels of dietary Cu. It is well documented that horses can tolerate high levels of dietary Cu (Smith et al. 1975; Belli et al. 2021) though Belli et al. (2021) reported cases of Cu poisoning in horses in Brazil. Smith et al. (1975) recorded an average liver Cu concentration of 3 870 mg/kg DM in ponies receiving a diet containing 791 mg Cu/kg for 183 days, with no visible ill-effects. Suttle et al. (1996) concluded that a liver Cu concentration for horses of 20 mg/kg DM indicates an adequate Cu status, though Suttle (2022) published the normal range for liver Cu concentration for horses to be 6.4-12.8 mg/kg DM as compared with 14-27 mg/kg DM (Table II) as suggested by Puls (1994). The normal range for plasma Cu concentration for horses is 0.5-0.8 mg/l (Suttle 2022).
We suggest that wild hindgut fermenters and monogastric species of southern Africa are likely to tolerate high dietary Cu levels, similar to the horse and domestic pig, respectively. In comparing the serum/plasma and liver Cu concentrations in rhinoceros species that died in captivity, viz. the black and white rhinos and the Indian and Sumatran rhinos, Dierenfeld et al. (2005) based their evaluation on "horse normal ranges", as published by Puls (1994). Liver samples were collected at necropsy of the animals that died in zoos. Although statistical analyses would not be valid, the livers of the grazer species, the Indian and white rhinos, contained dramatically more Cu (170 ± 296 and 83 ± 88 mg/kg ww, respectively) than those of the browsers, Black and Sumatran rhinos (6 ± 4 and 5 ± 0 mg/kg ww, respectively). The high Cu concentrations in the livers of the two grazer species compared with the browsers might suggest that they consumed excessive amounts of Cu.
Hepatic copper concentration and published reference ranges for ruminants
Since liver Cu concentration is a storage criterion (Suttle 1986; 1994), it is widely used to assess the Cu nutritional status of ruminants. However, Suttle (1994) pointed out that the conventional indices of Cu status in the animal give only an approximate measure of dietary adequacy, and he stated that sufficiency ranges are based on "accumulated wisdom" with a loose association between mineral status and animal health (Suttle 1988). López Alonso et al. (2000) also stated that there is no clear consensus as to what tissue concentrations of Cu indicate deficiency in cattle, and according to Strickland et al. (2019) Cu status in cattle is ill-defined because critical points or ranges often differ substantially between references.
The ranges of Cu concentrations in the livers of different domestic animal species as published by Puls (1994) are presented in Table II. Values have been converted to a liver DM basis, assuming that a normal healthy bovine liver contains ca. 28% DM (Van Ryssen et al. 2023). However, the livers of sheep that succumbed to Cu poisoning contain ca. 23% DM (Van Ryssen et al. 2023). Relatively wide ranges have been published, often making it difficult to attach a specific Cu nutritional status when interpreting laboratory results. Researchers have attempted to narrow down these values to more specific reference ranges and threshold values. Although "status" in Table II does not refer to specific dietary Cu concentrations, liver Cu concentrations in the table suggest linear increases with increasing Cu intake, also in pigs but not in the case of the horse. However, contrary to the values given for non-ruminants in Table II, the NRC (2005) stated that liver Cu concentrations normally found in most non-ruminants would be indicative of a Cu deficiency in ruminants.
Kendall et al. (2015) based their criteria to define Cu status according to the University of Wisconsin Veterinary Diagnostic Laboratory (WVDL 2015) standards. These levels seem to be adapted from Puls' criteria. Concentrations (converted from umol/kg DM) of < 90 mg Cu/kg DM are considered to indicate a deficient condition (including the marginal range) and 90 to 719 mg/kg DM as adequate. The toxic range is from 899 to 2 877 mg/kg DM. However, the authors also considered > 800 umol/kg DM (> 512 mg/kg DM) as the upper limit of the normal reference range. Likewise, López-Alonso et al. (2005a; 2017) and López-Alonso & Miranda (2020) quoted the normal range for cattle as 25-100 mg Cu/kg ww (89-357 mg Cu/kg DM) and a projected total liver Cu concentration of 450 mg/kg ww (1 607 mg/kg DM) at which progressive change in Cu distribution in the liver would indicate saturation of fractions of the liver cells. Strickland et al. (2019) quoted the reference ranges of the Michigan State University Veterinary Diagnostic Laboratory Lansing, Michigan, USA for dairy cows, considering 40-650 mg Cu/kg DM as adequate. Counotte et al. (2019) used a toxicological threshold for liver Cu concentration in bovine calves and older animals as > 1 000 mg/kg DM, while the NRC (2005) also stated that clinical signs of Cu toxicity in sheep do not usually occur until liver concentrations are > 1 000 mg/kg DM.
