Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the South African Veterinary Association
versión On-line ISSN 2224-9435
versión impresa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.94 no.1 Pretoria 2023
http://dx.doi.org/10.36303/JSAVA.535
ORIGINAL ARTICLE
Airborne bacteria in veterinary surgical theatres in South Africa
C van der Merwe; V Naidoo
Department of Paraclinical Sciences, Faculty of Veterinary Science, University of Pretoria, South Africa
ABSTRACT
The bioaerosol composition of the theatre environment plays a determining role in the development of surgical site infections (SSIs). It has been demonstrated that the concentration of viable airborne bacteria is influenced by the level of room occupancy, utilisation of surgical attire and importantly, proper ventilation systems, which are often lacking in the average veterinary facility.
The aim of this study was to evaluate the airborne bacterial load encountered in non-environmentally controlled small animal veterinary theatres during routine surgical sterilisations, and to correlate these findings with the managerial practices at the facility.
Four veterinary facilities with differing throughputs and managerial practices were recruited into the study. Blood agar settle plates, open from first incision to last suture, were used to quantify organisms that could settle in an incision.
The 45 plates yielded 487 bacterial isolates (53 species). The Micrococcus (28.8%) and Staphylococcus (16.8%) genera were predominant. Of the isolates 61.8% were classified as human/small animal commensals and 37.2% belonged to species previously implicated in small animal SSIs.
Specific trends were additionally evident in the bioaerosol loads. High room occupancy, lack of surgical attire and exposure to the outside environment were associated with higher bacterial counts. Accumulation from consecutive procedures was identified and linked to total occupancy time of the room. Current mitigation measures were not ideal to minimise the SSI risk. Routine, frequent and thorough cleaning in combination with surgical attire utilisation is recommended to reduce the bioburden for patient benefit.
Keywords: surgical site infection, non-environmentally controlled, open-air theatre, air-borne bacteria, veterinary theatre, clean procedures
Introduction
Veterinary medicine is dedicated to the promotion of health and the prevention of disease in animals. The surgical sterilisation of canines and felines achieves this goal by reducing the incidence of mammary tumours and eliminating the risk of pyometra in female patients, whilst decreasing the incidence of benign prostatic hyperplasia and prostatitis in males (McKenzie 2010). Despite the medical benefits from these procedures, the development of disease in the form of surgical site infections (SSIs) fails to meet a key goal in the principle of 'do no harm'.
In its least severe form, SSIs result in delayed wound healing and increased patient morbidity, whilst in other cases leading to protracted hospital stays, secondary complications and even death (Darouiche 2016; Badia et al. 2017; Nelson 2011). It has been estimated that 2 to 6% of veterinary patients undergoing what can be classified as 'clean' or non-contaminated surgery, develop surgical site infections (Spohrc et al. 2012; Eugster et al. 2004).
The development of a SSI is dependent on the interplay between the degree of bacterial contamination, the virulence of the inoculating organism, as well as the ability of the host's immune response to counteract or overcome this threat (Gawande et al. 2009; Nelson 2011). The bacterial contamination involved with said infections can arise from one of two sources, namely endogenously, referring to organisms which originated from within the body, either from sites of infection or from the normal flora, or exogenously from the surrounding environment (Owens & Stoessel 2008). In the case of a clean surgical procedure, as would be the case for a routine sterilisation, it has been shown that approximately 98% of the bacterial load is derived from airborne pathogens (Whyte et al. 1982). This is in line with the statement made by Sadrizadeh & Holmberg (2015) in which they state that "the infection risk of surgical patient is significantly correlated with the concentration of viable airborne bacteria". Therefore, based on current knowledge, air is considered the most important exogenous source of bacterial contamination (Chauveaux 2015). By extension, if the degree of airborne contamination can be controlled, the incidence of SSIs can be reduced.
