Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.95 n.1 Pretoria 2024
http://dx.doi.org/10.36303/JSAVA.572
ORIGINAL RESEARCH
Ketamine-butorphanol-medetomidine for the immobilisation of free-living hyenas (Crocuta crocuta)
A RougI; L MeyerII; L NetshitavhaduluIII; M LeiberichII; P BussI
IDepartment of Production Animal Studies and Centre for Veterinary Wildlife Research, Faculty of Veterinary Science, University of Pretoria, South Africa
IIDepartment of Paraclinical Sciences and Centre for Veterinary Wildlife Research, Faculty of Veterinary Science, University of Pretoria, South Africa
IIIWildlife Veterinary Services, Kruger National Park, South African National Parks, South Africa
ABSTRACT
Free-ranging spotted hyenas (Crocuta crocuta) are immobilised for a variety of purposes, including wildlife-human conflict mitigation, research, and veterinary treatment. Combinations of tiletamine-zolazepam (Zoletil) and medetomidine are commonly used for immobilisation of hyenas, however, recovery times are long.
In this descriptive study, a total of 20 adult or subadult free-ranging hyenas were immobilised near Skukuza in the Kruger National Park using ketamine, butorphanol, and medetomidine. The goal of the study was to evaluate a suitable dose and measure cardiorespiratory effects of this combination. The quality of induction and recovery were scored using an established scoring system from 1 (excellent) to (poor).
Twelve of the 20 hyenas were given an induction score of 1 (excellent), five an induction score of 2 (good), and three an induction score of 3 (fair). Of the animals with induction score = 1, the mean drug dose was 1.17 mg/kg ketamine, 0.25 mg/kg butorphanol and 0.03 mg/kg medetomidine, and the mean induction time and time to handling 6:25 minutes and 9:46 minutes respectively. The mean recovery time (from reversal to standing) was 10:16 min, which is shorter than what has been reported for tiletamine-zolazepam-based combinations in hyenas. Most hyenas were bradycardic (< 40 beats per minute) and the mean PaO2 69.5 mmHg. Three hyenas, one with induction score = 2, and two with induction scores = 3 spontaneously recovered at 33, 44 and 56 minutes post approach respectively. Regardless of induction time, all hyenas reached a level of surgical anaesthesia while immobilised.
Overall, ketamine-butorphanol-medetomidine (KBM) was effective in immobilising hyenas but induction times varied, and animals were bradycardic during immobilisation.
Keywords: spotted hyena, ketamine, butorphanol, medetomidine, capture
Introduction
Free-ranging spotted hyenas (Crocuta crocuta) are immobilised for various purposes, including wildlife-human conflict mitigation, research and medical interventions, such as snare removal. Tiletamine-zolazepam (Zoletil), a relatively long-acting cyclohexane-benzodiazepine combination, co-administered with medetomidine, an alpha-2 adrenergic agonist that is reversible with atipamezole, have previously been used for the immobilisation of free-living hyenas (Kock & Burroughs, 2021; Van Jaarsveld, 1988). Although effective and safe with relatively short induction times, this combination can result in prolonged recovery times and ataxia despite the reversal of medetomidine. Ideally, in free-living situations, recoveries should be as short as possible to limit attacks by other predators and to minimise environmental effects on the animal's body temperature. Further, a short induction time is critical when immobilising hyenas as they frequently run away after darting, even if immobilised near a bait. Combinations of ketamine (K), a short-acting cyclohexane, the opioid agonist/antagonist butorphanol (B) and medetomidine (M; KBM) have been successfully used for immobilisation of other carnivore species, including captive and free-ranging servals (Leptailurus serval) (Blignaut, 2021; Langan et al., 2000), captive red wolves (Canis rufus) (Larsen et al., 2002) and African lions (Panthera leo) (Donaldson et al., 2023). This combination has also been used in a wide range of other species including captive juvenile Thomson gazelles (Gazella thomsoni) (Chittick et al., 2001), Eurasian badgers (Meles meles) (De Leeuw et al., 2004), red kangaroos (Osphranter rufus) (Makrin-Dray et al., 2021) and zebra (Equus zebra) (Stemmet et al., 2019). An advantage of KBM over Zoletil-based combinations is that ketamine is shorter acting and recovery times is therefore shorter. This combination could potentially be of interest for the field immobilisation of hyenas.