However, in many other studies the reference ranges differ substantially from those of Puls (1994) and those based on the Puls' standards. Suttle (1986) suggested a marginal range for liver Cu concentration in sheep and cattle to be 5.1-20.5 mg/ kg DM, the normal range for the kidney cortex to be 13-19 mg/ kg DM and at > 26 mg/kg DM the likelihood of toxicity. Arnhold et al. (1998) reported that the liver Cu concentration limits at which Cu deficiency signs occur are < 20 mg/kg DM for roe deer, < 15 mg for red deer and aoudads, < 10 mg for sika deer and < 8 mg for domestic goats compared to < 35 mg/kg for cattle and sheep. Grace and Clark (1991) suggested as guidelines for diagnosing the Cu status of sheep and cattle in New Zealand: sheep are Cu deficient at < 21.7 mg/kg liver DM and normal at > 21.7 mg/kg DM, while cattle are deficient at a liver Cu concentration of < 10.3 mg/kg DM and sufficient at > 21.7 mg/kg DM. For red deer in New Zealand, Wilson and Grace (2001) considered a deficient range at < 13.7 mg/kg DM, marginal at 13.7-23 mg/kg DM and adequate at > 23 mg/ kg DM. According to Suttle (2022) marginal bands for liver Cu concentrations for sheep and cattle are 6.4-19.2 mg/kg DM; and for deer and goats, 11.5-19.2 mg/kg DM (converted from μmol/kg DM).
Strickland et al. (2019) pointed out that in cattle the range of potentially dangerous hepatic Cu reserves is ill-defined but
is suggested to be between 300 and 500 mg/kg DM. At the haemolytic crisis stage of Cu toxicosis, liver Cu concentrations of from 1 100 to 6 530 mg/kg DM have been recorded. According to the NRC (2005) sheep show histological and biochemical evidence of liver damage at Cu levels as low as 350 mg/kg DM though clinical signs of toxicosis usually occur at liver Cu concentrations of > 1 000 mg/kg DM. When CU concentrations exceed liver storage and export capacity, "free Cu" is more likely to occur. Free Cu has a prooxidant potential and in excess can lead to increased oxidative stress causing oxidative damage to hepatocellular lipids, proteins and DNA (Strickland et al. 2019).
Liver Cu concentrations of impala that were exposed to Cu pollution in the vicinity of the open-cast Cu mine close to the Kruger National Park provides valuable information on hepatic Cu concentrations in this game species. Gummow et al. (1991) measured liver Cu concentrations of 574 and 665 mg Cu/kg DM (assuming their livers contained 23% DM [Van Ryssen et al. 2023] plus probably some Cu depletion during the haemolytic crisis [Howell & Gooneratne 1987]) and elevated kidney Cu concentrations in two impala that were suggested to have died from Cu poisoning in the Cu-polluted area of the Kruger National Park, while the average Cu concentration in the impala shot in this high risk area was 810 ± 345 mg/kg DM.
Copper concentrations in serum/plasma in ruminants
Serum/plasma Cu concentration (60-70%) represents a functional pool (largely as caeruloplasmin, exporting Cu from the liver to tissues) and a transport pool (containing Cu in albumin- and amino acid-bound forms, transporting Cu from the intestine to the liver) (Herdt & Hoff 2011; EFSA Panel 2016). Some ceruloplasmin is lost during clotting, resulting in serum values at about 10% to 30% lower than the corresponding plasma values (Suttle 1994; EFSA Panel 2016; Luna et al. 2019). Consequently, Spears et al. (2022) suggested that for diagnostic purposes Cu concentrations should be measured in plasma rather than in serum.
López Alonso et al. (2006) and Clarkson et al. (2020) emphasised that neither serum Cu nor caeruloplasmin is significantly associated with hepatic Cu concentration. When hepatic stores are adequate, blood plasma/serum Cu concentrations remain relatively constant at between 0.6 and 1.5 mg/l and do not decline until absorption is less than minimum requirements and reserves in the liver are approaching exhaustion (Herdt & Hoff 2011). Therefore, a plasma Cu concentration of < 0.6 mg/l is a good indication of the onset of hypocupraemia in livestock (Suttle 1983), or marginal ranges for deer and goats at 0.32-0.51 mg/l (Suttle 2022). Herdt and Hoff (2011) stated that low blood (serum or plasma) concentrations are generally not observed until liver concentrations in cattle decrease to less than approximately 25 mg/kg DM (or 20 mg/kg DM as quoted by Spears et al. 2022). Mitscherlich relationships between plasma Cu and liver Cu concentrations, with plasma concentration plateauing at ca. > 0.6 mg/l, have been demonstrated for cattle (Claypool et al. 1975), sheep (Suttle 1994) and deer (Grace & Wilson 2002), though individual values scattered widely around the means.
