Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the South African Veterinary Association
versión On-line ISSN 2224-9435
versión impresa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.95 no.1 Pretoria 2024
http://dx.doi.org/10.36303/JSAVA.601
ORIGINAL RESEARCH
Parasites burden in peri-urban free-roaming pigs in Gert Sibande District Municipality, Mpumalanga Province, South Africa
P MunzheleleI; NPS SibekoII; JW OguttuIII; CA MbajiorguIII; FO FasinaIV, V
INooitgedacht Research Station, Animal Research, Non-ruminant Sub-directorate, Department of Agriculture, Rural Development, Land Administration and Environmental Affairs, South Africa
IIParasitology Unit, Department of Agriculture, Rural Development, Land and Environmental Affairs, Mpumalanga Provincial Veterinary Laboratory, South Africa
IIIDepartment of Agriculture and Animal Health, College of Agriculture and Environmental Science, University of South Africa, South Africa
IVFood and Agriculture Organization of the United Nations, Italy
VDepartment ofVeterinary Tropical Diseases, Faculty of Veterinary Science, University of Pretoria, South Africa
ABSTRACT
BACKGROUND: Parasite infections, unlike regulated animal diseases, do not often receive attention. In fact, parasites are major sources of financial losses in pig enterprises, particularly in subsistence and small-scale pig production systems.
OBJECTIVE: To identify and quantify the prevalence of ecto- and endo-parasites among peri-urban free-roaming pigs (FRP) in Gert Sibande District Municipality (GSDM), Mpumalanga.
METHOD: Pig owners were identified using the snowball sampling method since no sampling frame for FRP farmers exists. Stratified sampling was used to select pigs for sampling for ecto- and endo-parasites. A form was used to record the observations. Pairwise correlation analysis was performed using Stata 15.0. The SPSS V28.0 statistical package was used to perform the chi-square test (X2) to assess the distribution of parasites in different age groups. The prevalence of parasites was assessed in different age groups of pigs using multi-response crosstabs. Multivariate analysis of variance (MANOVA) was used to estimate the marginal mean of parasites according to municipality. Statistical significance was assessed at α < 0.05.
RESULTS: Over 90% (91.2%) of the pigs examined were infested with at least one parasite, including Haematopinus suis, Sarcoptes scabiei, Ascaris suum, Fasciola hepatica, Trichuris suis, Strongylids, Coccidia spp, Moniezia expansa, and Siphonaptera spp. The correlation between Ascaris suum and body condition was weak but statistically significant (r = 0.24; p < 0.05.
CONCLUSION: Policy makers, animal researchers and veterinary services must focus on developing policies, risk communication and community engagement materials, which target pig farmers in peri-urban areas such as Gert Sibande District Municipality, Mpumalanga Province.
Keywords: gastro-intestinal parasites, ecto-parasites, free-roaming pigs, disease, and zoonosis
Introduction
Pig farming has been recognised as a means of alleviating poverty, creating jobs, ensuring food security, providing a source of protein, and contributing to national GDPs (Adebisi 2008; Nonga & Paulo 2015; Odo et al. 2016; Nwafor et al. 2019). These recognitions are based on the fact that pigs have distinguishing traits, including rapid growth, excellent feed conversion, ease of housing and slaughter, need for smaller land area per unit, high fecundity, high protein content (pork), and ease of adaptation to diverse climatic situations. Despite the pig's outstanding performance, the pig industry is constrained by multiple yet overlooked challenges.
For many years, the South African pig industry has experienced numerous production-related and animal health issues with severe economic implications. Certain pig production systems used in the industry, particularly the extensive pig production system, act as catalysts for disease outbreaks. Such outbreaks may include, but are not limited to, classical swine fever (CSF), African swine fever (ASF) and salmonellosis. In the peri-urban areas of Mpumalanga Province (MP), including near human dwellings, open areas, along streams, and around garbage dumps, numerous pigs can be spotted roaming freely. In addition, a previous study conducted in MP confirmed that numerous pigs were slaughtered at home without the ante-mortem inspection by authorised veterinary services (Munzhelele 2015; Munzhelele et al. 2017; DCGTA, 2020). This situation portends high food safety and health risks to both humans and animals from the informal pig sector.
Parasitic diseases are commonly disregarded in smallholder pig farming, largely because unlike some rapidly spreading infectious diseases, parasitic diseases hardly cause enormous mortality. Furthermore, parasite infections, unlike regulated animal diseases, do not receive much attention in South Africa. Moreover, a high burden of parasite infestation can have severe negative economic impacts on the pig industry.
Ecto- and endo-parasites are major limitations to financial well-being and productivity in smallholder pig operation. In fact, previous research has shown that parasites have an ultimate effect on productivity and production outputs such as poor growth rate, litter size, low birth weight, reduced weight gain, poor feed conversion efficiency, decreased appetite, poor fertility, emaciation in pigs, condemnation of damaged organs after slaughter, respiratory distress, and high mortality and may lead to significantly high treatment costs. In addition, some parasites from pigs have zoonotic potential (Nsoso et al. 2000; Ngowi et al. 2004; Stewart & Hoyt 2006; Sowemimo et al. 2012; Kagira et al. 2012; Wilson & Swai 2014; Kumsa & Kifle 2014; Nonga & Paulo 2015; Nwafor et al. 2019).
Parasitic infections and infestations of the host may occur through contaminated feeds, water, pasture, and direct contact with pigs from infected areas, rearing pigs in filthy conditions, and poor farm management (Soulsby 1982; Damriyasa et al. 2004; Kagira et al. 2013; Nsoso et al. 2006; Tamboura et al. 2006). The lack of biosecurity and parasite control in small-scale farm settings exacerbates the situation in pig farming (Munzhelele 2015; Munzhelele et al. 2021). Furthermore, poor management, insufficient nutrition, a free-roaming pig (FRP) rearing system, and poor herd health, all of which predominate the smallholder farming system, are the leading causes of parasite endemicity in pig farming.
To date, the occurrence or prevalence of ecto- and endo-parasitism among FRP in the Gert Sibande District Municipality (GSDM)'s peri-urban areas (PUA) are unknown and hence require investigation. The purpose of this study was to identify and quantify the prevalence of ecto- and endo-parasites in free roaming pigs in the peri-urban areas of GSDM, Mpumalanga Province, and to assess the impact of parasitic load on the performance of FRP in the PUA of Mpumalanga Province.