The objective of this descriptive study was to evaluate whether KBM is suitable for immobilisation of free-living hyenas. Specifically, the aim was to determine an efficacious immobilisation dose by evaluating induction and recovery time and quality; and, assessing physiological effects. The observations and variables measured in the descriptive study were compared to what has been reported in the literature for other immobilisation combinations in hyena.
Methods
The study was conducted in collaboration with the South African National Parks (SANParks) Veterinary Wildlife Services and approved under the SANParks permit # SS666 and the University of Pretoria ethics protocol # REC015-22. Hyenas were captured by free-range darting or using cage traps near Skukuza in the Kruger National Park (24.9964° S, 31.5919° E). Two types of cage traps were used. One was a 2 m high fence of approximately 4 m (length) x 3 m (width) that surrounded a tree to which bait of impala meat was secured. The trap door was operated manually with a long rope and closed after one or more hyenas entered the trap to eat the bait. Several smaller cage traps of approximately 1.5 m x 3 m x 1.5 m were placed in the vicinity of the larger trap or in clearings frequented by hyena and near accessible service roads. Bait was tied to a release mechanism that triggered a fall door in the back of the trap when a hyena pulled on the bait. To attract hyena to both the larger and smaller traps, a recording of either a buffalo calf bellowing or hyena vocalising and feeding at a carcass were played using a loudspeaker system (Foxpro, Lewistown, Pennsylvania, USA). Trapping was only conducted for approximately six hours in the late afternoon and evening, and traps were continuously checked during that time. The exact trap times were unknown, but we estimate that no animal in the study remained in the trap for more than two hours. Traps were secured closed when not in use for the project. Regardless of whether in the trap or free-ranging, the hyenas were darted using a CO2 pressured dart rifle (JM Special, DanInject, Kolding, Denmark) and 3 ml DanInject darts with collared needles of 30 mm x 2 mm.
A pilot study was conducted with three hyenas that were darted with an approximate dose of KBM: 1.5 mg/kg ketamine + 0.25 mg/kg butorphanol + 0.05 mg/kg medetomidine. The pilot animals had marked bradycardia and bradypnoea and the dose was reduced to approximately 1.1 mg/kg ketamine + 0.22 mg/kg butorphanol + 0.03 mg/kg medetomidine. Hyenas' weights were estimated visually before they were darted from either a vehicle (if free-ranging) or outside a trap. After darting, the animals in the traps were observed quietly from a distance until recumbent. The free-ranging animals were quietly observed from the vehicle and approached on foot when recumbent. The induction time was measured as time from darting to the hyena becoming recumbent and the induction was scored using an established scoring system (Wenger et al., 2010) (Supplementary Table I). After recumbency, we waited an additional few minutes before approaching the animals (time to handling). The hyena was then blindfolded, the eyes lubricated with ophthalmic ointment, placed on a stretcher, and driven to a processing location approximately 500 metres from the site of capture.
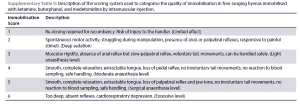
Once monitoring devices were attached to the hyenas, monitoring was started ten minutes after the initial handling (T0). Physiological variables were recorded at five minute intervals. Heart rate (HR) was measured by cardiac auscultation; respiratory rate (RR) by counting thoracic/abdominal excursions; and body temperature (RT) measured rectally with a digital thermometer (Hanna Checktemp 1, Hanna Instruments (Pty) Ltd., NE, USA). The oxygen haemoglobin saturation (SpO2%) was measured by attaching a pulse oximeter (Nonin PalmSat 2500, Nonin, Plymouth, MN, USA) to the tongue. Systolic, diastolic, and mean arterial pressures (SAP, DAP, and MAP) were measured by inserting a 22-gauge x 1 inch catheter (B. Braun Medical, Johannesburg, Gauteng, South Africa) into the palmar digital artery and connecting it via a pressure transducer (Deltran, Utah Medical Products, Midvale, UT, USA) placed at heart level to an intra-arterial blood pressure monitor (IntraTorr, IntraVitals, Coventry, United Kingdom). The depth of immobilisation was scored from 1-6 using a scoring system previously used in a study on lions (Panthera leo) (Supplementary Table II; (Wenger et al., 2010).