Certain situations cause elevations in plasma/serum concentrations: i) As an acute-phase protein, caeruloplasmin concentrations in the plasma are elevated with the occurrence of various inflammatory and infection conditions in the body (Suttle 1994; López-Alonso et al. 2006; Hepburn et al. 2009). This is an important reason to avoid analysing tissue Cu concentrations of sick animals to obtain an indication of Cu status of the animal (Van Ryssen et al. 2023): ii) When Cu storage in the liver is overloaded and develops into a haemolytic crisis of Cu toxicosis, a sudden release of Cu in the blood occurs, causing transient high plasma/serum Cu concentrations (Howell & Gooneratne 1987; López Alonso et al. 2006), and (iii) If the molybdenum plus sulphate intake of an animal is high, complexes containing Cu thiomolybdates are present in tissues and plasma, resulting in elevated plasma Cu concentrations (Clarkson et al. 2020). The Cu in this complex, the so-called "systemic effect" of this interaction, is unavailable to the animal and plasma Cu concentration does not reflect a real Cu status except if direct reacting Cu (10% TCA soluble Cu) content is measured (Van Ryssen & Stielau 1981).
Conclusion: Proposed guidelines for wild ruminants in southern Africa
Considerations
Since limited information is available on the Cu nutritional status of wild Bovidae in southern Africa, assumptions have to be made in order to suggest reference ranges indicating their Cu nutritional status. Considering that there are 78 species of extant African Bovidae (Gagnon & Chew 2000) it is accepted that wide variations would probably exist among species and even within species. This suggests that rigid threshold values indicating deficiencies or toxicities would not be valid.
From existing data it is assumed that liver Cu concentrations in ruminant ungulates would follow a linear increase with increasing Cu intakes, at least within the adequate range. Liver Cu concentrations should therefore be a fairly reliable measure of the Cu nutritional status of the animal and the presence of consumed sources of Cu in a region.
The following cases of Cu nutritional status among game have been recorded:
• The serum of two sable antelope bulls from Hoedspruit displaying faded coat colours, contained 0.21 and 0.43 μg Cu/ ml (UP Nutrilab).
• Dierenfeld et al. (2002) quoted the Cu concentration in plasma/serum of a number of free ranging and captive-held duiker species, to be 0.7-1.2 and 0.6-1.2 μg/ml, respectively. These levels correspond well with concentrations in cattle and sheep.
• Penrith et al. (1996) reported swayback in a black wildebeest (Connochaetes gnou) and swayback and faded coat colour in a blesbok (Damaliscus pygargus phillipsi) from the Karoo Nature Reserve near the town of Graaff-Reinet. The Cu concentrations in the livers of the two affected animals were 3.0 mg/kg ww (10.7 mg/kg DM, assuming 28% DM in livers) compared to Cu concentrations in unaffected blesbok (n = 6) of 21.6 mg/kg ww (77.1 mg/kg DM), and other unaffected species (n = 5) of 21.06 mg/kg ww (75.2 mg/kg DM).
• In an investigation into a possible Cu deficiency in blesbok at the same location as that reported by Penrith et al. (1996), Quan (2001) recorded an average liver Cu concentration of 16.1 mg/kg DM in unhealthy blesbok compared to 178 and 97 mg/kg DM in the livers of blesbok in two separate game reserves in the Free State province. Plasma Cu concentrations were 0.07, 1.64 and 1.41 mg/l, respectively.
• Van Ryssen (2006) recorded a black wildebeest and a red hartebeest (Alcelaphus buselaphus) shot in the Vrede vicinity (eastern Free State province), with liver Cu concentrations of 14 mg/kg DM.
• Gummow et al. (1991) recorded liver Cu concentrations ranging from 461 to 1 586 (average 810) mg/kg DM in impala exposed to Cu pollution in the Kruger National Park.
• UP Nutrilab recorded the liver Cu concentrations in a young roan antelope and a young Sable antelope at 855 mg/kg DM and 820 mg/kg DM, respectively. This suggests that these highly valued animals might have taken in excess Cu through supplements.
Proposed reference ranges
Serum/plasma concentrations
A plasma/serum concentration of < 0.6 mg Cu/l can be used to indicate deficiency.
In the case of excessive Cu intake and high liver Cu concentrations during the prehaemolytic stage, plasma Cu concentrations would remain unchanged at between 0.6 and 1.5 mg Cu/l. However, increased liver enzyme (GLDH, GGT and AST) activities at this stage would indicate a pending haemolytic crisis. Considering the wide variation in the relationship between liver and plasma Cu concentrations (Claypool et al. 1975; Suttle 1994), it is considered unnecessary to allow for the difference between serum and plasma Cu concentrations in this recommendation for ungulates.
Hepatic Cu concentrations
It is proposed that liver Cu concentrations of < 20 mg Cu/kg DM indicate a potentially marginal to acute Cu deficiency, suggesting that the animal would respond to Cu supplementation.