Materials and methods
Study area
South Africa has nine provinces with a total area of 1 221 037 km2. Mpumalanga is South Africa's second-smallest province after Gauteng, occupying approximately 79 494 km2. Mpumalanga Province has three district municipalities, including: Gert Sibande, Nkangala and Ehlanzeni. The district shares boundaries with three provinces locally, and including Free State, Gauteng and Kwazulu Natal and one international border with eSwatini. The current study was conducted in the Gert Sibande District Municipality (GSDM). The GSDM (26.5471oS, 29.9741oE) is the largest district in MP covering 31 845,9 km2 (41% of the total land area of Mpumalanga). It consists of seven municipalities, including Msukaligwa, Govan Mbeki, Dipaliseng, Lekwa, Pixley ka Seme, Mkhondo and Chief Albert Luthuli (Figure 1). Mining (26%) is the largest economic sector in GSDM, followed by manufacturing (18%), community services (15%), and agriculture with only 3%. The district is hampered by high poverty rates (46.5%) and large unemployment (38.4%) (COGTA, 2020).
Data collection
The participants were identified using the snowball sampling method since no sampling frame exists for free-roaming pig farmers (FRP). The first FRP farmer in each community was identified through the assistance of the district or municipal animal health professional, and thereafter, snowball sampling was utilised to recruit other farmers into the study. For the pigs, the stratified random sampling method was used to select pigs used for ecto- and endo-parasite samples. The pig population that made up the study population was divided into five strata (groups) and included: 33 boars (12 months + male), 78 sows (12 months + female), 33 finishers (8-11 months), 64 growers (5-7 months) and 75 piglets/weaners (1-4 months). In each stratum, one pig was randomly selected. A total of 283 samples were collected from 283 pigs from seven different municipalities in GSDM. In addition, data was collected from 124 farmers using structured questionnaires.
An outbreak of African Swine Fever (ASF) in most study regions followed by an outbreak of Foot and Mouth disease in cattle in a nearby province delayed the onset of the study. Dipaliseng, Lekwa, Govan Mbeki, and Msukaligwa were among the municipalities devastated by the earlier outbreaks of ASF, which occurred between 2020 to early 2022. To minimise the risk of spreading infectious diseases (ASF), this investigation was therefore postponed, re-planned and a heightened biosecurity protocol integrated into the survey plan. Permission to begin data collection was acquired from the state veterinarian. All biosecurity protocols were strictly followed at all times, including separating farm areas into low and high-risk areas, visiting the low-risk farms first, changing shoe covers where necessary, reducing interaction directly with the pigs, not visiting more than three farms per day, using disinfectant to sanitise hands, shoes and other equipment used such as pig holder and pig handling boards. No single case of ASF or any other infectious disease in pigs was recorded in all the areas where the research was carried out, both during and after data collection.
Pig handling
To reduce the risk of trauma and injury to the pigs and the handler, standard pig handling practices were followed. Pigs were managed in line with the Animal Protection Act 71 of 1962 by following standard procedures for handling violent animals in the pig industry. To restrain the pigs during data collection, plastic handling boards and pig snares were used. To minimise stressing the pigs, data was collected as quickly as feasible taking cognisance of the five welfare principles of Webster (Webster 2016).
Small pigs were captured by their hind legs and pulled up by placing a hand beneath the piglet's chest to support its weight. To restrict movement during handling, the piglets were carefully restrained. By doing so, the researcher and other team members were able to collect the samples without compromising the welfare of the piglets. After the procedures of collecting samples were completed, the piglet was placed on the floor as soon as possible, by letting both front limbs contact the floor surface before being gently lowered onto their hind legs. To minimise the risk of spreading infections between pigs, the equipment used was disinfected between procedures in water containing F10 (an antiseptic solution containing benzalkonium chloride and polyhexanide).
Pigs weighing more than 10 kg were caught using a pig snare. As shown in Appendix 1a, the snare restraint was manoeuvred into the mouth and placed behind the canine teeth (tusks), and held gently, safely, yet firmly without compromising the welfare of the pig. The pig was freed from the pig snare as quickly as possible by gently releasing the snare.
Faecal sample collection
Fresh faeces were collected directly from the pig rectum through gentle rectal massage. But if the pig voided faeces in the presence of the researcher, the core of the freshly voided faeces was collected. Approximately 5 g of faeces were collected from the rectum of each pig. A new sterile latex glove was used for each pig, or from the freshly voided faeces. The gloves were lubricated with liquid paraffin before insertion of the gloved fingers into the rectum. The samples were stored in a sterile cooler bag with ice packs to maintain the integrity of the sample. The collected samples were delivered to Mpumalanga Provincial Veterinary Laboratory (MPVL) on the same day of collection and stored in the refrigerator (2-8 °C). The examination of the samples was done within three days of collection.
Collection of skin scrapings
Skin scrapings were done in different areas of the pigs (i.e. on the neck, in the inner part of the ear, the tip of the tail or back of the ear). Care was taken to minimise bleeding from the skin by rubbing a few drops of liquid paraffin into the skin before the surface was extensively scraped with the scalpel. Masking tape was used after the samples had been assembled to seal the slides. The slides were examined under a stereo microscope.
The collected lice were placed in 5% formalin or methylated spirits in the plastic specimen following the procedure used by Laha (2015). Procedures describe by previous researchers were used to identify the lice (Lapage 1968; Odo et al. 2016). Standard laboratory protocols and the standard operating procedures (SOPs) of MPVL were used to process all samples. All the sampled pigs were sprayed with wound spray (Zoetis Terramycin wound spray [oxytetracycline hydrochloride 4 g and blue marker dye]), to avoid flies and infection after taking skin scrapings.
The animal samples were accompanied by a form, which contained the following information: unique code, municipality, age, sex, weight, breed, and body condition. The form was completed for each pig that was sampled (see Appendix 2).
Faecal egg count
The McMaster approach as simplified by Roepstroff and Nansen (1998) and Kaufmann (1996), was used to conduct faecal egg counts (FECs). The FECs were expressed as eggs per gram (EPG) of faeces. An empty bottle was placed on a weighing scale tared to 0 g for each sample, and 2 g of faeces was inserted into the empty bottle. The 2 g of faeces were thoroughly mixed with 58 ml of 40% sugar solution (400 g sugar mixed with 1 000 ml hot water and 20 ml of 10% formalin to prevent growth of fungus) using a mortar and pestle. A plastic pipette was used to pick the mixture and fill the McMaster counting chamber. The McMaster was loaded, and then left to stand for five minutes to allow the eggs to float to the surface of the 40% sugar solution. Using the microscope (10x magnification), the eggs were counted in the grid of the two chambers on the McMaster slide. The sheep counter was used to count the eggs. Then, all of the available eggs were identified, and the number of eggs was recorded.