Arterial blood samples were drawn from the catheter into pre-heparinised 1 ml syringes at T0, T20 and T30. Samples were immediately analysed with a blood gas analyser (EPOC, Heska, Loveland, CO, USA) that measures arterial pH, partial pressure of carbon dioxide (PaCO2), partial pressure of oxygen (PaO2), base excess (BE), bicarbonate (HCO3-), total carbon dioxide (tCO2), sodium (Na+), potassium (K+), calcium (Ca++), chloride (Cl-), glucose, haemoglobin, haematocrit, and lactate. Hyena's gender was determined by palpating the scrotum/pseudoscrotum, and an approximate age determined by assessing tooth wear. After monitoring for 30 minutes, animals were weighed, moved approximately 100 m from the processing site, and the immobilisation was partly antagonised by intramuscular injection of 5 mg atipamezole per mg medetomidine and 2 mg naltrexone per mg butorphanol. After reversal, the animals were observed from a distance until ambulatory. The recovery time and the recovery scores were assessed as described in Wenger et al., 2010 (Supplementary Table III).
Alveolar-arterial oxygen partial pressure gradient P(A-a)O2 was determined as previously described (Meyer et al., 2015). Normality of variables was assessed using histograms. The drug doses, induction, and recovery times were summarised by induction score. Significant differences in the drug dose (mg/kg) between induction scores was assessed using the Kruskal-Wallis test. The physiological variables measured were summarised using descriptive statistics, and key variables were graphed over time using ggplot2 in R (Wickham, 2016). Mixed effects logistic regression models using the package lme4 in R (Bates et al., 2015) were used to evaluate the effect of time of sampling, sex, and estimated age on the HR, RR, T, SpO2, PaO2, PaCO2, A-a gradient, and MAP. The ID of the animal was inserted as a random effect to account for repeated measurements from each animal. Non-normally distributed variables (HR and RR) were log transformed. The normality of residuals was estimated using qq-plots. P-values < 0.05 were considered significant.
Results
A total of 20 hyenas, nine males and eleven females with a weight range of 30 to 67.5 kg, were successfully enrolled into the study. Of these, 16 were trapped (six in a large trap, ten in small traps) and four were free-range darted. Induction scores varied; twelve animals (six males and six females; one free-range darted and eleven trapped) had induction score = 1 (excellent), five animals (one male, four females; two free-ranged darted and three trapped) induction score = 2 (good), and three animals (one female, two males; one free-range darted, two trapped) induction score = 3 (fair). Three hyenas, one with induction score 2, and two with induction scores = 3 spontaneously recovered at 33, 44 and 56-minutes post approach respectively. The mean drug dose of hyenas with induction score = 1 was 1.17 mg/kg ketamine, 0.25 mg/kg butorphanol and 0.035 mg/kg medetomidine (Table I, Figure 1). All but one of the hyenas with induction scores 2 or 3 received less or equal to 1.06 mg/kg ketamine, 0.22 mg/kg butorphanol, 0.033 mg/kg medetomidine (Figure 1, Table I). One of the hyenas that spontaneously recovered and had induction score = 3 received 1.33 mg/kg ketamine, 0.28 mg/kg butorphanol, and 0.04 mg/kg medetomidine (Figure 1, Table I), however, the dart placement was suboptimal in this animal, which may have influenced drug absorption. There were no significant differences in the drug doses (mg/kg) between induction scores (p > 0.08 for ketamine, butorphanol, and medetomidine).