Liver Cu concentrations of between 20 and 300 mg Cu/kg DM indicate an adequate and healthy Cu nutritional status.
Liver Cu concentrations of 300 to 500 mg/kg DM might not indicate a pending haemolytic crisis but are undesirable and potentially unhealthy because of the possibility of increased hepatic oxidative stress evident in hepatocellular lipids, proteins and DNA.
Liver Cu concentrations of > 500 mg/kg DM indicate that the animals are probably consuming more Cu than required and are at risk of developing Cu toxicosis.
Kidney cortex
A Cu concentration of the kidney cortex of 13-19 mg/kg DM can be considered as normal, > 19 mg/kg DM as abnormal, and < 13 mg Cu/kg DM as possibly a deficiency (Suttle 1986).
It should be noted that Puls (1994) stated in a foreword in his book that all kidney values in his tables refer to analyses done on the kidney cortex. Suttle (2022) also presented mineral concentrations of the kidney cortex. Cu concentrations in the kidney cortex of sheep were found to be three time higher than in the kidney medulla (Van Ryssen 2016).
Final remarks
It should be stressed that many other signs are associated with Cu poisoning and deficiency, making bodily tissue and fluid analyses only one of the tools to be used to diagnose an ill-health situation.
Conflict of interest
The authors declare no conflict of interest.
Funding source
No funding was required.
Ethical approvement
The author/s declare that this submission is in accordance with the principles laid down by the Responsible Research Publication Position Statements as developed at the 2nd World Conference on Research lntegrity in Singapore, 2010.
ORCID
JBJ van Ryssen https://orcid.org/0000-0002-9603-1869
GF Bath https://orcid.org/0000-0002-9496-2176
References
Ackerman, DJ., Reinecke, A.J., Els, H.J., et al., 1999, Sperm abnormalities associated with high copper levels in impala (Aepyceros melampus) in the Kruger National Park, South Africa, Ecotoxicol Environ Safety 43(3), 261-266. https://doi.org/10.1006/eesa.1999.1787. [ Links ]
Arnhold, W., Anke, M., Glei, M., et al., 1998, Determination of copper status in ruminants, Trace Elements and Electrolytes 15(2), 65-69. [ Links ]
Bartoskewitz, M.L., Hewitt, D.G., Laurenz, J.C., et al., 2007, Effect of dietary copper and zinc concentrations on white-tailed deer antler growth, body size, and immune system function, Small Rumin Res 73(1), 87-94. https://doi.org/10.1016/j.smallrumres.2006.11.005. [ Links ]
Bath, G.F., 1979, Enzootic icterus - A form of chronic copper poisoning, J S Afr Vet Ass 50(1), 3-14. [ Links ]
Belli, C.B., Fernandes, W.R., Torres, L.N., et al., 2021, Copper toxicity in horses: Does it exist? J Equine Vet Sci 106, 103752. https://doi.org/10.1016/j.jevs.2021.103752. [ Links ]
Boyazoglu, P.A., 1973, Mineral imbalances of ruminants in Southern Africa, S Afr J Anim Sci 3, 149-152. [ Links ]
Bracewell, C.D., 1958, A note of jaundice in housed sheep, Vet Rec 70, 342-344. [ Links ]
Bremner, I., Beattie, J.H., 1995, Copper and zinc metabolism in health and disease: speciation and interactions, Proc Nutr Soc 54, 489-499. https://doi.org/10.1079/PNS19950017. [ Links ]
Clarkson, A.H., Paine, S., Martin-Tereso, J., et al., 2020, Copper physiology in ruminants: trafficking of systemic copper, adaptations to variation in nutritional supply and thiomolybdate challenge, Nutr Res Rev 33(1), 43-49. https://doi.org/10.1017/S0954422419000180. [ Links ]
Claypool, D.W., Adams, F.W., Pendell, H.W., et al., 1975, Relationship between the level of copper in the blood plasma and liver of cattle, J Anim Sci 41(3), 911-914. https://doi.org/10.2527/jas1975.413911x. [ Links ]
Counotte, G., Holzhauer, M., Carp-van Dijken, S., 2019, Levels of trace elements and potential toxic elements in bovine livers: A trend analysis from 2007 to 2018, PLoS One 14(4), e0214584. https://doi.org/10.1371/journal.pone.0214584. [ Links ]
