Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the South African Veterinary Association
versão On-line ISSN 2224-9435
versão impressa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.95 no.1 Pretoria 2024
http://dx.doi.org/10.36303/JSAVA.584
ORIGINAL RESEARCH
Effect of asiatic acid supplementation in tris-extender on post-thaw functional competence, antioxidant enzyme activity and in vivo fertility of bull sperm
M JameelI; IS SheikhII; N KakarIII; MR YousufI; A RiazI; W ShehzadIV; D KhanV; M IqbalVI; AM TareenVII
IDepartment of Theriogenology, University of Veterinary and Animal Sciences, Pakistan
IICenter for Advanced Studies in Vaccinology and Biotechnology, University of Balochistan, Pakistan
IIIDepartment of Natural and Basic Sciences, University of Turbat, Pakistan
IVInstitute of Biochemistry and Biotechnology, University of Veterinary and Animal Sciences, Pakistan
VLivestock and Dairy Development Department, Government of Balochistan, Pakistan
VISemen Production Unit, Livestock and Dairy Development Department, Government of Balochistan, Pakistan
VIIDepartment of Microbiology, University of Baluchistan, Quetta Pakistan
ABSTRACT
Reactive oxygen species at supra-physiological levels trigger oxidative stress during cryopreservation, which can be neutralised by incorporating suitable antioxidants into the semen extender medium. This study was intended to explore the effect of asiatic acid (AA) as an antioxidant in semen extender on frozen-thawed sperm quality and in vivo fertility of bull sperm.
Semen was collected from Holstein Friesian bulls for 10 consecutive weeks (total ejaculates = 60). Semen was cryopreserved with a Tris citric acid egg yolk-based extender supplemented with 0 (control), 20, 40, 60, and 100 μM AA.
The supplementation of the extender with 40 and 60 μM AA improved (p < 0.05) post-thaw motility kinematics, plasma membrane integrity, acrosome integrity, sperm viability, and DNA integrity of bull sperm. Mitochondrial membrane potential was high (p < 0.05) with 60 μM of AA concentration in extender media. The catalase activity in seminal plasma was maintained (p < 0.05) when semen was added with 20, 40, and 60 μM of AA. The in vivo fertility was found to be significantly high with the semen extended with 60 μM AA.
Conclusively, this study showed that AA supplementation in semen extender significantly improved sperm motility kinematics and cell integrity, conserved antioxidant enzyme activity, and improved in vivo fertility.
Keywords: antioxidants, asiatic acid, computer-assisted sperm analysis, Holstein Friesian, sperm cryopreservation
Introduction
Sperm cryopreservation maintains the sperm's viability and functionality as well as assisting in the storage and transport of spermatozoa for their use in artificial insemination and other assisted reproductive technologies (Grötter et al. 2019). During the cryopreservation process, spermatozoa are subjected to radical changes in temperature, ice crystal formation and osmotic and oxidative stresses that drastically compromise sperm quality and subsequent fertility (Kowalczyk & Czerniawska Piątkowska 2021).
Excessive reactive oxygen species (ROS) cause the oxidation of lipids, proteins, and nucleic acids which results in the production of electrophilic lipid aldehydes that bind to DNA and protein molecules involved in the functional competence of spermatozoa. Uncontrolled ROS production affects all parameters of sperm function, including motility, acrosome configuration, plasma membrane integrity, and fertilising ability. Furthermore, oxidative stress affects the integrity of DNA, with potential impacts on the developmental competence of embryos and the well-being of the progeny (Aitken 2020).
Intracellular antioxidants, such as catalase and glutathione, can resist oxidation by reduction when the concentration of ROS is low or at a normal physiological level (Alhayaza et al. 2020). However, at higher concentrations of ROS, these cellular antioxidants are incapable of balancing the redox reactions.
Antioxidants are potent substances to neutralise ROS and are incorporated into extender media to resist oxidative stress and diminish the damage from ROS during cryopreservation (Chen & Li 2020).