Statistical analysis
Matched variables and the laboratory data from the parasitological analysis were entered in a Microsoft Excel spreadsheet version 2016 (Microsoft Corporation, Redmond, Washington, USA). Pairwise correlation (r) analysis was conducted to determine correlated association of pig characteristics (sex, age, body conditions score and breed) with the parasites from the faecal and skin scrapping samples. Significant p values was set at < 0.05 using Bonferroni-adjusted significance level in Stata 15.0 (StataCorp LLC, College Station, Texas, United States). Furthermore, the prevalence of parasites was assessed in different age groups of pigs using multi response crosstabs of SPSS V28.0 (IBM corp 2021). The chi-square test (X2) was used to assess the distribution of parasites in different age groups of FRP in GSDM peri-urban areas. The multivariate analysis of variance (MANOVA) was used to estimate the marginal mean of parasites according to municipality. The cross tabulation was used to assess the relationship between parasites and the age of the pig, parasites and the municipality, parasites and the type of breed, and finally parasites and the body condition score of the pig (as indicated by Coffey et al. 1999).
Results
Animal characteristics correlated with parasites
A total of 283 pigs of various ages were sampled. The association between Ascaris suum and body condition was weak but significant (r = 0.24; p < 0.05). Similarly, the association between Ascaris suum and H. suis was weak but significant (r = 0.20; p < 0.05), while the correlation between Ascaris suum and Coccidia spp. was moderate but significant (p < 0.05). In addition, Trichuris suis was a moderate positive predictor for Coccidia spp. (r = 0.37; p < 0.05) but a weak positive predictor for Strongylids (r = 0.24; p < 0.05). Finally, the association between the Strongylids and T. suis was moderate predictor (r = 0.48; p < 0.05) (Table I).
Distribution of parasites in different free-roaming pig age groups
Sarcoptes scabiei was commonly isolated in all age categories, with the highest prevalence observed in pigs aged > 12 months (41.6%) and < four months (28.0%) (Tables II and III). Coccidia spp. showed a similar pattern, with a high prevalence observed in pigs aged > 12 months (40.8%) and < 4 months (29.6%). The prevalence of Haematopinus suis did not change significantly across three age groups: > 12 months and older (30.7%), four months (29.5%), and five to seven months (25.0%). Trichuris suis, Strongylids, Fasciola hepatica, and Moniezia expansa were all quite predominant in pigs 12 months or older, with 50.0%, 51.7%, 50.0%, and 50.0%, respectively (Table II).
Observational findings in peri-urban areas of Gert Sibande District Municipality
Pigs were seen free-roaming in sewerage areas, streams, immobile dams and crossing roads. In terms of feeding, the following were observed: pigs fed dead chicken and swill, feeders used without cleaning, or disinfecting and being fed from the soiled floor. The use of unwashed drums as storage for swill. Bones, plastics and kitchen utensils were also observed in the pig pens.
Prevalence of parasites in the peri-urban free-roaming pigs of Gert Sibande District Municipality, Mpumalanga Province
Sarcoptes scabiei and Coccidia spp. were the most frequently recovered parasites in peri-urban FRP of GSDM accounting for 29.2% and 25.0% of the total parasites recovered and observed in 62.9% and 53.7% of the pigs respectively (Table III). The following parasites were also observed in the study area: A. suum (37.8%), H. suis (31.1%), Trichuris suis (13.4%), and Strongylid nematodes (10.2%) (Table III). Other parasites (Siphonaptera spp., Moniezia expansa and Fasciola hepatica) were less frequently recovered from the FRP in the GSDM.
The prevalence of parasites in FRP from the different municipalities varied. Mkhondo had the highest prevalence of H. suis (50.0%), followed by Msukaligwa (37.5%) and Govan Mbeki (12.5%). On the other hand, H. suis was not isolated in certain municipalities such as Chief Albert Luthuli Municipality (CALM), Lekwa, Pixley ka Seme, and Dipaliseng (Table IV). Sarcoptes scabiei was found in 9.6% of the pigs tested in each of CALM and Dipaliseng. Govan Mbeki (28.7%) and Mkhondo (15.2%), each yielded a higher number of positive samples. Only 7.2% of the pigs from each of Pixley ka Seme and Lekwa were positive.
The municipalities with a high percentage of pigs positive for Ascaris suum were Mkhondo (41.1%), followed by Govan Mbeki (30.8%). Dipaliseng (11.2%), Msukaligwa (9.3%), and CALM (7.5%) had lower A. suum prevalence. All pigs tested negative for A. suum in the municipalities of Pixley ka Seme and Lekwa (Table IV).
In GSDM peri-urban areas, Govan Mbeki (39.5%) reported the highest prevalence of FRP that were positive for Coccidia spp. This was followed by Mkhondo (22.4%) and Dipaliseng (9.7%), with the other municipalities showing lower numbers of positive FRPs (Table IV). None of the pigs (0%) in Pixley ka Seme tested positive for Coccidia spp. Trichuris suis was discovered in only three municipalities, with Dipaliseng having the highest percentage of positive cases (71.0%). Mkhondo (23.7%) and CALM (5.3%), on the other hand, had lower parasite burdens than Dipaliseng (Table IV). Trichuris suis was not found in the other four municipalities. Strongylid was the least observed parasite in the GSDM, with Dipaliseng having (37.9%) the highest prevalence rate, followed by Mkhondo (31.1%), Govan Mbeki (20.7%), and Msukaligwa (10.3%). In the remaining three municipalities, strongylid detection was negative.
Surprisingly, Fasciola hepatica and Siphonaptera fleas were only reported in Mkhondo and Msukaligwa respectively (Table IV). Moniezia expansa was found in two municipalities, CALM and Govan Mbeki, with each reporting a 50% prevalence (Table IV).
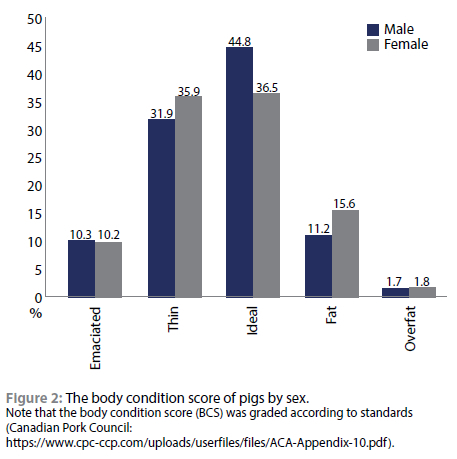
Slightly less than half of the male (44.8%) and fewer females (36.5%) had an ideal BCS (Figure 2). Approximately 42.2% and 46.1% of the male and female FRP in GSDM had a BCS that ranged from emaciated to thin and only 12.9% and 17.4% male and female had fat to overfat BCS respectively.