Cousins, R.J., 1996, Zinc. In: Present Knowledge in Nutrition. Ed. Filler, L.J., Ziegler, E.E., 7th edn International Life Science Institute-Nutrition Foundation, Washington, DC. (cited by Suttle 2022 p. 417). [ Links ]
Dehority, B.A., 2002, Gastrointestinal tracts of herbivores, particularly the ruminant: Anatomy, physiology and microbial digestion of plants, J Appl Anim Res 21(2), 145-160. https://doi.org/10.1080/09712119.2002.9706367. [ Links ]
Dick, A.T., 1954, Studies on the assimilation and storage of copper in crossbred sheep, Austr J Agric Res 5(3), 511-544. https://doi.org/10.1071/AR9540511. [ Links ]
Dierenfeld, E.S., Mueller, P.J., Hall, M.B., 2002, Native food composition, micronutrient assessment, and implications for improving captive diets, Zoo Biol 21, 185-196. https://doi.org/10.1002/zoo.10037. [ Links ]
Dierenfeld, E.S., Atkinson, S., Craig, A.M., et al., 2005, Mineral concentrations in serum/plasma and liver tissue of captive and free-ranging rhinoceros species, Zoo Biol 24, 51-72. https://doi.org/10.1002/zoo.20043. [ Links ]
EFSA Panel (European Food Safety Authority Panel on Additives and Products or Substances used in Animal Feed)., 2016, Scientific opinion on the revision of the currently authorised maximum copper content in complete feed, EFSA J 14(8), e4563 100. https://doi.org/10.2903/j.efsa.2016.4563. [ Links ]
Fraser, A.J., 1982, Production related reference ranges. In: Laboratory diagnosis of trace element efficiency diseases. Surveillance 9 (Special issue), 4-7. [ Links ]
Gagnon, M., Chew, A.E., 2000, Dietary preferences in extant African Bovidae, J Mamm 81(2), 490-511. https://doi.org/10.1644/1545-1542(2000)081<0490:DPIEAB>2.0.CO;2. [ Links ]
Gopinath, C., Howell, J.McC., 1975, Experimental chronic copper toxicity in sheep. Changes that follow the cessation of dosing at the onset of haemolysis, Res Vet Sci 19(1), 35-43. https://doi.org/10.1016/S0034-5288(18)33551-3. [ Links ]
Grace, N.D., Clark, R.G., 1991, Trace element requirements, diagnosis and prevention of deficiencies in sheep and cattle. Physiology aspects of digestion and metabolism in ruminants. Proc Seventh Int Symp Ruminant Physiology Eds Tsuda, T., Sasaki, Y., Kawashima, R., pp. 321-341. Academic Press Inc., Tokyo. https://doi.org/10.1016/B978-0-12-702290-1.50022-9. [ Links ]
Grace, N.D., Wilson, P.R., 2002, Trace element metabolism, dietary requirement, diagnosis and prevention of deficiencies in deer, New Zealand Vet J 50(6), 252-259. https://doi.org/10.1080/00480169.2002.36321. [ Links ]
Grobler, D.G., Swan, G.E., 1999, Copper poisoning in the Kruger National Park: Field investigation in wild ruminants, Onderstepoort J Vet Res 86, 157-168. [ Links ]
Gummow, B., Botha, C.J., Basson, A.T., et al., 1991, Copper toxicity in ruminants: Air pollution as a possible cause, Onderstepoort J Vet Res 58(1), 33-39. [ Links ]
Hemingway, R.G., MacPherson, A., 1967, The accumulation of copper in the liver of housed Blackface lambs, Vet Rec 81, 695-696. [ Links ]
Henry, R.B., Liu, J., Choudhuri, S., et al., 1994, Species variation in hepatic metallothionein, Toxicol Lett 74(1), 23-33. https://doi.org/10.1016/0378-4274(94)90071-X. [ Links ]
Hepburn, J.J., Arthington, J.D., Hansen, S.L., et al., 2009, Technical note: Copper chaperone for copper, zinc superoxide dismutase: A potential biomarker for copper status in cattle, J Anim Sci 87(12), 4161-4166. https://doi.org/10.2527/jas.2009-1978. [ Links ]
Herdt, T.H., Hoff, B., 2011, The use of blood analysis to evaluate trace mineral status in ruminant livestock, Veterinary Clinics: Food Animal Practice 27(2), 255-283. https://doi.org/10.1016/j.cvfa.2011.02.004. [ Links ]
Hill, G.M., Shannon, M.C., 2019, Copper and zinc nutrition issues for agricultural animal production, Biol Trace Elem Res 188, 148-159. https://doi.org/10.1007/s12011-018-1578-5. [ Links ]
Howell, J.McM., Gooneratne, S.R. 1987. The pathology of copper toxicity in animals. Ch 9 in: Copper in Animal and Man. Ed. Howell, J.McM., Gawthorne, J.M., 1987 Volume II, pp. 71-78, CRC Press Inc. Boca Raton, Florida, USA. [ Links ]