Asiatic acid (AA), a potent antioxidant, is a triterpenoid that naturally occurs in many herbs, most commonly in Indian pennywort (Centella asiatica). AA shows various important pharmacological properties, such as anti-inflammatory, antioxidative, antiapoptic, neuroprotective, gastroprotective, and anti-cancer. Antioxidative activities of AA are dose-dependent; they act against hydroxyl radicals, superoxide anions, and reactive species of oxygen, and diminish myeloperoxidase activation and lipid peroxidation (Nagoor Meeran et al. 2018). AA improved the developmental competence of porcine embryos through a reduction in ROS production, mitigating mitochondrial dysfunction, and regulating the expression of genes related to the antioxidant system Sod-1 and genes related to blastocyst formation Cox-2, while downregulating the gene related to apoptosis, Caspase-9 (Qi et al. 2020). AA supplementation in high-fed obese rats improved spermatogenesis and sperm quality, including motility kinematics (Miao et al. 2018).
This study aimed to investigate the effects of AA in semen extender as an antioxidative agent. The study evaluates the effects of AA on post-thaw sperm motility and motion kinematics, oxidative status and functional competence. Further, this study assesses the in vivo fertility of bull sperm using the best treated semen.
Materials and methods
Semen source and cryopreservation
Semen was obtained from Holstein Friesian (ßos taurus taurus) bulls (n = 6, age = 3-6 years). The bulls were born in Pakistan, regular semen donors, and maintained at the semen production unit in Quetta, Pakistan (30° 10' 59.7720'' N and 66° 59' 47.2272'' E). Animals were provided with a balanced diet (alfalfa and corn fodder, corn silage, and concentrate) and clean drinking water. The semen production unit has a regular health check programme that includes timely vaccination and deworming of all breeder bulls.
Semen was collected using an artificial vagina once a week from each bull for a total of 10 consecutive weeks (replicates = 10). Each ejaculate was initially assessed for volume by calibrated glass tubes, gross motility through a microscope (BX41, Olympus, Japan), and sperm concentration by a semen photometer (SDM5, Minitub, Germany). The ejaculate having normal volume (2-8 ml), concentration (> 1 × 109/ml), and initial motility (> 75%) was selected for further experiments (Barth 2007). The samples from all bulls that met the established criteria (n = 58) were pooled to avoid individual bull variations, and those that did not meet these criteria (n = 02) were discarded and excluded from the study. Each pooled semen sample was divided into five equal parts and diluted with Tris egg yolk-based extender (Tris [hydroxymethyl-amino-methane] 3.81 g, D fructose 1.25 g, citric acid 1.97 g, glycerol 7 ml, egg yolk 20 ml, penicillin 1000 IU, streptomycin 1 mg/ml and distilled water 100 ml) (Baloch et al. 2019) supplemented with 0 (control) or 20, 40, 60, and 100 μM AA with a final sperm cell concentration of 50 x 106 sperm/ml. AA (Sigma-Aldrich) was dissolved in dimethyl sulfoxide [(CH3)2SO] to prepare working solution (Qi et al. 2021). Dimethyl sulfoxide (0.1%) was also added in the control group. Extended semen samples were cooled to 4 °C within two hours. After cooling, the semen was filled into 0.5 ml straws (IMV, L'Aigle, France), sealed with polyvinyl alcohol powder, and equilibrated for four hours at 4 °C. Semen-filled straws were arranged horizontally in a nitrogen box at 5 cm above the surface of liquid nitrogen in vapour phase for 12 minutes, then plunged into liquid nitrogen and stored for one week before being used for post-thaw analysis.
Post-thawed sperm quality analyses
For post-thaw semen analysis, at least five straws per replicate of each experimental group were thawed at 37 °C for 30 seconds and processed for analysis as follows:
Sperm motility, velocity, and kinematics
Post-thaw sperm motility, velocity, and kinematics were assessed through computer-assisted sperm analysis (Sperm Vision™, version 3.5.5, Minitub, USA). The software was set for bull semen analysis standard settings (frame rate: 60 Hz/sec, number of frames: 30, minimum cell contrast: 15, minimum cell size (pixel): 5, cell intensity: 80, threshold straightness: 80, medium VAP: 25 mm/s, low VAP cutoff: 5.0, VSL: 0.05 mm/s). An undiluted 5 μl of semen was loaded into a normal grease-free slide and covered with a coverslip. Six fields on a slide were counted from each sample for average motility kinematics assessments. The variables evaluated were progressive motility (%), total motility (%), average path velocity (VAP, μm/s), straight line velocity (VSL, μm/s), curvilinear velocity (VCL, μm/s), the amplitude of lateral head displacement (ALH, um), linearity (LIN), beat-cross frequency (BCF, Hz), straightness (STR), and wobble (WOB).