The exotic breeds raised in the peri-urban free-roaming settings were more vulnerable to different parasites. As shown in Table V, a high number of exotic pigs were positive for H. suis (68.2%), Sarcoptes scabiei (79.8%), Ascaris suum (73.8%), Coccidia spp (75.7%), Trichuris suis (68.4%), Strongylids (79.3%), Moniezia expansa (100%) and Siphonaptera spp (100%). The exception was Fasciola hepatica, which had a higher proportion (50%) of positive pigs among the crosses as compared to both the exotic breed and indigenous breeds. The indigenous breeds had the least parasite burden.
In terms of sex, female pigs (167) had a higher parasite load than the male pigs (116) (Table V). The exception was Fasciola hepatica which was found in equal proportions (50%) in both male and female pigs (Table V).
The pigs that had a thin or ideal BCS tended to carry a higher parasite burden than those with an emaciated BCS. The least parasite burden was found among the fat and overfat pigs. Moniezia expansa (33.3%) was predominant in pigs with an ideal and fat BCS (Table VI), while thin pigs (75.0%), had a higher proportion of Fasciola hepatica-positive pigs. Siphonaptera infestation was also high in pigs with ideal body conditions (71.4%) (Table VI).
Discussion
Free-roam pig keeping appears to contribute to the prevalence of the various endo- and ecto-parasites observed in this study. Although, results reported here are not as high as those found in free-roaming pigs elsewhere, they are consistent with results from a study that was done in Botswana in which the following was observed: Ascaris suum - 54.55%; Trichostrongylus spp. -20.45% and Trichuris suis - 6.82% (Nsoso et al. 2000). Results of the present study are also consistent with those from Romania (Băieş et al. 2022). Based on our observations, pigs were discovered scavenging in sewage, streams, dumping areas, being fed dead chickens, feeding from soiled flooring, and being fed swill during the course of the study (results not shown). The overall prevalence of ecto- and endo-parasites was 91.2%. This is similar to findings of a study from Nepal that reported a prevalence of 91% (Adhikari et al. 2021). They are also consistent with results of a study from Burkina Faso in which a prevalence of 92.7% was observed (Tamboura et al. 2006). However, findings of the present study contrasted with those from a study conducted in the Free State Province (SA) that observed a lower prevalence (79.2%) (Nwafor et al. 2019).
Sarcoptes scabiei as well as Coccidia spp. Ascaris suum and Haematopinus suis were the most common parasites among FRP in the peri-urban areas of Mpumalanga Province. On the other hand, Siphonaptera, Moniezia expansa and Fasciola hepatica were the least prevalent parasites in the study area. These finding were not surprising as most of these parasites are soil-borne and the FRP may encounter them frequently while searching for food in the environment (Omudu & Amuta 2007). The recovery of Moniezia expansa, a large tapeworm commonly associated with the small intestines of ruminant livestock (i.e. sheep, goats and cattle) is a significant finding. This parasite has been reported as an incidental finding in pigs from a few studies globally. For example, Gomez-Puerta et al. (2008) in Peru, Matos et al. (2011) in Mozambique. and Adelakun et al. (2021) in Nigeria have reported it. The present study focused on FRP, and because some farmers in the study area employ mixed farming methods, FRP interact freely with these other livestock species; this could explain the finding of Moniezia expansa in our study. This view is supported by Matos et al. (2011) who linked the occurrence of Moniezia expansa in pigs to a scenario in which pigs picked up the parasite while grazing on the same pasture with cattle and goats. It is plausible that this Moniezia expansa can gain entry into pigs through food and water sources (Soulsby 1982). This is supported by the fact that the prevalence of Moniezia expansa was significantly higher in CALM (50%, p < 0.05) and Govan Mbeki (50%, p > 0.05), areas where pigs and goats shared facilities for night kraaling. This observation will need further evaluation.
Fasciola hepatica was observed in 1.4% of the pigs. However, this was lower than previously reported in Ethiopia (2.8%) by Tomass et al. (2013). The researchers in the Ethiopian study linked the occurrence of F. hepatica in pigs to irrigated urban and peri-urban crop production practices, in which crops are watered with wastewater that may contain the intermediate hosts of F. hepatica. In this study, pigs were observed (results not shown) consuming water from dumping sites, burst sewerage streams, and still rain dams. Therefore, some of the pigs might have inadvertently eaten the snail hosts. However, a similar study in Uganda failed to detect F. hepatica in pigs (Roesel et al. 2017).
The proportion of pigs carrying ecto-parasites (96.5%) in this study was higher than those reported in Mbeya in Tanzania (84%) (Braae et al. 2013) and in Nigeria (50.75%) (Odo et al. 2016). H. suis was predominantly identified around the ears, neck, and abdomen (Braae et al. 2013), and these may have been contracted through direct contact with neighbouring pigs and/or during pasturing (Damriyasa et al. 2004; Braae et al. 2013). According to Islam et al. (2006), H. suis causes hair loss, dandruff, and thickening of the skin in pigs. Although, previous researchers have suggested a link between H. suis and ASF or swine pox (Sanchez & Badiola 1966; Doster 1995; Permin et al. 1999; Damriyasa et al. 2004), our results did not confirm this suggestion. Based on the veterinary health records in the province, the Mkhondo municipality has never had an ASF outbreak, yet the prevalence of H. suis was 50.0%. However, the other two municipalities that had H. suis (Msukaligwa at 37.5% and Govan Mbeki at 12.5%) both had ASF outbreaks in the previous years. Furthermore, Dipaliseng and Lekwa have reported numerous outbreaks of ASF, yet, all pigs from these municipalities tested negative for H. suis. It is worth noting that this is the first report of H. suis in pigs from Mpumalanga Province.
According to findings reported here, Sarcoptes scabiei was prevalent among FRPs (62.9%). Around the world, Sarcoptic mange is the most frequent external parasite of pigs. It is not unusual to report more than 50% prevalence for Sarcoptic mange in pigs (Odo et al. 2016). For example, Islam et al. (2006) reported a prevalence of 57.30% in Bangladesh, while Kagira et al. (2013) reported 63.7% in Kenya. A high proportion of infected sows infested with ecto-parasites has been related to a high rate of piglet mortality (Ózsvári 2018). However, Jufare and colleagues have linked variation in the proportion of pigs infested with parasite to changes in management systems, pig breed, nutrition, climatic conditions, animal health practices, and government policies, which are the key barriers in pig farming. In this study, the high occurrence of parasites is attributed to the fact that pigs were allowed to roam freely in the peri-urban area.
The overall the burden of A. suum infection in the peri-urban FRP of GSDM in Mpumalanga Province was 37.8%. However, research in the neighbouring province of Free State (SA) found a higher prevalence of Ascaris suum (44.5%). Pigs aged 12 months and older had a higher percentage with A. suum (33.6%).