Huang, Y.L., Wang, Y., Spears, J.W., et al., 2013, Effect of copper on performance, carcass characteristics, and muscle fatty acid composition of meat goat kids, J Anim Sci 91(10), 5004-5010. https://doi.org/10.2527/jas.2012-5820. [ Links ]
Humphries, W.R., Morrice., Mitchell, A.N., 1987, Copper poisoning in Angora goats, Vet Rec 121, 231. [ Links ]
Ishmael, J., Gopinath, C., Howell, J.McC., 1972, Experimental chronic copper toxicity in sheep. Biochemical and haematological studies during the development of lesions in the liver, Res Vet Sci 13, 22-29. https://doi.org/10.1016/S0034-5288(18)34084-0. [ Links ]
Jenkins, K.J., Hidiroglou, M., 1988, Tolerance of the calf excess copper in milk replacer, J Dairy Sci 72(1), 150-156. https://doi.org/10.3168/jds.S0022-0302(89)79090-1. [ Links ]
Kendall, N.R., Holmes-Pavord, H.R., Bone, P.A., et al., 2015, Liver copper concentration in cull cattle in the UK: are cattle being copper loaded, Vet Rec. https://doi.org/10.1136/vr.103078. [ Links ]
Kincaid, R.L., 1999, Assessment of trace mineral status of ruminants: A review, Proc Am Soc Anim Sci 77, 1-10. https://doi.org/10.2527/jas2000.77E-Suppl1x. [ Links ]
Kowalczyk, T., Pope, A.L., Sorensen, D.K., 1962, Chronic copper poisoning in sheep resulting from free-choice, trace-mineral salt ingestion, J Am Vet Med Assoc 141, 362-366. [ Links ]
Lintzenich, B.A., Ward, A.M., 1997, Hay and pellets: Considerations in feeding ungulates. Nutrition Advisory Group, Fact sheet 006. (Google Scholar). [ Links ]
López-Alonso, M., Miranda, M., 2020, Copper supplementation, a challenge in cattle, Animals 10(10), 1890. https://doi.org/10.3390/ani10101890. [ Links ]
López-Alonso, M., Benedito, J.L., Miranda, M., et al., 2000, Arsenic, cadmium, lead, copper and zinc in cattle from Galicia, NW Spain, Sci Tot Environ 246(2-3), 237-248. https://doi.org/10.1016/S0048-9697(99)00461-1. [ Links ]
López-Alonso, M., Prieto, F., Miranda, M., et al., 2005a, The role of metallothionein and zinc in hepatic copper accumulation in cattle, Vet J 169(2), 262-267. https://doi.org/10.1016/j.tvjl.2004.01.019. [ Links ]
López-Alonso, M., Prieto, F., Miranda, M., et al., 2005b, Intercellular distribution of copper and zinc in the liver of copper-exposed cattle from northwest Spain, Vet J 170(3), 332-338. https://doi.org/10.1016/j.tvjl.2004.07.007. [ Links ]
López-Alonso, M., Crespo, A., Miranda, M., et al., 2006, Assessment of some blood parameters as potential markers of hepatic copper accumulation in cattle, J Vet Diagn Invest 18(1), 71-75. https://doi.org/10.1177/104063870601800109. [ Links ]
López-Alonso, M., Carbajales, P., Miranda, M., et al., 2017, Subcellular distribution of hepatic copper in beef cattle receiving high copper supplementation, J Trace Elements Med Biol 42, 111-116. https://doi.org/10.1016/j.jtemb.2017.05.001. [ Links ]
Luginbuhl, J.M., Poore, M.H., Spears, et al., 2000, Effect of dietary copper level on performance and copper status of growing meat goats, Sheep Goat Res J 16(2), 65-71. [ Links ]
Luna, D., López-Alonso, M., Cedeño, Y., et al., 2019, Determination of essential and toxic elements in cattle blood: Serum vs plasma, Animal 9, 465. https://doi.org/10.3390/ani9070465. [ Links ]
McDowell, L.R., Conrad, J.H., Ellis, G.L., 1984, Mineral deficiencies and imbalances, and their diagnosis. Article 3 in Herbivore Nutrition in the Subtropics and Tropics. Eds Gilchrist, F.M.C., Mackie, R.I. 1984. pp. 67-88, The Science Press (Pty) Ltd Craighall, South Africa. ISBN 0 907997 03 1. [ Links ]
Mehra, R.K., Bremner, I., 1984, Species differences in the occurrence of copper-metallothionein in the particulate fractions of the liver of copper-loaded animals, Biochem J 219, 539-546. https://doi.org/10.1042/bj2190539. [ Links ]
Mills, C.F., 1987, Biochemical and physiological indicators of mineral status in animals: Copper, cobalt and zinc, J Anim Sci 65(6), 1702-1711. https://doi.org/10.2527/jas1987.6561702x. [ Links ]