Plasma membrane integrity
Plasma membrane integrity (PMI) of frozen-thawed semen was determined by methods described (Rasul et al. 2001). To determine the integrity of plasma membrane of the sperm cells, a hypo-osmotic swelling test (HOST) was performed. For HOST, 50 μl of frozen, thawed semen was mixed with 500 μl of HOST solution (sodium citrate 0.735 g, fructose 1.35 g, and 100 ml of distilled water). This mixture was kept at 37 °C for 40 minutes, and a drop (40 μl) of this mixture was placed on a microscopic slide and observed through a microscope (Olympus, BX41, Japan) at 40x magnifications. A minimum of 200 sperm cells were counted for each semen sample. Sperm cells with a swollen head, twisted tail, or coiled tail were considered to have intact PMI.
Acrosome integrity
For the acrosome integrity assay, 50 μl normal apical ridge (NAR) solution (1 ml of 37% formaldehyde, 2.92 g of tri-sodium citrate dehydrate, and 100 ml of distilled water) was mixed in thawed semen (500 μl). At least 200 sperm were counted for acrosome integrity using a phase contrast Olympus BX41 microscope at 100x magnification. Sharp crescent appearances of the sperm acrosome were considered positive for NAR and had intact acrosome integrity (Rasul et al. 2001).
Sperm viability
The propidium iodide fluorescent staining method was used for the assessment of sperm viability, as described earlier (Tariq et al. 2020). For this assay, 50 μl semen was added to 50 μl of Tris Citrate Fructose (TCF) buffer and centrifuged for five minutes at 800 relative centrifugal force (xg). After centrifugation, the supernatant was removed, and the sperm pellet was re-diluted with TCF buffer up to 47.5 μl in which 2.5 μl of propidium iodide (10 μg/ml) was mixed and incubated at 37 °C in the dark for five minutes. Just after incubation, a drop from this mixture was placed on a glass slide, and a minimum of 200 sperm were analysed through a fluorescent microscope (Olympus BX41). Red fluorescence at the head region of sperm was considered non-viable, whereas sperm without fluorescence was noted as viable.
Mitochondrial membrane potential
Frozen thawed semen mitochondrial membrane potential (MMP) was determined by modified Rhodamine fluorescence microscopy (Tariq et al. 2020). For the rhodamine assay, 50 μl of semen was added to 50 μl TCF buffer and centrifuged for five minutes at 800 xg. After centrifugation, 5 μl of rhodamine (10 μg/ml) was added to the sperm-TCF mixture and incubated for 20 minutes at room temperature (25 °C) in the dark. A minimum of 200 sperm cells were analysed from each sample for MMP. Green fluorescence at the midpoint of a piece of sperm was considered sperm with active mitochondria.
DNA integrity
Acridine orange (AO) fluorescence microscopy was used to assess the post-thaw semen DNA integrity as described previously (Ahmad et al. 2017; Tariq et al. 2020). In brief, the AO stock solution (40 ml of citric acid anhydrous mixed with 2.5 ml of 0.3 M disodium phosphate) was used to make an AO stain by mixing 1 g of AO with one litre of the AO stock solution. For the assay, 200 ul thawed semen was added with a drop of distilled water and centrifuged at 1180 xg for seven minutes at room temperature. A sperm smear was prepared from this suspension by placing a drop on the glass slide. The slide was allowed to air dry and fixed for two hours at 4 °C with a newly prepared Carnoy's solution (ethanol and glacial lactic acid in a 3:1 ratio). After fixation, the slide was again air-dried and placed in AO stain-containing glass jar for about three minutes. A sperm smear slide was washed with tap water and examined for DNA integrity using a fluorescent microscope. At least 200 sperm cells were assessed for DNA integrity. Sperm with green fluorescence at head regions were considered positive for the AO assay and had normal DNA, whereas sperm with other than green (yellow to red) fluorescence at head regions were counted as having damaged DNA content.