The airborne bacterial composition of a theatre environment is both complex and dynamic in nature. Through the shedding of squamous epithelial cells, hair and respiratory excretions, all occupants of the room contribute directly to the bioburden (Al-Waked 2010; Roy et al. 2018; Shaw et al. 2018). This correlation between the room occupancy and bacterial load is so strong, that it has been shown that each additional person increases the SSI risk 1.3 fold (Eugster et al. 2004). In order to 'contain' shedding, staff are required to wear appropriate surgical attire which consists of masks, gloves, caps, scrub suits and gowns (Gawande et al. 2009). Despite these cautionary measures, it has been estimated that surgical staff can still shed approximately 10 000 squamous epithelial cells per person per minute, with 10% of these cells being expected to carry microorganisms (Al-Waked 2010).
To further mitigate the introduction of bacteria, theatres can be equipped with heating ventilation and air conditioning (HVAC) systems. These units not only serve to exchange the entire volume of air in the operating room on a regular basis, but through the utilisation of high efficiency particulate air (HEPA) filters, remove particles larger than 0.3 μm with an efficiency of 99.97% (Mangram et al. 1999). Despite the value of HVACs, most veterinary facilities do not have the financial resources available for an appropriate ventilation system. These less than ideal conditions could theoretically lead to veterinary theatres being contaminated with high bacterial loads. This can in turn increase the risk of SSIs and thereby the utilisation of otherwise unnecessary prophylactic antimicrobials as a compensatory measure. To investigate if this is the case in South Africa, this study evaluated the airborne bacterial load encountered in non-environmentally controlled veterinary theatres during routine canine and feline sterilisation. The secondary objectives were to identify isolated organisms to ascertain their potential for surgical site infections, and their antimicrobial susceptibility.
Material and methods
Four veterinary facilities were evaluated in the study. Included were three first opinion, small animal veterinary practices without ventilation systems, which were considered to have high, intermediate and low surgical caseloads. The Biomedical Research Centre (Onderstepoort, University of Pretoria, Facility D), made up of four theatres, served as a control facility. Because only a limited amount of data was collected at Facility D, theatres 1 and 2, which were essentially identical and contained HEPA filtration systems, were treated as a single unit - namely D1. Whilst the non HEPA filtered theatres, 3 and 4, formed D2.
The details of the various theatres are reported in Table I. The study was approved by the Animal Ethics Committee of the University of Pretoria (V049-18) and the Research Ethics Committee of the University of Pretoria (REC036-18).
Sample collection
Samples were taken during routine canine ovariohysterectomies and orchidectomies, as well as during feline ovariohysterectomies. No procedures were booked specifically for the purpose of this study. Sampling was through the use of settle plates as described by Tršan et al. (2019), on blood agar prepared specifically for this study. Settle plates (one per procedure) were placed at the same height as the patient and as close as possible to the incision site (maximum of one metre), without affecting the sterile field. Plates were placed on the lateral aspect on the patient to ensure that patients were not breathing directly onto the plate. The plates were opened upon first incision and closed upon placement of the last suture. The samples reached the laboratory for further processing within four hours of collection. Facility D was sampled once during a training workshop. To simulate the average duration of a surgical sterilisation, two plates, placed in each of the four theatres, were opened upon first incision and closed after 20 minutes. The Department of Veterinary Tropical Diseases bacteriology laboratory and the Potchefstroom Veterinary Laboratory assisted with the processing of these samples.
Bacterial identification
Plates were incubated at 37 ± 2 °C for 48 hours, at which point the number of colony-forming units were manually counted. Pure cultures were subject to primary identification, with the Sensititre ARIS 2x automated bacterial identification system being used to identify organisms to the genus or species level. Bacterial identification was done for all collected samples. Once identified, organisms were grouped into Gram-positive and Gram-negative. The natural habitat of each of the sampled species was researched, allowing isolates to be further subcategorised into commensals (listed as forming part of the normal microflora of humans/canines and felines), and non-commensals. Organisms which are occasionally isolated as commensals, but commonly found in the environment, were categorised as non-commensals. Resources used to classify these organisms are listed in Appendix A.