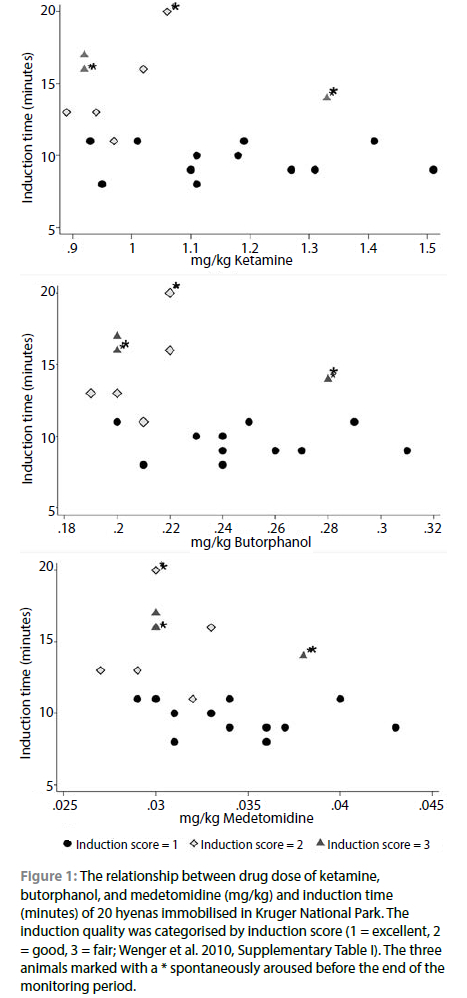
Of the animals with induction score = 1, the mean induction time was 6:25 minutes (SD = 1:40, range = 4:09-9:00 min) and 0.25 mg/kg butorphanol and 0.035 mg/kg medetomidine (Table I, Figure 1). All but one of the hyenas with induction scores 2 or 3 received less or equal to 1.06 mg/kg ketamine, 0.22 mg/kg butorphanol, 0.033 mg/kg medetomidine (Figure 1, Table I). One of the hyenas that spontaneously recovered and had induction score = 3 received 1.33 mg/kg ketamine,0.28 mg/kg butorphanol, and 0.04 mg/kg medetomidine (Figure 1, Table I), however, the dart placement was suboptimal in this animal, which may have influenced drug absorption. There were no significant differences in the drug doses (mg/kg) between induction scores (p > 0.08 for ketamine, butorphanol, and medetomidine).
Of the animals with induction score = 1, the mean induction time was 6:25 minutes (SD = 1:40, range = 4:09-9:00 min) and the mean time to handling 9:46 minutes (SD = 1:11, range = 8:00-11:26 min). The recovery time averaged 10:16 min (SD = 6:33 min, range = 4-22 min). The hyenas showed some ataxia immediately after becoming ambulatory, but gained stability within a few minutes, and all were able to leave the area immediately. For hyena with induction score = 2 the mean induction time was 10 minutes (SD = 1:59 min, range = 8-15 min) and the mean recovery time was 5:00 min. For hyenas with an induction score = 3 (fair), the mean induction time was 12:40 minutes (SD = 2:04 min, range = 11-15 min) and the recovery time for the one animal that did not spontaneously recover was 3:00 min.
Regardless of the induction score, all hyenas were completely immobilised after induction and had immobilisation scores of 5 at all measured time points, as evident by smooth, complete relaxation, extractable tongue, loss of palpebral reflex and jaw tone, no involuntary tail movements, no reaction to blood sampling, safe handling. (Surgical anaesthesia level, Supplementary Table II). This included the three animals that spontaneously recovered.
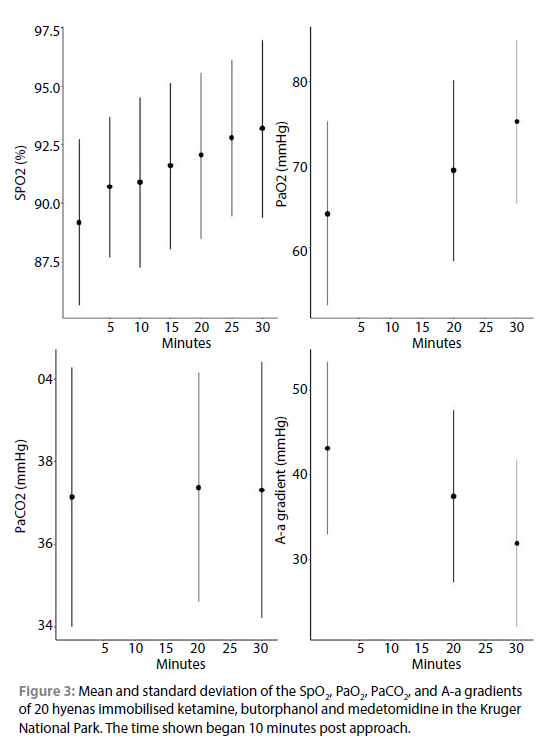
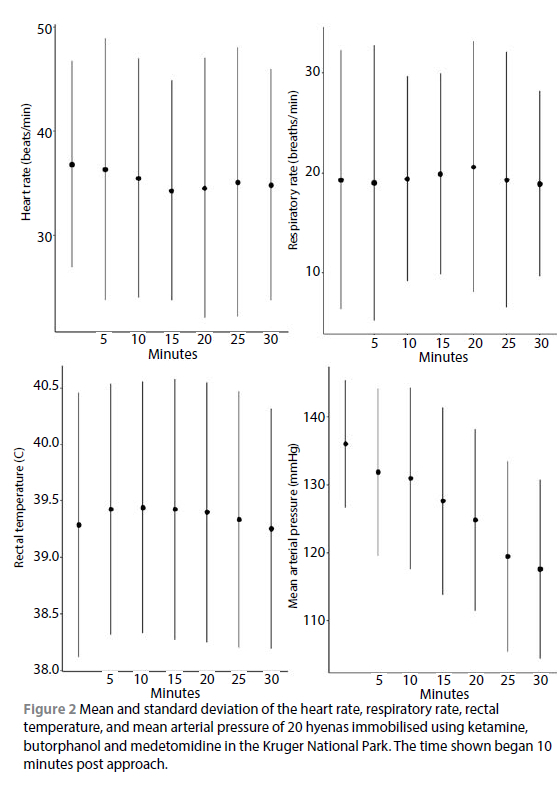
Most hyenas had a low HR, which decreased significantly over time (p = 0.006); for 11/20 of the hyenas the HR remained under 40 beats per minute for the entire immobilisation (Table II, Figure 2). Most hyenas were mildly hypertensive (mean = 126.6 mmHg, SD = 14.0 mmHg and overall the MAP significantly decreased over time (p < 0.001, Figure 2). Further, the MAP was significantly higher in the females compared to the males (p = 0.010). The PaO2 readings ranged widely (36.9-91.2 mmHg) with a mean (SD) of 69.5 ± 11.5 mmHg , but overall, increased over the duration of the immobilisation (p < 0.001) (Table II, Figure 