Minervino, A.H.H., Barrêto Junior, R.A., Ferreira, R.N.F., et al., 2009, Clinical observations of cattle and buffalos with experimentally induced chronic copper poisoning, Res Vet Sci 87(3), 473-478. https://doi.org/10.1016/j.rvsc.2009.05.002. [ Links ]
NRC 2005, Copper. Chapter 13 in Mineral Tolerance of Animals. National Research Council (NRC), 2nd revised edition. 2005. pp. 134-153. The National Academies Press, Washington, D.C., USA. [ Links ]
Penrith, M-L, Tustin, R.C., Thornton, D.J., et al., 1996, Swayback in a blesbok (Damaliscus dorcas phillipsi) and a black wildebeest (Connochaetes gnou), J S Afr Vet Ass 67(2), 93-96. [ Links ]
Puls, R., 1994, Mineral levels in animal health. Diagnostic data, (2nd ed.) Sherpa Int., Canada. [ Links ]
Quan, M., 2001, Copper deficiency in blesbok (Damaliscus pygargus phillipsi) from the Karoo Nature Reserve. MSc (Veterinary Science) dissertation, University of Pretoria, Pretoria, South Africa. (Google Scholar). [ Links ]
Ross, D.B., 1964, Chronic copper poisoning in lambs, Vet Rec 76, 875-876. [ Links ]
Schulz, K.C.A., Van der Merwe, P.K., Van Rensburg, P.J.J., et al., 1951, Studies demyelinating diseases of sheep associated with copper deficiency, Onderstepoort J Vet Res 25(2), 35-75. [ Links ]
Shepstone, C.A., Lubout, P.C., Van Zyl, J.H.C., 2022, Horn growth characteristics of sable antelope (Hippotragus niger niger) in South Africa, S Afr J Anim Sci 52(3), 374-382. [ Links ]
Smith, J.D., Jordan, R.M., Nelson, M.L., 1975, Tolerance of ponies to high levels of dietary copper, J Anim Sci 41(6), 1645-1649. https://doi.org/10.2527/jas1975.4161645x. [ Links ]
Solaiman, S.G., Maloney, M.A., Qureshi, M.A., et al., 2001, Effect of high copper supplements on performance, health, plasma copper and enzymes in goats, Small Rumin Res 41(2), 127-139. https://doi.org/10.1016/S0921-4488(01)00213-9. [ Links ]
Solaiman, S.G., Shoemaker, C.E., Jones, W.R., et al., 2006, The effect of high levels of supplemental copper on the serum lipid profile, carcass traits, and carcass composition of goat kids, J Anim Sci 84(1), 171-177. https://doi.org/10.2527/2006.841171x. [ Links ]
Spears, J.W., Brandao, V.L.N., Heldt, J., 2022, Invited review: Assessing trace element status in ruminants, and factors that affect measurements of trace elements, Appl Anim Sci 38, 252-267. https://doi.org/10.15232/aas.2021-02232. [ Links ]
Strickland, J.M., Lyman, D., Sordillo, L.M., et al., 2019, Effects of super nutritional hepatic copper accumulation on hepatocyte health and oxidative stress in dairy cows, Vet Med Int Article 3642954. https://doi.org/10.1155/2019/3642954. [ Links ]
Suttle, N.F., 1983, The nutritional basis for trace element deficiency in ruminant livestock, in Trace elements in animal production and veterinary practice, Occasional Publication No 7 - British Society of Animal Production. Eds Suttle, N.F., Gunn, R.G., Allen, W.M., et al. 1983. https://doi.org/10.1017/S0263967X00030056. [ Links ]
Suttle, N.F., 1986, Problems in the diagnosis and anticipation of trace element deficiencies in grazing livestock, Vet Rec 119(7), 148-152. [ Links ]
Suttle, N.F., 1988, Predicting the risk of mineral deficiencies in grazing animals, S Afr J Anim Sci 18(1), 15-22. http://dx.doi.org/10.4314/sajas.v18iL3. [ Links ]
Suttle, N.F., 1994, Meeting the copper requirements of ruminants. Chapter 9 in Recent Advances in Animal Nutrition. Eds Carnworthy, P.C., Cole, D.J.A., 1994, pp. 173-187, Nottingham University Press, Leicestershire, U.K. [ Links ]
Suttle, N.F., 2022, Mineral Nutrition of Livestock. 5th ed. CABI Publishing, CABI International, Wallingford, Oxon OX10 8DE, UK. [ Links ]
Suttle, N.F., Small, J.N.W., Collins, E.A., et al., 1996, Serum and hepatic copper concentrations used to define normal, marginal and deficient copper status in horses, Equine Vet J 28(6), 497-499. https://doi.org/10.1111/j.2042-3306.1996.tb01624.x. [ Links ]
Taylor, W.A., Child, M.F., Lindsey, P.A., et al., 2021, South Africa's private wildlife ranches protect globally significant populations of wild ungulates, Biodivers Conserv 30, 4111-4135. https://doi.org/10.1007/s10531-021-02294-5. [ Links ]