Antioxidant enzyme activity
The spectrophotometer method was used for the determination of the intracellular antioxidant enzyme (catalase) profile as described (Hadwan & Abed 2016). Thawed semen straws were put in a glass beaker and sonicated with the help of an ultrasonic processor (JY92-IIN, Ningbo Scientz, Biotechnology Co., Ltd., China). Thawed semen straws were sonicated for 20 seconds with a 30 second cooling period between each cycle, and a total of eight cycles were completed. Then semen was placed in 3 ml microtubes and centrifuged at 800 xg for five minutes. The supernatant was transferred to another microtube for analysis of catalase activity. For the catalase test, four cuvettes were prepared: the cuvette for the test sample contained 50 μl supernatant with 500 μl of (20 mM) hydrogen peroxide (H2O2); the control-test cuvette was added with 50 μl supernatant and 500 μl distilled water; the standard cuvette was filled with 50 μl distilled water and 500 μl H2O2 and a blank tube was prepared by adding 550 μl distilled water to it. All the cuvettes were incubated for three minutes at 37 °C. After incubation, 2 000 μl of ammonium molybdate (32.4 mmol/l) was added to each cuvette to stop the chemical reaction, and absorbance was checked at 374 nm through a spectrophotometer (UV-1700; Shimadzu; Japan). The concentration of catalase was calculated by the following equation.
Catalase activity (kL/U) = 2.303 / t [logS°/S-M] Vt / Vs
where t = total time, SH = absorbance of standard tubes, S = absorbance of test tubes, M = absorbance of control test, Vt = total volumes of reagents in test tubes, and Vs = volume of the semen sample.
In vivo fertility
A fertility trial was conducted to evaluate the effect of AA on sperm fertility. Adult cyclic Holstein Friesian cows (n = 46, age = 2-6 years, parity = 1-3) possessing no reproductive problems were selected. Based on the post-thaw results, the two groups, 0 (control) and the best treatment group (60 uM AA), were selected for the fertility trial. Cows were inseminated (n = 21 for control; n = 25 for 60 uM asiatic acid) by the same inseminator. A pregnancy diagnosis was performed by ultrasonography (KX5600, Kaixin, Xuzhou Kaixin Electronic Instrument Co., Ltd.) at least 35 days post-insemination.
Data analysis
All the data was managed and analysed using statistical software (SPSS, version 20.0, IBM Corp., Armonk, NY) and presented as the standard error of the mean (± S.E.M). The mean ± S.E.M of post-thaw sperm motility kinetics, velocity parameters, acrosome integrity, plasma membrane integrity, sperm viability, mitochondrial membrane potential, DNA integrity, and catalase level were compared through one-way analysis of variance (ANOVA). The difference between measured variables in all groups was analysed through Duncan's multiple range post-hoc tests. The fertility data were analysed using the chi-square test. A probability level of p < 0.05 was considered significant.
Results
Effect of AA on sperm motility and velocity kinematics
There was a positive effect of AA supplementation in extender media on sperm motility, velocity, and kinematic variables of frozen-thawed Holstein Friesian semen. The results revealed that the total motility was high (p < 0.05) when the extender was supplemented with 40 and 60 μM AA as compared to the control. Progressive motility showed a significant (p < 0.05) positive effect with 0, 20, and 60 μM AA concentrations compared to other concentrations (Figure 1).
Results for sperm motion kinematics were analysed using CASA (Table I). The highest values (p < 0.05) of VAP, VCL, and VSL were observed in semen containing 20 and 60 uM AA and semen without AA supplementation as compared to other concentrations. Lateral head displacement was significantly different (p < 0.05) when semen was supplemented with 60 uM AA or without any supplementation of AA. Straightness, wobble, and BCF were non-significant with or without AA supplementation. Linearity was lowest (p < 0.05) in semen supplemented with 60 uM AA as compared to the control.