3). The mean PaCO2 was < 40 mmHg for 17/20 of the hyenas. The mean (SD) A-a gradient was 37.5 ± 11.0 mmHg and the A-a gradient decreased significantly over time (p < 0.001, Figure 3). The HR, RR, SpO2, PaO2, PaCO2, A-a gradient and RT did not differ significantly between males and females or by age. The haematocrit, haemoglobin, sodium, potassium, calcium, glucose, chloride and lactate values were within clinically published ranges (Suedmeyer, 2015) (Table II).
Discussion
The combination of ketamine, butorphanol and medetomidine administered to free-ranging hyenas produced adequate immobilisation for safe handling for up to 60 minutes in 17/20 of the animals, but resulted in variable induction times. A dose of 1.17 mg/kg ketamine, 0.25 mg/kg butorphanol, and 0.03 mg/kg medetomidine provided the best induction quality and shortest induction times (mean = 6:25 minutes, range 4-9 minutes). Regardless of induction score, once immobilised all animals were adequately deep to perform minor procedures such as blood draws and ear notching.
A rapid induction time is important when immobilising free-ranging hyenas as they can run a long distance after darting. As exact weights were not known before preparing the dart, the drug doses administered ranged slightly, and most animals with excellent induction scores were in the upper end of the range administered. This likely indicates that the animals with longer induction times simply received a slightly lower drug dose on average. However, based on the immobilisation quality criteria used in the study, the quality of immobilisation after induction was indistinguishable regardless of induction time in most cases. Trapping and darting are stressful events for any animal, and high levels of endogenous catecholamines can reduce the effectiveness of the alpha-2 agonists in reducing excitatory neurotransmitter release (Sinclair, 2003), which may explain the slower induction with lower doses. The induction times observed in this study for animals that had excellent induction scores were similar to what was reported in several other studies of hyenas. Brown hyenas darted in the field in Namibia with 3-4 mg/kg ketamine and 0.035-0.045 mg/kg medetomidine, showed effects within three minutes and were recumbent within seven minutes (Hahn et al., 2014). Similarly, Anthony (2018) reported mean (SD) induction times of 7 (5.5) minutes when using 1 mg/kg zoletil plus 0.02 mg/kg medetomidine in captive hyenas, and the induction time was reduced to 4.2 (2.1) minutes when increasing the medetomidine dose to 0.04 mg/kg. Further, hyenas immobilised with a mean (SD) of 10.7 (1.9) mg/kg ketamine plus 0.5 (0.1) mg/kg xylazine reportedly became recumbent in a mean (SD) of 7 (2.36) minutes (Van Jaarsveld et al., 1984).