Previous results have also noted A. suum as the most common parasite among scavenging pigs (Kumar et al. 2002; Ngowi et al. 2004; Tamboura et al. 2006; Matos et al. 2011; Kagira et al. 2013; Odo et al. 2016). The high infestation with A. suum among growing pigs has been documented as a cause of poor growth, anaemia and even death (Nsoso et al. 2000; Ngowi et al. 2004; Tamboura et al. 2006; Tomass et al. 2013; Taylor 2015; Jufare et al. 2015). The A. suum eggs can survive inclement weather and some chemicals, and they may last for extended periods of time while still being viable and infectious (Roepstorff & Nansen 1998). This could explain the high prevalence of A. suum in FRPs in the present and previous studies.
Results reported in Table I revealed that the relationship between A. suum and Coccidia spp. was statistically significant (p < 0.05). This suggests that a high prevalence of A. suum in young pigs seven months and younger could be linked to a high prevalence of Coccidia spp in these age groups. Furthermore, this can be understood to mean that pigs infected with A. suum are more likely to be infected with Coccidia spp. or vice versa. Furthermore, according to Nonga and Lugendo (2015), A. suum infestation is linked somewhat with pig body condition (p < 0.05).
Results of the present study did not demonstrate the existence of an association between age and different parasites (p > 0.05). This contrasts with findings of studies conducted in Northern Ethiopia and Bangladesh, which found an association between parasites and age (Islam et al. 2006). On the other hand, this study agreed with findings of the study by Tomass et al. (2013), who found no association between sex and parasites.
Strongylid nematodes were observed in only 10.2% of the pigs tested in the present study. However, this was slightly higher than the 6.6% reported in South Korea (Lee et al. 2022). Mkhondo municipality with a percentage of 31.1% had the highest prevalence of Strongylids. Furthermore, Strongylids were most prevalent in emaciated to thin pigs (58.6%) and pigs older than 12 months (51.7%). The high percentage of Strongylids may be attributed to Mkhondo municipality's high rainfall, which ranges from 701 mm to 1 200 mm when compared to other municipalities. As previously stated, the majority of Strongylids were detected in older pigs, and this could be attributable to the fact that older pigs are usually large and hence are not usually included in the pigs that are treated. This is because it is difficult to handle large pigs without proper handling equipment.
Coccidia, a protozoan parasite was observed in 53.7% of the peri-urban FRP in GSDM. This is much higher than the 0.3% recorded in South Korea (Lee et al. 2022); 5.6% in Ethiopia (Abdu & Gashaw 2010); 29.9% in China (Gong et al. 2021) and 12% in Kenya (Jufare et al. 2015). However, findings of the present study are lower than the 72.7% observed in Free State (Nwafor et al. 2019). The highest proportion of pigs positive for Coccidia was seen in Govan Mbeki municipality (39.5%; p < 0.05), Msukaligwa (22.4%; p < 0.05), and Dipaliseng (19.7%; p < 0.05). Coccidia spp. was more prevalent in female pigs (61.2%) and pigs with a thin to ideal body condition score (74.4%). Exotic breeds (75.67%) and 12-month-old pigs (40.8%) were the most afflicted by Coccidia spp. Meanwhile, in China, the prevalence of Coccidia infection was 19.9% in the suckling and 26.2% in the finishing age groups.
Coccidia infections had a moderately positive correlation with the presence of other parasites including T. suis (r = 0.366; p < 0.05) and Strongylids (r = 0.244; p < 0.05). The prevalence of Coccidia in this study was associated with the observed feeding of dead chicken (poultry), continuous use of feeders without cleaning, and drums used for swill storage that were not cleaned or sterilised. The high prevalence of Coccidia in pigs causes diarrhoea, anorexia, weight loss in piglets, haemorrhage, reduced weight gain (Dione et al. 2018; Nwafor et al. 2019; Gong et al. 2021). In addition, Strongylids have previously been linked to diarrhoea in pigs, which can lead to weight loss and even death. This could explain why pigs in this study that had Strongylids were found to have BCS that varied from emaciated to thin. The negative effects of the parasites causes stunted growth in the pig, hence delaying marketing which can exacerbate financial loss in the pig industry.
Trichuris suis, had a lower prevalence in the GSDM (13.4%) but a higher prevalence in Dipaliseng (71.0%; p < 0.05). Female pigs and pigs older than 12 months were the most infested with T. suis, (63.2% and 50%, respectively). Furthermore, T. suis was most prevalent in exotic breeds (68.4%) and in pigs with BCS ranging from emaciated to thin (65.8%). However, no pigs with BSC ranging from fat to overfat were infected with T. suis. These findings suggest that T. suis is associated with loss of weight and poor BCS among pigs, which can result in low profit and poor pig performance.
The high percentage of pigs with T. suis observed in this study, can be linked to poor management or husbandry techniques among peri-urban FRP. It could also be explained by the fact that T. suis eggs are resilient and can tolerate unfavourable environmental circumstances for up to four years (Urquhart et al. 2003; Nwafor et al. 2019). This increases the risk of exposure of FRPs to the eggs of T. suis.
Siphonaptera flea is not a common parasite and hence it has rarely been studied in pigs. Of note, Siphonaptera flea was discovered in several peri-urban areas of Msukaligwa. The flea was most common in female pigs (57.1%), exotic breeds (100%), pigs with thin to ideal BCS (85.7%), and pigs younger than seven months (100%). Considering that Siphonaptera feeds on the skin, this study discovered that it occurs in exotic breeds and young pigs with ideal BCS. Siphonaptera was most likely discovered in young pigs, with soft skins like that of exotic breeds, rather than indigenous breeds.
Finally, previous workers have confirmed co-infestations with various parasites among free-roaming and intensively managed pigs, with a significant impact on their productivity (Kagira 2010; Greve 2012; Băieş et al. 2022). Co-infections may lead to severe negative effects (clinical signs, duration, and treatment) associated with the impact of multi-parasitism on pig health either through synergistic or antagonistic interaction, which may be complicated by resident microbial pathogens (Vaumourin et al. 2015; Kouam et al. 2022). Parasites, on the other hand, play a significant role as a hidden cause of economic loss, including slower growth rate, weight loss, and fewer litters of small size (Pattison et al. 1980; Wilson & Swai 2014; Odo et al. 2016). There is an urgent need to educate farmers who allow pigs to roam freely in peri-urban areas about the threat of parasites and preventative measures they can adopt.