Antimicrobial susceptibility testing
Susceptibility testing was undertaken with appropriate antibiotic discs, including kanamycin (30 μg), amoxicillin/clavulanic acid (30 μg), cephalothin (30 μg), enrofloxacin (5 μg), sulfisoxazole (300 μg), trimethoprim sulpha (25 μg), erythromycin (15 μg), tetracycline (30 μg), gentamicin (10 μg) and ampicillin (10 μg). Antimicrobials were selected based on the guidelines set out by the Clinical and Laboratory Standard Institute (CLSI). The quality control of the test was assured using an individual ATCC strain for each organism tested. Result interpretation was based on the definitions provided by this standard. Organisms are classified as susceptible when the zone diameter is at or above the susceptible breakpoint, i.e. is at or above the drug level that can be achieved at the site of infection when used at recommended dosages. The intermediate category implies reduced efficacy when compared to susceptible organisms. A resistant isolate on the other hand would not be inhibited by usually attainable drug concentrations at the site of infection due to a zone diameter below the susceptible breakpoint or specific antimicrobial resistance mechanisms (CLSI 2020). Multidrug resistance was based on the definition utilised by Siegel et al. (2007) whereby organisms were considered to be multidrug resistant when resistance to two or more antimicrobial classes was demonstrated.
Data analysis
All results were analysed using simple descriptive statistics. The average deposition rate for each procedure/facility was calculated by dividing the total collection time by the total number of colony forming units (CFU) to give the time per CFU. For the expected contribution that each person made to the bioload per time period, the sum of the procedure duration multiplied by the occupants per procedure, provided the total occupancy time for the facility. The total number of commensal organisms isolated at the facility was then divided by this number. This is summarised by the equation:
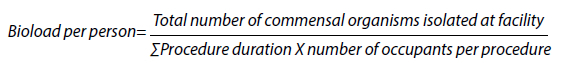
The bioaerosol load, being the colony forming units per volume of air (cfu/m3) was calculated using the Omeliansky formula:
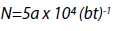
whereby N = colony forming unit per cubic meter of air (cfu/m3), a = number of colonies per settle plate, b = surface area of settle plate in cm2, and t = time exposure (minutes) (Najotra et al. 2017).
Results
Settle plate results
The total number of procedures and total sampling times were 12 (209 min), 12 (196 min) and 13 (278 min) for facilities A to C. Facility D was sampled for 160 minutes, representing two plates in each of the four theatres for 20 min. Overall the 45 settle plates, covering 843 minutes, yielded 487 bacterial isolates (53 species) with no plate being negative on culture. Average deposition rates (time/CFU) were 1 min 18 sec, 59 sec, 4 min 36 sec and 2 min 30 sec for facilities A-D respectively; whilst mean air contamination (cfu/m3) was calculated to be 691 ± 357, 788 ± 338, 153 ± 109 and 314 ± 94 for these same facilities. An overview of the results obtained is available in Table II. A more detailed breakdown of the mean air contamination is available in Figure 1 thereafter.
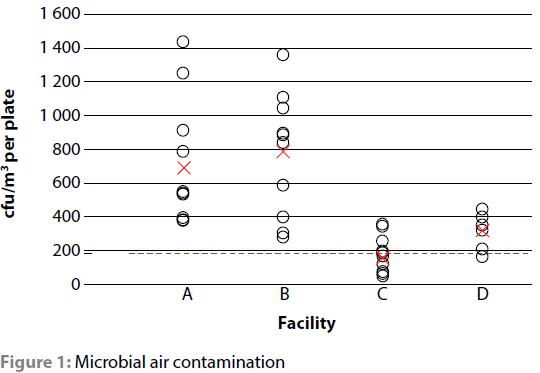
Each dot represents a single settle plate, whereas the red crosses represent the mean microbial air contamination for each facility. The broken line at 180 cfu/m3 represents the maximum acceptable standard of air contamination in a working theatre as set by the Healthcare Infection Society (Stauning et al. 2018; Hoffman et al. 2002). Dots above this line are above the maximum recommended levels.