A short recovery time is especially important for free-ranging hyenas who need to be able to navigate obstacles and avoid predators after being released in the field. The recovery times observed in this study were similar to what was reported when using ketamine-medetomidine and reversing with atipamezole in brown hyenas (Hahn et al., 2014), however, they were shorter than what was reported for captive hyenas immobilised with Zoletil-medetomidine (Anthony, 2018) or Zoletil alone (Van Jaarsveld, 1988). The relatively short recovery time observed with the KBM combination is one clear advantage over Zoletil-based combinations.
HRs remained < 40 bpm in over half of the hyenas. Slow HR is a common adverse effect of alpha-2 agonists, such as medetomidine, which initially causes vasoconstriction and reflex bradycardia followed by a decrease in systemic vascular resistance through vasodilatation (Posner, 2018). Normal resting HRs for awake hyenas are not available, but Anthony (2018) proposed a normal HR of 85 beats per minute using the Schmidt-Nielson equation. In comparison, Anthony (2018) also reported HRs of a mean of 40-42 bpm for captive hyenas immobilised with Zoletil-medetomidine. In brown hyenas immobilised with ketamine-medetomidine in Namibia, HRs averaged 40-60 bmp (Hahn et al., 2014).
Respiratory depression and hypoxaemia are also commonly observed in animals immobilised with combinations containing alpha-2 agonists (Kock & Burroughs, 2021). Hypoxaemia is generally defined as a PaO2 < 80 mmHg and an SpO2 < 95%. Severe hypoxaemia is defined as a PaO2 < 60 mmHg and an SpO2 of less than 90% (Haskins, 2015). The PaO2 of most of the hyenas fell in between these two categorisations with a mean (SD) PaO2 of 69.5 (11.5). In most cases the PaCO2 was not elevated (mean PaCO2 was < 40 mmHg), indicating adequate ventilation and riddance of CO2. Alveolar-arterial oxygen difference (A-a gradient) describes the difference in oxygen partial pressure between the gas in the alveoli and the blood in the pulmonary capillaries. An elevated gradient indicates suboptimal alveolar-arteriolar oxygen transfer, which can be caused by pulmonary hypertension resulting in congestion and alveolar-interstitial oedema, or ventilation/perfusion mismatching (Kock & Burroughs, 2021). A reference value for normal A-a gradients is not available for hyenas but in domestic dogs a gradient around 10-15 mmHg is considered normal (Rieser 2013). The A-a gradients were mildly elevated (mean = 37.5, SD = 11.0) indicating some reduction in the effective gas exchange, however the exact cause could not be determined with the data collected.
The level of hypoxaemia observed in the hyenas in this study was lower than what was reported in two other studies, for example, Anthony (2018) reported mean PaO2 values of 56.0 and 57.7 mmHg in captive hyenas immobilised with two doses of Zoletil-medetomidine, despite PaCO2 values of 35.1-37.1 mmHg. Arterial partial pressure of oxygen was not measured for brown hyenas immobilised in Namibia with ketamine-medetomidine, but pulse oximetry was initially low (< 75%) and improved to >90% with 20-30 min of anaesthesia (Hahn et al., 2014).
One adult female hyena had comparatively high HRs (range 54-76 beats per minute), high RRs (range 56-76 breaths per minute), and low PaO2 (range 36.9-50.7 mmHg). The A-a gradient of this hyena was also high (range of 55-71 mmHg) indicating very poor oxygen exchange, however the PaCO2 was not elevated (34-36 mmHg), likely due to the rapid RR. These findings could be attributed to the hyena having eaten a large amount of bait prior to the immobilisation. In free-ranging animals, it is naturally not possible to fast animals prior to darting, and the data from this hyena illustrates how compromising a full stomach and intestines can be during immobilisation, likely due to pressure on the thoracic cavity. Reducing down time as much as possible if the animal has just consumed large amounts of food, or if possible, avoiding immobilisation altogether, is paramount for animal safety.
Normal arterial blood pressures in hyenas are not known, but blood pressures of SAP of 125-160 mmHg, MAP of 90-110 and DAP of 75-95 mmHg is considered normal in healthy dogs and cats (Clarke et al., 2014). Therefore, the hyenas were likely mildly hypertensive (mean = 126.6, SD = 14.0, which is similar to the MAPs of 123.5-134.5 mmHg that was observed by Anthony (2018) immobilising hyenas with Zoletil and two doses of medetomidine. Blignaut et al. (2021) worked on African serval using 8.7 mg/kg ketamine, 0.4 mg/kg butorphanol and 0.09 mg/kg medetomidine and recorded MAP's of 137, 130, and 112 mmHg 20-30, 35-45, and 50-60 minutes after immobilisation respectively. However, this level of hypertension for a brief period is likely of limited clinical concern.