According to Sowemimo et al. (2012), parasite infestation can be prevented or minimised by adopting appropriate management strategies such as routine daily cleaning and disinfection of the environment, feeding of high-quality commercial feed, and timely application of efficient anthelminthic drugs. Furthermore, according to Nonga and Lugendo (2015), understanding the disease's spectrum and epidemiology is critical in developing effective control strategies targeted at enhancing the country's pig industry. At the end of this investigation, an itinerary with the occurrence of parasites in each municipality and the necessary control measures will be established for FRP farmers.
Free-roaming pigs carry a variety of ecto- and endo-parasites in the peri-urban areas of GSDM, Mpumalanga Province with implications on animal and human health. This may directly negatively affect the performance and productivity of pigs reared under FRPs farming system.
Ascaris suum and T. suum were observed in this study. These parasites have zoonotic potential and hence the observed prevalence of these parasites in this study is of serious public health concern (Poudel 2018). According to Taylor (2015), zoonotic infections are a major threat for public health and other animals. Furthermore, de Silva et al. (2003) linked the T. suis and A. suum to the severity of A. lumbricoides and T. trichiura, known to infect 1 221 and 795 million individuals worldwide, respectively. This adds to the significance of observing A. suum and T. suum in the free-roaming pigs in the study area.
Conclusion
A total of nine different parasites were found including H. suis, S. scabiei, A. suum, F. hepatica, T. suis, Strongylids, Coccidia spp, M. expansa, and Siphonaptera. Parasitic infestation and infection is very high among FRPs, with approximately nine (9) out of every 10 FRP in Gert Sibande District Municipality positive for at least one or more parasites. These findings have implications for performance in these pigs characterised by lower growth rates, lower sow and boar reproductive efficiency, and lower feed conversion efficiencies (Hale & Stewart 1979; Polley & Mostert 1980; Hale et al. 1985; Ózsvári 2018) with resultant decreased income return in the pig farming industry.
Findings of this study confirmed that sex, age, breed, and body condition scores are not good predictors of internal and external parasitosis in pigs. This conclusion was also reached by Roesel et al. (2017) and Symeonidou et al. (2020) who also observed that these indicators do not predict pig parasitosis in small-scale pig enterprises in Central and Eastern Uganda, and in Greek farrow-to-finish pig farms respectively.
As found in this study, pigs older than 12 months were more susceptible to parasites than younger pigs. We also found that the most troublesome parasites in the peri-urban free-roaming practice of GSDM, Mpumalanga Province, were S. scabiei (69.3%) and Coccidia spp. (59.1%). Female pigs, pigs with thin ideal body condition scores, and exotic breeds were most likely to have parasite infestations under the peri-urban free-roaming practice. We attributed these findings to poor management and unrestrained movement. With the advent of numerous diseases in the Republic of South Africa, we suggest that agricultural regulations (peri-urban farming) be revised in order to control and prevent parasites in FRPs in peri-urban areas. Policy makers, animal researchers and veterinary services must focus on developing policies and risk communication and community engagement (RCCE) materials, which target pig farmers in peri-urban areas such as Gert Sibande District Municipality, Mpumalanga Province to reduce disease burdens in humans and animals.
Acknowledgements
We would like to thank the management of the M & D Bursary at the University of South Africa (UNISA) and the Department of Agriculture, Rural Development, Land and Environmental Affairs (DARDLEA) for funding this research. All peri-urban pig participants for allowing us to collect samples from their pigs, as well as the South African Society for Veterinary Epidemiology and Preventive Medicine (SASVEPM) for sponsoring attendance at the 19th SASVEPM conference in East London in 2022, where this work was presented to the wider scientific community in South Africa. Finally, yet importantly, we would like to thank the Mpumalanga Provincial Veterinary Laboratory (MPVL) for allowing us to analyse the ecto- and endo-parasite samples in their facilities.
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
The University of South Africa under the College of Agriculture and Environmental Science approved this study's ethical clearance with reference numbers 2019/CAES_HREC/108 and REC-170616-051. Section 20 was approved by the National Department of Agriculture in South Africa with reference number 12/11/1(1237).
ORCID
P Munzhelele https://orcid.org/0000-0002-0074-163X
JW Oguttu https://orcid.org/0000-0001 -6810-4437
CA Mbajiorgu https://orcid.org/0000-0002-6612-7982
FO Fasina https://orcid.org/0000-0003-3088-8752
References
Abdu, S., Gashaw, A., 2010, Production system dynamism and parasitic interaction of swine in and around Holetta, Ethiopia, Ethiopian Veterinary Journal 14(1), 71-82. https://doi.org/10.4314/evj.v14i2.63886. [ Links ]
Adebisi, O.R., 2008, Gastro-intestinal helminths and public health: Overview of a neglected sector, The Internet Journal of Veterinary Medicine 4(2), 72-78. [ Links ]
Adelakun, O.D., Abiola, J.O., Akande, F.A., 2021, Moniezia expansa in intensively raised pigs: A Possible First Report in Nigeria, Nigerian Veterinary Journal 42(2), 123-128. https://doi.org/10.4314/nvj.v42i2.3. [ Links ]
Adhikari, R.B., Adhikari Dhakal, M., Thapa, S., et al., 2021, Gastrointestinal parasites of indigenous pigs (Sus domesticus) in south-central Nepal, Veterinary Medicine and Science 7(5), 1820-1830. https://doi.org/10.1002/vms3.536. [ Links ]
Băieş, M.H., Boros, Z., Gherman, C.M., et al., 2022, Prevalence of swine gastrointestinal parasites in two free-range farms from nord-west region of Romania, Pathogens 11(9), 954. https://doi.org/10.3390/pathogens11090954 [ Links ]