Classification of the organisms
Across all facilities, Gram-positive isolates were significantly more abundant, with the trends in the predominant genera being evident between facilities. A detailed breakdown is presented in Table III.
When the isolates were categorised into commensals (i.e. those that form part of the normal microflora of humans/small animals) and non-commensals based on published literature, commensals accounted for 53.1% of the isolates at facility D (50.0% in the HEPA-equipped theatres and 55.6% in the non HEPA-equipped theatres); 59.7% at facility B; 63.1% at facility A and 74.2% at facility C. This can be visualised in Figure 2.
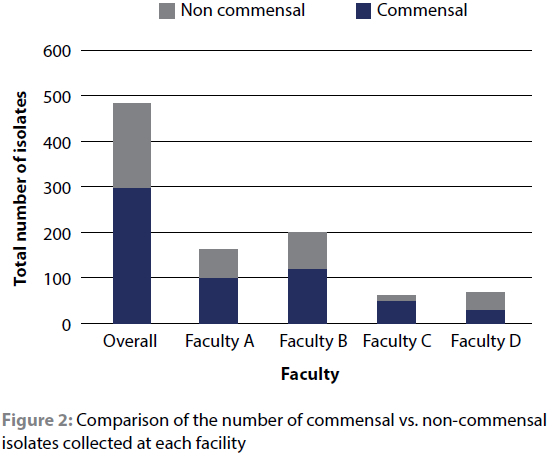
For the commensal bioload per person per minute, facility D was the lowest at 0.05 commensals per person per minute, with facility C, B and A at 1.2x, 2.6x and 5.0x that of facility D. When the isolates are evaluated in terms of potential pathogenicity, 10 species that have previously been implicated in small animal surgical site infections were identified. Implicated species included Micrococcus luteus, Micrococcus species, Pseudomonas species, Streptococcus species, Enterococcus faecalis, Enterobacter cloacae, Staphylococcus pseudintermedius, S. aureus, coagulase-positive staphylococci (COPS) and coagulase-negative staphylococci (CONS). Despite forming only 37.2% of the total isolates, at least one pathogenic organism was isolated in 88.9% of procedures.
Antimicrobial susceptibility
Though antimicrobial susceptibility testing was performed, overall, due to sample loss (i.e. pure cultures which did not survive cold storage for further antimicrobial susceptibility testing), too few samples were available to obtain statistically valid results. To illustrate the potential for air-borne bacteria to carry resistance, the pooled results of some of the more commonly encountered genera are presented in Table IV.
Discussion
As expected, a diverse group of bacterial organisms were isolated during the course of this study. Of the isolates, 61.8% could be classified as human and/or companion animal commensals, while 37.2% belonged to species that have previously been implicated in small animal SSIs.
Considering that a single patient was present in each room at the time of sampling and that these patients were draped (albeit drapes may have been applied slightly differently), one can expect the level of shedding from the animals between procedures and facilities to be similarly low, thereby making the person(s) in theatre and their level of surgical attire, the major contributor to the bioload.
As a way of comparing the effect that personnel had on the bacterial bioload between facilities, the commensal deposition rate per total occupancy time (i.e. the bioload per person per time period) was calculated. Based purely on the degree of utilisation of surgical attire, it would be expected that facility D would have the lowest commensal bacterial load, followed by facility A and then B, with facility C, due to its relative lack of protective clothing, having the highest commensal bioload per person. The calculated result did however not follow the expected trend, instead, at A>B>C>D, it was nearly the opposite. A likely explanation would be the extended survival of organisms in the environment and the consequent cumulative effect that room occupancy and consecutive procedures had on bacterial counts.