Limitations of the study included variability in weight estimates, amount of food the hyena consumed before being immobilised, dart placement, and differences in the individual stress level of the animals. However, such variability is inherent when immobilising free-ranging animals that cannot be weighed or starved before the immobilisation.
Conclusion
In conclusion, KBM at a dose of 1.17 mg/kg ketamine, 0.25 mg/ kg butorphanol, 0.03 mg/kg medetomidine was adequate for immobilising spotted hyenas for up to 60 minutes and resulted in induction times similar to what has been reported when using Zoletil-medetomidine or ketamine-medetomidine combinations in hyenas. The majority of the animals had low HRs, were moderately hypoxaemic, and had mild hypertension during immobilisation. Animals were slightly ataxic after reversal, which largely resolved within minutes, and the time from reversal to ambulation was shorter than what has been reported for Zoletil-medetomidine combinations.
Acknowledgements
We sincerely thank SANparks Wildlife Veterinary Services staff for their help with the study. The study was funded by the Keith Richardson Foundation.
Conflict of interest
The authors have no conflict of interests to disclose.
Ethical approval
The study was conducted in collaboration with the South African National Park (SANParks) Veterinary Wildlife Services and approved under the SANParks permit # SS666 and the University of Pretoria ethics protocol # REC015-22.
ORCID
A Roug https://orcid.org/0000-0002-6844-4076
L Meyer https://orcid.org/0000-0002-5122-2469
L Netshitayhadulu https://orcid.org/0009-0002-8832-7405
M Leiberich https://orcid.org/0000-0001-7381 -2446
P Buss https://orcid.org/0000-0001-5614-0975
References
Anthony, T., 2018, Physiological effects of high and low medetomidine doses in combination with zolazepam/tiletamine for the immobilisation and anaesthesia of spotted hyenas (Crocuta crocuta). University of Pretoria, Available at: https://repository.up.ac.za/bitstream/handle/2263/71648/Anthony_Physiological_2018.pdf?sequence=1&isAllowed=y. [ Links ]
Bates, D., Mächler, M., Bolker, B., et al., 2015, Fitting linear mixed-effects models using lme4, J Stat Softw, 67(1), 1-48. https://doi.org/10.18637/jss.v067.i01. [ Links ]
Blignaut, C.J., Steenkamp, G., Loock, D., et al., 2021, Ketamine-butorphanol-medetomidine versus butorphanol-midazolam-medetomidine immobilisation of serval (Leptailurus serval), Vet Anaesth Analg 48, 707-715. https://doi.org/10.1016/j.vaa.2021.01.011. [ Links ]
Chittick, E., Horne, W., Wolfe, B., et al., 2001, Cardiopulmonary assessment of medetomidine, ketamine, and butorphanol anesthesia in captive Thomson's gazelles (Gazella thomsoni), J Zoo Wildl Med 32, 168-175. https://doi.org/10.1638/1042-7260(2001)032[0168:CAOMKA]2.0.CO;2. [ Links ]
Clarke, K.W., Trim, C.M., Hall, L.W., 2014, Veterinary Anaesthesia, 11th Edition. W.B. Saunders, Oxford, UK. [ Links ]
De Leeuw, A., Forrester, G., Spyvee, P., et al., 2004, Experimental comparison of ketamine with a combination of ketamine, butorphanol and medetomidine for general anaesthesia of the Eurasian badger (Meles meles L), Vet J 167, 186-193. https://doi.org/10.1016/S1090-0233(03)00113-8. [ Links ]
Donaldson, A.C., Meyer, L.C.R., Fuller, A., et al., 2023, Comparison of the cardiovascular effects of immobilization with three different drug combinations in free-ranging African lions, ConservPhysiol 11, coac077. https://doi.org/10.1093/conphys/coac077. [ Links ]