Effect of AA on the plasma membrane, acrosome integrity, viability and mitochondrial membrane potential of bull sperm
The sperm plasma membrane, acrosome integrity, and viability findings showed that semen extenders supplemented with 40 and 60 uM AA concentrations showed the highest (p < 0.05) percentage of normal sperm cells with integrity and viability as compared to controls (Figure 2). The HOST, NAR positive and viable sperm images are depicted in (Figure 4). A significantly high MMP was observed with 60 μM AA compared to other concentrations.
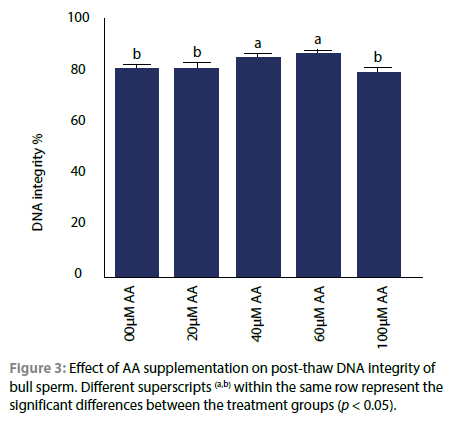
Effect of AA on DNA integrity of bull sperm
Significantly more sperm cells with intact DNA were observed in the treatment groups containing 40 or 60 μM AA, while no effect was seen with 20 and 100 μM or without supplementation of AA (Figures 3 and 4).
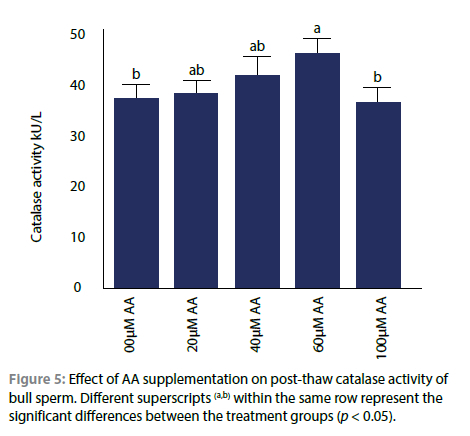
Effect of AA on catalase enzyme activity
The effect of AA on intracellular catalase enzyme activity in frozen-thawed bull semen is exhibited in Figure 5. Interestingly, the catalase enzyme activity was significantly elevated when the semen extender was supplemented with the 20, 40, and 60 μM AA.
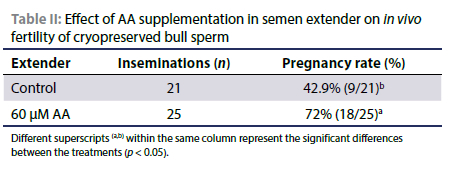
Effect of AA on in vivo fertility
Significantly more cows inseminated with treated semen were pregnant 72% (18/25) compared to the cows inseminated with control semen 42.9% (9/21). These results were obtained from one insemination per cow (Table II).
Discussion
This study was based on the hypothesis that AA supplementation in extender media can improve the post-thaw quality of cryopreserved bull semen through its anti-oxidative properties. This is the first study to reveal that AA supplementation led to better sperm quality indicators in bull sperm. Post-thaw sperm motility, structural integrity, and catalase enzyme activity were sustained, and in vivo, fertility was enhanced by the addition of AA in the semen extender.
Antioxidants in extender media have been extensively employed to counter the ROS concentration exceeding the physiological limit during cryopreservation (Liu et al. 2021). AA has been thought to have powerful free radical scavenging and antioxidative properties (Hu et al. 2021) which promote spermatogenesis and improve semen quality (Miao et al. 2018). The mammalian spermatozoa contain high levels of lipids in the plasma membrane in the form of polyunsaturated fatty acids (PUFAs). The unconjugated double bounds in these PUFAs, which are separated by methylene groups, make hydrogen tremendously vulnerable to oxidative damage. Reactive oxygen species attack these PUFAs at supraphysiological levels within the cells, disrupting the membrane's integrity (Dutta et al. 2019). AA captures these ROS and makes the cells more resistant to oxidative stress (Qi et al. 2021). The present study found similar effects, as AA exerted significant positive effects on the plasma membrane and acrosome integrity of bull sperm.