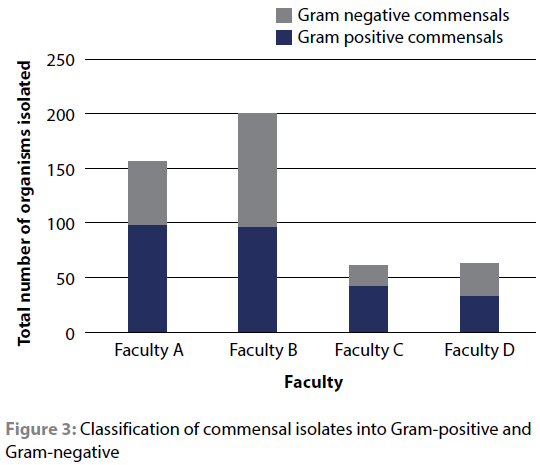
Of the 24 species that were classified as commensals, all but four were Gram-positive. Gram-positive organisms, due to their relatively thick peptidoglycan layer of highly cross-linked chains (Salton & Kwang-Shin 1996), have a superior ability to survive adverse environmental conditions (Tolabi et al. 2019). Staphylococcus epidermidis, which was isolated at three of the facilities for example, has been shown to remain viable in the environment for five days (Thompson et al. 2011).
When re-evaluating the commensal bioload per person per time period with this knowledge in mind, a trend emerges. Due to the high procedure load, the cumulative effect of theatre occupancy at facility A would be quite high, explaining the high bacterial load despite the relatively good level of surgical attire utilisation. This is in direct contrast to the low throughput facility C which yielded a low bioload per person despite almost no surgical attire being worn. This leads to the conclusion that, in the veterinary surgical theatres evaluated, the level of contamination is linked to the total amount of time persons spend in the theatre, a trend which is evident in Figure 4.
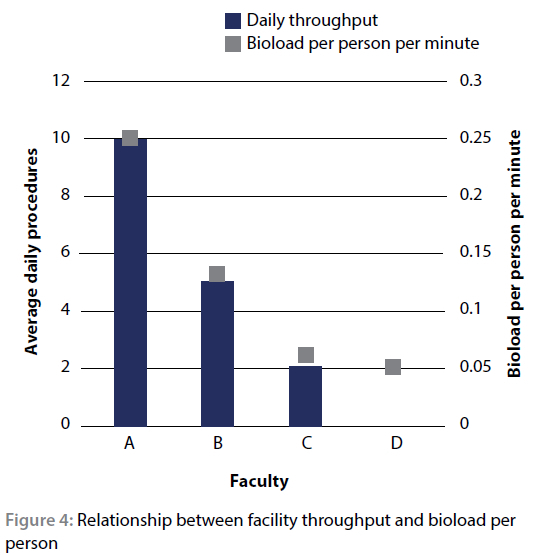
Facility D was noted as having a daily throughput of '0' as it is only used approximately once per month.
In a theatre environment with a HVAC system which replaces the volume of air, this bioload would be reduced by the air system before organisms have the chance to settle into the wound site. Since theatres often do not have installed ventilation systems, the one area of debate focuses on opening windows and doors to allow for a degree of fresh air-flow as a way of diluting some of the already present commensal organisms. Considering however that "outdoor air is thought to be the most important source of indoor micro flora" (Lina et al. 2019), this may not necessarily be true. The current study offers some insight into this.