Hahn, N., Parker, J.M., Timmel, G., et al., 2014, Hyenidae, In: Zoo Animal and Wildlife Immobilization and Anesthesia, 627-633. https://doi.org/10.1002/9781118792919.ch44. [ Links ]
Haskins, S.C., 2015, Chapter 15 - Hypoxemia, In: Silverstein, D.C., Hopper, K. (Eds.) Small Animal Critical Care Medicine (Second Edition). W.B. Saunders, St. Louis, USA. 81-86. https://doi.org/10.1016/B978-1-4557-0306-7.00015-5. [ Links ]
Kock, M.D., Burroughs, R., 2021, Chemical and physical restraint of African wild animals, 3rd Edition. IVWS. Greyton, South Africa. [ Links ]
Langan, J.N., Schumacher, J., Pollock, C., et al., 2000, Cardiopulmonary and anesthetic effects of medetomidine-ketamine-butorphanol and antagonism with atipamezole in servals (Felis serval), J Zoo Wildl Med 31, 329-334. https://doi.org/10.1638/1042-7260(2000)031[0329:CAAEOM]2.0.CO;2. [ Links ]
Larsen, R.S., Loomis, M.R., Kelly, B.T., et al., 2002, Cardiorespiratory effects of medetomidine-butorphanol, medetomidine-butorphanol-diazepam, and medetomidine-butorphanol-ketamine in captive red wolves (Canis rufus), J Zoo Wildl Med 33, 101-107. https://doi.org/10.1638/1042-7260(2002)033[0101:CEOMBM]2.0.CO;2. [ Links ]
Makrin-Dray, A.G., Lapid, R., Kafri, A., et al., 2021, Medetomidine-ketamine-midazolam versus medetomidine-ketamine-butorphanol for immobilization of red kangaroos (Osphranter rufus), J Zoo Wildl Med 52, 1175-1184. https://doi.org/10.1638/2021-0046. [ Links ]
Meyer, L.C.R., Hetem, R.S., Mitchell, D., et al., 2015, Hypoxia following etorphine administration in goats (Capra hircus) results more from pulmonary hypertension than from hypoventilation, BMC Vet Res 11, 18. https://doi.org/10.1186/s12917-015-0337-5. [ Links ]
Posner, L.P., 2018, Chapter 14. Sedatives and tranquilizers, In: Riviere, J.E., Papich, M.G. (Eds.) Veterinary Pharmacology and Therapeutics. John Wiley & Sons, Incorporated, Newark, United States, 324-368. [ Links ]
Rieser, T.M., 2013, Arterial and venous blood gas analyses, Top Companion Anim Med 28, 86-90. https://doi.org/10.1053/j.tcam.2013.04.002. [ Links ]
Sinclair, M.D., 2003, A review of the physiological effects of alpha2-agonists related to the clinical use of medetomidine in small animal practice, Can Vet J 44, 885-897. [ Links ]
Stemmet, G.P., Meyer, L.C., Bruns, A., et al., 2019, Compared to etorphine-azaperone, the ketamine-butorphanol-medetomidine combination is also effective at immobilizing zebra (Equus zebra), Vet Anaesth Analg 46, 466-475. https://doi.org/10.1016/j.vaa.2019.01.008. [ Links ]
Suedmeyer, W.K., 2015, Hyaenidae. In: Fowler's Zoo and Wild Animal Medicine, Vol 8, 509-514. https://doi.org/10.1016/B978-1-4557-7397-8.00051-7. [ Links ]
Van Jaarsveld, A., 1988, The use of Zoletil for the immobilization of spotted hyaenas, S Afr J Wildl Res 18, 65-66. [ Links ]
Van Jaarsveld, A., McKenzie, A.A., Meltzer, D., 1984, Immobilization and anaesthesia of spotted hyaenas Crocuta Crocuta, S Afr J Wildl Res 14, 120-122. [ Links ]
Wenger, S., Buss, P., Joubert, J., et al., 2010, Evaluation of butorphanol, medetomidine and midazolam as a reversible narcotic combination in free-ranging African lions (Panthera leo), Vet Anaesth Analg 37, 491-500. https://doi.org/10.1111/j.1467-2995.2010.00569.x. [ Links ]
Wickham, H., 2016, Elegant graphics for data analysis. Springer-Verlag New York. ISBN 978-3-319-24277-4, https://ggplot2.tidyverse.org. [ Links ]
 Correspondence:
Correspondence:
email: roug.ats@gmail.com
Supplementary information

Supplementary Table I - Click to enlarge
Supplementary Table II - Click to enlarge