Mitochondria are highly susceptible to cryodamage, and any anomalies in their structure or function are directly linked to a decline in sperm quality (Madeja et al. 2021). Mitochondrial function is extensively assessed through mitochondrial membrane potential (MMP). During cryopreservation, exceeding ROS causes an alteration in MMP, which results in the reduced fertilising ability of the sperm. Our results are in line with previous studies, which showed that AA increased MMP activity by reducing ROS in porcine oocytes (Hu et al. 2021; Qi et al. 2021) and embryos (Qi et al. 2021).
DNA integrity is one of the objective biomarkers for sperm function and fertilising ability. Antioxidants could mitigate DNA damage by resisting oxidative stress (Gilmore et al. 2021). The current study points out that AA supplementation during cryopreservation resulted in improved DNA integrity. This is possible due to the counteracting effects of AA on ROS production, which had a beneficial impact on cell DNA integrity as described earlier by Ribero et al. (2021).
Among the various sperm parameters, the motility of sperm is one of the very fundamental characteristics associated with the fertilising ability of spermatozoa in vitro and in vivo (Gilmore et al. 2021). Our results revealed that AA had positive effects on post-thawed semen motility and velocity kinetics. These were in accordance with the fact that the high motility and velocity can be attributed to a large number of sperm cells with an intact plasma membrane and MMP (Nagy et al. 2015). These were found to be significantly high in the semen extended with AA in our study. Sperm motility and velocity parameters had a strong correlation with sperm viability (Gilmore et al. 2021). Miao et al. (2018) also observed that AA promotes spermatogenesis and improves sperm motility kinematics. In the present study, sperm viability was significantly high in the semen samples supplemented with AA.
The application of antioxidants during cryopreservation can reduce oxidative stress, suppress ROS, and promote antioxidative enzyme activity (Liu et al. 2021). In our study, AA supplementation significantly preserved the catalase enzyme level in frozen and thawed semen. Previous studies demonstrated that AA can sustain the activities of catalase and superoxide dismutase (SOD) in liver tissues (Qi et al. 2017) and improve glutathione levels in porcine oocytes (Qi et al. 2021) and embryos. Moreover, AA upregulated the expression of genes linked to the antioxidant system Sod-1, while down-regulating the expression of the gene associated with apoptosis, Caspase-9 (Qi et al. 2020).
Cryopreservation and the freeze-thawing process of bovine sperm lessen the activity of antioxidants, which is why the in vivo fertility of cryopreserved semen is lower compared to fresh semen (Bilodeau et al. 2001). The current study revealed that in vivo fertility rates were significantly enhanced with 60 |M AA supplementation. Previous studies suggest that supplementation with AA improves oocyte quality (Hu et al. 2021) and embryo developmental competence in pigs (Qi et al. 2020). These findings also demand further large-scale in vivo fertility investigations.
Implications
This study highlights the potential benefits of incorporating AA as an antioxidant in semen extenders for cryopreservation. The findings suggest that AA supplementation improves sperm quality, conserves antioxidant enzyme activity, and enhances in vivo fertility. These results provide valuable insights for developing improved cryopreservation protocols and optimising the use of antioxidants in reproductive technologies.
Study limitations
While the study presented promising results regarding the effects of AA supplementation on frozen-thawed sperm quality and in vivo fertility of bull sperm, it is important to consider the limitations of the study. The study focused on a specific breed of bulls (Holstein Friesian), which may limit the applicability of the results to other breeds. The study assessed the effects of AA supplementation on sperm quality and fertility immediately after thawing. Long-term effects or the impact on pregnancy rates were not investigated.