Thompson, K.G., Audigé, L., Arthur, D.G., et al., 1994, Osteochondrosis associated with copper deficiency in young farmed red deer and wapiti X red deer hybrids, New Zealand Vet J 42(4), 137-143. https://doi.org/10.1080/00480169.1994.35804. [ Links ]
Underwood, E.J., Suttle, N.F., 1999, The mineral nutrition of livestock, 3rd ed. CABI Publishing, CABI International, Wallingford, Oxon OX10 8DE, UK. [ Links ]
Van Deventer, R., 2019, Association analysis of coat colour in Blue Wildebeest (Connochaetes taurinus taurinis), Master of Science thesis, Stellenbosch University, Stellenbosch, South Africa. (Google Scholar) [ Links ]
Van Ryssen, J.B.J., 2006, An evaluation of the trace element nutritional status of grazers in the eastern regions of the Free State and Mpumalanga. SA-ANIM SCI 7, 22-30. http://www.sasas.co.za/Popular/Popular.html [ Links ]
Van Ryssen, J.B.J., 2016, Background information when using bodily fluid and tissue analyses to assess the mineral nutritional status of livestock, RuVASA congress March 2016, Protea Ranch Hotel, Polokwane, South Africa, pp. 80-88. [ Links ]
Van Ryssen, J.B.J., Stielau, WJ., 1980, The influence of dietary sulphur on copper and molybdenum metabolism in sheep, S Afr J Anim Sci 10(1), 49-57. [ Links ]
Van Ryssen, J.B.J., Stielau, W.J., 1981, Effect of different levels of dietary molybdenum on copper and Mo metabolism in sheep fed on high levels of Cu, Br J Nutr 45(1), 203-210. https://doi.org/10.1079/BJN19810092. [ Links ]
Van Ryssen, J.B.J., Barrowman, P.R., 1988, Effect of ionophores on the accumulation of copper in the livers of sheep, Anim Prod 44(2), 255-261. https://doi.org/10.1017/S0003356100018626. [ Links ]
Van Ryssen, J.B.J., Webb, E.C., Myburgh, J.G., 2023, Liver moisture content in animals and possible causes of variations in hepatic dry matter content, J S Afr Vet Assoc 94(1), 7-15. https://doi.org/10.36303/JSAVA.518. [ Links ]
Van Saun, R.J., 2009, Nutritional diseases of llama and alpacas, Vet Clin Food Anim 25, 797-810. https://doi.org/10.1016/j.cvfa.2009.07.013. [ Links ]
Van Saun, R.J., 2012, Understanding copper nutrition in small ruminants, Am Assoc Bovine Pract Proc. https://doi.org/10.21423/aabppro20123890. [ Links ]
Wiener, G., Suttle, N.F., Field, A.C., et al., 1978, Breed differences in copper metabolism in sheep, J Agric Sci Camb 91, 433-441. https://doi.org/10.1017/S0021859600046530. [ Links ]
Wilson, P.R., Grace, N.D., 2001, A review of tissue reference values used to assess the trace element status of farmed red deer (Cervus elaphus), New Zealand Vet J 49, 126-132. https://doi.org/10.1080/00480169.2001.36219. [ Links ]
Woolliams, J.A., Suttle, N.F., Wiener, G., et al., 1982, The effect of breed of sire on the accumulation of copper in lambs, with particular reference to copper toxicity, Anim Prod 35(3), 299-307. https://doi.org/10.1017/S0003356100000969. [ Links ]
Woolliams, J.A., Suttle, N.F., Wiener, G., et al., 1983, The long-term accumulation and depletion of copper in the liver of different breeds of sheep fed diets of differing copper content, J Agric Sci 100(2), 441-449. https://doi.org/10.1017/S0021859600033608. [ Links ]
Woolliams, C., Suttle, N.F., Woolliams, J.A., et al., 1986, Studies on lambs from lines genetically selected for low and high copper status. 1. Differences in mortality, Anim Prod 43(2), 293-301. https://doi.org/10.1017/S0003356100002488. [ Links ]
WVDL (University of Wisconsin Veterinary Diagnostic Laboratory), 2015. Available from: www.wvdl.wisc.edu/wp-content/uploads/2013/06/WVDL.Info_Toxicology_Normal_Ranges.pdf. (Cited by Kendall et al. 2015). [ Links ]
Zervas, G., Nikolaou, E., Manzios, A., 1990, Comparative study of chronic copper poisoning in lambs and young goats, Anim Prod 50(3), 497-506. https://doi.org/10.1017/S0003356100004980. [ Links ]
 Correspondence:
Correspondence:
email: jvryssen@up.ac.za