Braae, U.C., Ngowi, H.A., Johansen, M.V., 2013, Smallholder pig production: incidence and risk factors of ectoparasites, Veterinary Parasitology 196(1-2), 241-244. https://doi.org/10.1016/j.vetpar.2012.12.058. [ Links ]
Coffey, R.D., Parker, G.P., Laurent, K.M., 1999, Assessing sow body condition. Ky. Coop. Ext. Serv. Rep. No. ASC-158. http://www2.ca.uky.edu/agcomm/pubs/asc/asc158/asc158.pdf. Accessed 13 October 2023. [ Links ]
Damriyasa, I.M., Failing, K., Volmer, R., et al., 2004, Incidence, risk factors and economic importance of infestations with Sarcoptes scabiei and Haematopinus suis in sows of pig breeding farms in Hesse, Germany, Medical and Veterinary Entomology 18(4), 361-367. https://doi.org/10.1111/j.0269-283X.2004.00520.x. [ Links ]
De Silva, N.R., Brooker, S., Hotez, P.J., Montresor, et al., 2003, Soil-transmitted helminth infections: updating the global picture, Trends in Parasitology 19(12), 547-551. https://doi.org/10.1016/j.pt.2003.10.002. [ Links ]
Department of Cooperative Governance and Traditional Affairs (DCGTA), 2020, A profile and Analysis of Gert Sibande District. Retrieved August 2023, from Final-Edited-Gert-Sibande-DM_26-June-2020-FINAL.pdf (cogta.gov.za). [ Links ]
Dione, M., Masembe, C., Akol, J., et al., 2018, The importance of on-farm biosecurity: Sero-incidence and risk factors of bacterial and viral pathogens in smallholder pig systems in Uganda, Acta Tropica 187, 214-221. https://doi.org/10.1016/j.actatropica.2018.06.025. [ Links ]
Doster, A.R., 1995, Skin diseases of swine, Swine Health Production 3(6), 256-261. [ Links ]
Gómez-Puerta, L.A., Lopez-Urbina, M.T., González, A.E., 2008, Occurrence of Moniezia expansa (Rud, 1810) Blanchard, 1891 (Cestoda: Anoplocephalidae) in domestic pig (Sus scrofa domestica Linnaeus, 1758) in Perú, Veterinary Parasitology 158(4), 380-381. https://doi.org/10.1016/j.vetpar.2008.08.019. [ Links ]
Gong, Q.L., Zhao, W.X., Wang, Y.C., et al., 2021, Incidence of coccidia in domestic pigs in China between 1980 and 2019: a systematic review and meta-analysis, Parasites & Vectors 14(1), 248. https://doi.org/10.1186/s13071-021-04611-x. [ Links ]
Greve, J.H., 2012, Internal parasites: helminths, Diseases of Swine, 10th ed.; Zimmerman, JJ, Karriker, LA, Ramirez, A., Schwartz, KJ, Stevenson, GW, Eds, pp.908-920. [ Links ]
Hale, O.M., Stewart, T.B., 1979, Influence of an experimental infection of Trichuris suis on performance of pigs, Journal of Animal Science 49(4), 1000-1005. https://doi.org/10.2527/jas1979.4941000x. [ Links ]
Hale, O.M., Stewart, T.B., Marti, O.G., 1985, Influence of an experimental infection of Ascaris suum on performance of pigs, Journal of Animal Science 60(1), 220-225. https://doi.org/10.2527/jas1985.601220x. [ Links ]
Islam, A., Majumder, S., Anisuzzaman, R.A., et al., 2006, Incidence and pathology of ticks and lice of pigs in relation to age and management systems in Bangladesh, International Journal of BioResearch 1, 22-27. [ Links ]
Jufare, A., Awol, N., Tsegaye, Y., et al., 2015, Parasites of pigs in two farms with poor husbandry practices in Bishoftu, Ethiopia, Onderstepoort Journal of Veterinary Research 82(1), 1-5. https://doi.org/10.4102/ojvr.v82i1.839. [ Links ]
Kagira, J.M., Kanyari, P.W., Maingi, N., et al., 2010, Characteristics of the smallholder free-range pig production system in western Kenya, Tropical Animal Health and Production 42, 865-873. https://doi.org/10.1007/s11250-009-9500-y. [ Links ]
Kagira, J.M., Kanyari, P.N., Githigia, S.M., et al., 2012, Risk factors associated with occurrence of nematodes in free range pigs in Busia District, Kenya, Tropical Animal Health and Production 44, 657-664. https://doi.org/10.1007/s11250-011-9951-9. [ Links ]
Kagira, J.M., Kanyari, P.N., Maingi, N., et al., 2013, Relationship between the incidence of ectoparasites and associated risk factors in free-range pigs in Kenya, International Scholarly Research Notices 2013. https://doi.org/10.1155/2013/650890. [ Links ]
Kaufmann, J., 1996, Parasitic infections of domestic animals: a diagnostic manual. ILRI (aka ILCA and ILRAD). https://doi.org/10.1007/978-3-0348-7666-7. [ Links ]
Kouam, M.K., Ngueguim, F.D., 2022, Prevalence, intensity, and risk factors for helminth infections in pigs in Menoua, Western Highlands of Cameroon, with Some Data on Protozoa, Journal of Parasitology Research 2022. https://doi.org/10.1155/2022/9151294. [ Links ]
Kumar, S., Prasad, K.D., Singh, S.K., 2002, Incidence of common gastrointestinal parasites in pigs at and around Ranchi, Jharkhand, Indian Journal of Animal Sciences 72(1), 35-37. [ Links ]
Kumsa, B., Kifle, E., 2014, Internal parasites and health management of pigs in Burayu District, Oromia Regional State, Ethiopia, Journal of the South African Veterinary Association 85(1), 1-5. [ Links ]
Laha, R., 2015, Sarcoptic mange infestation in pigs: an overview, Journal of Parasitic Diseases 39, 596-603. https://doi.org/10.1007/s12639-014-0419-5. [ Links ]
Lapage, G., 1968, Veterinary Parasitology. 2nd edn. Oliver and Boyd, Edinburgh and London. Pp. 1082-1120. [ Links ]
Lee, S., Alkathiri, B., Kwak, D., et al., 2022, Distribution of Gastrointestinal Parasitic Infection in Domestic Pigs in the Republic of Korea: Nationwide Survey from 2020-2021. The Korean Journal of Parasitology, 60(3), 207-211. https://doi.org/10.3347/kjp.2022.60.3.207. [ Links ]
Matos, C., Sitoe, C., Banze, J., et al., 2011, A pilot study of common health problems in smallholder pigs in Angónia and Boane districts, Mozambique, Journal of the South African Veterinary Association 82(3), 166-169. https://doi.org/10.4102/JSAVA.v82i3.55. [ Links ]
Munzhelele, P., 2015, Evaluation of the production systems and constraints of smallholder pig farming in three agro-ecological zones of Mpumalanga province, South Africa (Masters dissertation). https://doi.org/10.1007/s11250-016-1158-7. [ Links ]
Munzhelele, P., Oguttu, J., Fasanmi, O.G., et al., 2017, Production constraints of smallholder pig farms in agro-ecological zones of Mpumalanga, South Africa, Tropical Animal Health and Production 49, 63-69. https://doi.org/10.1007/s11250-016-1158-7. [ Links ]