Conclusion
In conclusion, this study reports that AA supplementation at dose rate of 60 μM concentration in semen extender significantly reduced cryodamage and improved post-thaw sperm motility kinematics and structural integrity, preserved antioxidant enzyme activity, and enhanced in vivo fertility.
Conflict of interest
No conflict of interest exists.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
All the experimental procedures followed the ethical guidelines and were approved by the local ethical review committee, University of Veterinary and Animal Sciences, Lahore 54000, Pakistan, and Livestock and Dairy Development Department Balochistan, Pakistan.
ORCID
M Jameel https://orcid.org/0000-0003-2310-100X
IS Sheikh https://orcid.org/0000-0003-0616-0411
N Kakar https://orcid.org/0000-0003-0966-4658
MR Yousuf https://orcid.org/0000-0001-7506-4015
A Riaz https://orcid.org/0000-0002-0963-7118
W Shehzad https://orcid.org/0009-0006-7548-0135
D Khan https://orcid.org/0000-0001-7169-4351
M Iqbal https://orcid.org/0009-0009-7037-3367
References
Ahmad, M., Ahmed, M., Ahmad, N., 2017, Optimization of acridine orange staining for buffalo sperm, cryopreserved in egg yolk based extender to detect DNA fragmentation, Pakistan Journal of Zoology 49(5). https://doi.org/10.17582/journal.pjz/2017.49.5.sc6. [ Links ]
Aitken, R.J., 2020, Impact of oxidative stress on male and female germ cells: implications for fertility, Reproduction 159(4): R189-R201. https://doi.org/10.1530/REP-19-0452. [ Links ]
Alhayaza, R., Haque, E., Karbasiafshar, C., et al., 2020, The relationship between reactive oxygen species and endothelial cell metabolism, Frontiers in Chemistry 8. https://doi.org/10.3389/fchem.2020.592688. [ Links ]
Baloch, A.H., Kunbhar, H.K, Memon, M.I., et al., 2019, Fresh and post-thaw quality characteristics of Holstein Friesian bull semen maintained at semen production unit Quetta Balochistan, Pakistan, Pure and Applied Biology 8(1), 780-789. https://doi.org/10.19045/bspab.2019.80020. [ Links ]
Barth, A.D., 2007, Evaluation of potential breeding soundness of the bull. In. Current Therapy in Large Animal Theriogenology. Elsevier. p. 228-240. https://doi.org/10.1016/B978-072169323-1.50034-9. [ Links ]
Bilodeau, J.-F., Blanchette, S., Gagnon C., et al., 2001, Thiols prevent H2O2-mediated loss of sperm motility in cryopreserved bull semen, Theriogenology 56(2), 275-286. https://doi.org/10.1016/S0093-691X(01)00562-3. [ Links ]
Chen, W., Li, D., 2020, Reactive oxygen species (ROS)-responsive nanomedicine for solving ischemia-reperfusion injury, Frontiers in Chemistry 8, 732. https://doi.org/10.3389/fchem.2020.00732. [ Links ]
Dutta, S., Majzoub, A., Agarwal, A., 2019, Oxidative stress and sperm function: a systematic review on evaluation and management, Arab Journal of Urology 17(2), 87-97. https://doi.org/10.1080/2090598X.2019.1599624. [ Links ]
Gilmore, A., Hitit, M., Ugur, M.R., et al., 2021, Functional variables of bull sperm associated with cryotolerance, Kafkas Üniversitesi Veteriner Fakültesi Dergisi 27(3). [ Links ]
Grötter, L.G., Cattaneo, L., Marini, P.E., et al., 2019, Recent advances in bovine sperm cryopreservation techniques with a focus on sperm post-thaw quality optimization, Reproduction in Domestic Animals 54(4), 655-665. https://doi.org/10.1111/rda.13409. [ Links ]
Hadwan, M.H., Abed, H.N., 2016, Data supporting the spectrophotometric method for the estimation of catalase activity, Data in brief 6, 194-199. https://doi.org/10.1016/j.dib.2015.12.012. [ Links ]
Hu, W.-Y., Li, X.X., Diao, Y.F., et al., 2021, Asiatic acid protects oocytes against in vitro aging-induced deterioration and improves subsequent embryonic development in pigs, Aging (Albany NY) 13(3), 3353. https://doi.org/10.18632/aging.202184. [ Links ]
Kowalczyk, A., Czerniawska Piątkowska, E., 2021, Antioxidant effect of Elamipretide on bull's sperm cells during freezing/thawing process, Andrology. https://doi.org/10.21203/rs.3.rs-58891/v1. [ Links ]
Liu, X., Xu, Y., Liu, F., et al., 2021, The feasibility of antioxidants avoiding oxidative damages from reactive oxygen species in cryopreservation, Frontiers in Chemistry 9, 64. https://doi.org/10.3389/fchem.2021.648684. [ Links ]
Madeja, Z.E., Podralska, M., Nadel, A., et al., 2021, Mitochondria content and activity are crucial parameters for bull sperm quality evaluation, Antioxidants 10(8), 1204. https://doi.org/10.3390/antiox10081204. [ Links ]
Miao, X.L., Gao, G.M., Jiang, L., et al., 2018, Asiatic acid attenuates high-fat diet-induced impaired spermatogenesis, Experimental and Therapeutic Medicine 15(3), 2397-2403. https://doi.org/10.3892/etm.2017.5672. [ Links ]
Nagoor Meeran, M.F., Goyal, S.N., Suchal, K., et al., 2018, Pharmacological properties, molecular mechanisms, and pharmaceutical development of asiatic acid: a pentacyclic triterpenoid of therapeutic promise, Frontiers in Pharmacology 9, 892. https://doi.org/10.3389/fphar.2018.00892. [ Links ]
Nagy, Á., Polichronopoulos, T., Gáspárdy, A., et al., 2015, Correlation between bull fertility and sperm cell velocity parameters generated by computer-assisted semen analysis, Acta Veterinaria Hungarica 63(3), 370-381. https://doi.org/10.1556/004.2015.035. [ Links ]
Qi, J.-J., Li, X.-X., Zhang, Y., et al., 2021, Supplementation with asiatic acid during in vitro maturation improves porcine oocyte developmental competence by regulating oxidative stress, Theriogenology 172, 169-177. https://doi.org/10.1016/j.theriogenology.2021.06.013. [ Links ]
Qi, J.-J., Li, X.X., Diao, Y.F., et al., 2020, Asiatic acid supplementation during the in vitro culture period improves early embryonic development of porcine embryos produced by parthenogenetic activation, somatic cell nuclear transfer and in vitro fertilization, Theriogenology 142, 26-33. https://doi.org/10.1016/j.theriogenology.2019.09.027. [ Links ]
Qi, Z., Ci, X., Huang, J., et al., 2017, Asiatic acid enhances Nrf2 signaling to protect HepG2 cells from oxidative damage through Akt and ERK activation, Biomedicine & Pharmacotherapy 88, 252-259. https://doi.org/10.1016/j.biopha.2017.01.067. [ Links ]
Rasul, Z., Ahmad, N., Anzar, M., 2001, Changes in motion characteristics, plasma membrane integrity, and acrosome morphology during cryopreservation of buffalo spermatozoa, Journal of Andrology 22(2), 278-283. https://doi.org/10.1002/j.1939-4640.2001.tb02181.x. [ Links ]
Ribeiro, A.B., Ozelin, S.D., da Silva, L.H., et al., 2021, Influence of asiatic acid on cell proliferation and DNA damage in vitro and in vivo systems, Journal of Biochemical and Molecular Toxicology 35(4), e22712. https://doi.org/10.1002/jbt.22712. [ Links ]
Tariq, A., Ahmad, M., Iqbal, S., et al., 2020, Effect of carboxylated poly L-Lysine as a cryoprotectant on post-thaw quality and in vivo fertility of Nili Ravi buffalo (Bubalus bubalis) bull semen, Theriogenology 144, 8-15. https://doi.org/10.1016/j.theriogenology.2019.12.012. [ Links ]
 Correspondence:
Correspondence:
email: niamatullah.kakar@uot.edu.pk