Munzhelele, P., Oduniyi, O.S., Scheltens, M.L., et al., 2021, Determinants of market choice and strategies adopted by small-scale pig producers in redline areas of Mpumalanga Province, South Africa: A Fractional Outcome-Tobit Model Approach, Journal of Hunan University Natural Sciences 48(12). [ Links ]
Ngowi, H.A., Kassuku, A.A., Maeda, G.E.M., et al., 2004, A slaughter slab survey for extra-intestinal porcine helminth infections in northern Tanzania, Tropical Animal Health and Production 36(4), 335-340. https://doi.org/10.1023/B:TROP.0000026663.07862.2a. [ Links ]
Nonga, H.E., Lugendo, F., 2015, Incidence of mange infestation in smallholder pig farms in selected areas of Mpwapwa town, Tanzania. [ Links ]
Nonga, H.E., Paulo, N., 2015, Incidence and intensity of gastrointestinal parasites in slaughter pigs at Sanawari slaughter slab in Arusha, Tanzania. [ Links ]
Nsoso, S.J., Mosala, K.P., Ndebele, R.T., et al., 2000, The incidence of internal and external parasites in pigs of different ages and sexes in Southeast District, Botswana, Onderstepoort Journal of Veterinary Research 2000 Sep, 67(3), 217-20. [ Links ]
Nsoso, S.J., Mannathoko, G.G., Modise, K.G., 2006, Monitoring production, health and marketing of indigenous Tswana pigs in Ramotswa village of Botswana, Livestock Research in Rural Development 18(9). [ Links ]
Nwafor, I.C., Roberts, H., Fourie, P., 2019, Incidence of gastrointestinal helminths and parasites in smallholder pigs reared in the central Free State Province, Onderstepoort Journal of Veterinary Research 86(1), a1687. https://doi.org/10.4102/ojvr.v86i1.1687. [ Links ]
Odo, G.E., Agwu, EJ., Ossai, N.I.K., et al., 2016, A survey of ectoparasites of local pigs, Sus scrofa domesticus at Emene Town area in Enugu State, Academia Journal of Biotechnology 4, 126-137. [ Links ]
Omudu, E.A., Amuta, E.U., 2007, Parasitology and urban livestock farming in Nigeria: prevalence of ova in faecal and soil samples and animal ectoparasites in Makurdi, Journal of the South African Veterinary Association 78(1), 40-45. https://doi.org/10.4102/JSAVA.v78i1.285. [ Links ]
Ózsvári, L., 2018, Production impact of parasitisms and coccidiosis in swine, Journal of Dairy, Veterinary and Animal Research 7(5), 217-222. https://doi.org/10.15406/jdvar.2018.07.00214. [ Links ]
Pattison, H.D., Thomas, RJ., Smith, W.C., 1980, A survey of gastrointestinal parasitism in pigs, The Veterinary Record 107(18), 415-418. [ Links ]
Permin, A., Yelifari, L., Bloch, P., et al., 1999, Parasites in cross-bred pigs in the Upper East Region of Ghana, Veterinary Parasitology 87(1), 63-71. https://doi.org/10.1016/S0304-4017(99)00159-4. [ Links ]
Polley, L.R., Mostert, P.E., 1980, Ascaris suum in Saskatchewan pigs: An abattoir survey of incidence and intensity of infection, The Canadian Veterinary Journal 21(11), 307. [ Links ]
Roepstorff, A., Nansen, P., 1998, Epidemiology, diagnosis and control of helminth parasites of swine, Rome, Food and Agricultural Organization, 36, 245-257. [ Links ]
Roesel, K., Dohoo, I., Baumann, M., et al., 2017, Prevalence and risk factors for gastrointestinal parasites in small-scale pig enterprises in Central and Eastern Uganda, Parasitology Research, 1 16, 335-345. https://doi.org/10.1007/s00436-016-5296-7. [ Links ]
Sanchez-Botija, C., Badiola, C., 1966, Presencie of the African swine pest virus in Haematopinus suis, Bulletin-Office International des épizooties 66, 699-705. https://doi.org/10.20506/bull.art.66.L2968 [ Links ]
Poudel, S., 2018, Prevalance of gastrointestinal parasites of domestic pig (Sus Scorfa Domesticus Carl Linnaeus, 1758) in two farms of Pokhara Valley (Masters dissertation, Department of Zoology). https://elibrary.tucl.edu.np/handle/123456789/15298. [ Links ]
Soulsby, E.J.L., 1982, Helminths, arthropods and protozoa of domesticated animals, 7th Edition, VetBooks. [ Links ]
Sowemimo, O.A., Asaolu, S.O., Adegoke, F.O., et al., 2012, Epidemiological survey of gastrointestinal parasites of pigs in Ibadan, Southwest Nigeria, Journal of Public Health and Epidemiology 4(10), 294-298. https://doi.org/10.5897/JPHE12.042. [ Links ]
SPSS, 2021, IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp. [ Links ]
Stata Corp. 2019, Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC. [ Links ]
Stewart, B.T., Hoyt, P.G., 2006, Internal parasites of swine: in Diseases of Swine. Symeonidou, I., Tassis, P., Gelasakis, A.I., et al., 2020, Prevalence and risk factors of intestinal parasite infections in Greek swine farrow-to-finish farms, Pathogens 9(7), 556. https://doi.org/10.3390/pathogens9070556. [ Links ]
Tamboura, H.H., Banga-Mboko, H., Maes, D., et al., 2006, Incidence of common gastrointestinal nematode parasites in scavenging pigs of different ages and sexes in eastern centre province, Burkina Faso, Onderstepoort Journal of Veterinary Research 73(1), 53-60. https://doi.org/10.4102/ojvr.v73i1.169. [ Links ]
Taylor, M.A., Coop, R.L., Wall, R.L., 2015, Veterinary parasitology. John Wiley & Sons. https://doi.org/10.1002/9781119073680. [ Links ]
Tomass, Z., Imam, E., Kifleyohannes, T., et al., 2013, Incidence of gastrointestinal parasites and Cryptosporidium species in extensively managed pigs in Mekelle and urban areas of southern zone of Tigray region, Northern Ethiopia, Veterinary World 6(7). https://doi.org/10.5455/vetworld.2013.433-439. [ Links ]
Urquhart, G.M., Armour, J., Duncan, J.L., et al., 2003. Veterinary Parasitology 2nd ed. Black well science Ltd.Oxford. [ Links ]
Vaumourin, E., Vourc'h, G., Gasqui, P., et al., 2015, The importance of multiparasitism: examining the consequences of co-infections for human and animal health, Parasites and vectors 8(1), 1-13. https://doi.org/10.1186/s13071-015-1167-9. [ Links ]
Webster, J., 2016., Animal welfare: freedoms, dominions and "A life worth living", Animals 6(6), 35. https://doi.org/10.3390/ani6060035. [ Links ]
Wilson, R.T., Swai, E.S., 2014, Pig production in Tanzania: a Critical Review, Tropicultura 32(1). [ Links ]
 Correspondence:
Correspondence:
email: priscilla.munzhelele@gmail.com
Appendix 1: Pictures taken during data collection.






Appendix 2: Score sheet for weighing pigs, faecal and skin scrape samples