At facility A, the theatre door and windows remained closed whether or not the room was in use; while facility B, in general, had one door and at times three doors left open with large amounts of movement. Though dilution was evident at facility B, as it had the lowest proportion of commensal organisms, overall it had the highest plate count, higher even than facility A which recorded double the number of procedures on a daily basis. The introduction of contaminated outside air is therefore likely a major contributing factor. In order to find the balance between a completely closed system, where no dilution takes place, and seeding the environment with additional pathogens through contaminated outside air, it is recommended that veterinary theatres without adequate mechanical ventilation systems allow fresh air introduction for a few hours following completion of the procedures for the day, as seen in facility C. Thereafter, proper mechanical cleaning should take place before the theatre is used again, whilst other mitigating measures such as performing patient preparation outside of the theatre, correctly draping the patient once in the operating room (OR) and utilising appropriate surgical attire (gloves, masks, caps and gowns) should be used to decrease initial seeding.
In order to be truly effective, theatre cleaning should not be thought of as a singular event, but rather a continuously implemented process. All horizontal surfaces should be damp dusted at the start of each day, spills and biological waste should be taken care or intraoperatively. All surfaces and equipment in the immediate vicinity of the operative area or that have been in contact, either directly or indirectly, with the patient or staff, should be cleaned between procedures. At the end of the day, or at least once every 24 hours, terminal cleaning, in which all exposed surfaces, including but not limited to lights, sinks, bins, and equipment wheels are disinfected, should take place (Roy et al. 2018; WRHA 2017; Wood 2016). All of the above should be done following the principle of cleaning from higher to lower surfaces and moving from clean to dirty areas (WRHA 2017; Roy et al. 2018). Apart from adequately addressing all surfaces, cleaning cannot be considered thorough unless an appropriate disinfectant is used correctly, with dilution, time and degree of biological material being determinants of overall efficacy.
Overall the combination of daily throughput, theatre occupancy, surgical attire utilisation, fresh air introduction and cleaning protocols resulted in a mean air contamination 1.7, 3.8 and 4.4 times higher at facilities D, A and B than the 180 cfu/m3 maximum recommended by the Healthcare Infection Society (EAI4). The higher than expected levels in Facility D was associated with the HVAC only being used intermittently and not constantly as one would expect. Facility C, at 153 cfu/m3, was the only facility where the average bioaerosol load was within the standards for a working theatre; though some procedures did exceed the recommended limit. One of the concerns with having high circulating levels of bacteria in a theatre environment is their potential ability to be infectious. Considering that counts above 700 cfu/m3 have been associated with a significant risk of airborne infection in the human medical field (Parker 1978), that 37.2% of isolates belonged to species that have previously been implicated in canine or feline surgical site infections, and that at least one of these pathogens was isolated in 88.9% of procedures, the results for this study are concerning.
Though, due to various technical reasons, only a small number of isolates were evaluated for their antimicrobial susceptibility, resistance was clearly evident in the sampled population.
Considering that these organisms would not only require more extensive treatment should a surgical site infection develop, but that they could harbour and potentially disseminate antimicrobial resistance genes, concern is not unjustified. It should additionally be noted that the CLSI standard only included information for 23 of the 41 species that were tested for their antimicrobial susceptibility. Since the zone of inhibition or the minimum inhibitory concentration is based on the interaction between the pharmacodynamics and pharmacokinetics of a specific drug in the species of interest, airborne infections from these other organisms may be more difficult to treat than the common veterinary pathogenic bacteria.
Another concern evident in this study was the manner of use of perioperative antimicrobials as a way of mitigating SSI risk. When employed correctly, prophylactic antimicrobial therapy ensures that adequate plasma concentrations are reached prior to the first incision, suppressing bacterial multiplication to the extent that the host defence mechanisms can prevent the progression to infection (WHO 2016; Verwilghen & Singh 2015). In order to achieve adequate plasma concentrations at first challenge (i.e. upon first incision), the World Health Organization strongly recommends that intravenous antimicrobial administration should take place no later than two hours prior to the start of the procedure (WHO 2016), with the exact pre-surgical dosing interval being dependant on the antimicrobial agent's half-life (WHO 2016). Two facilities made use of a procaine benzylpenicillin and benzathine benzylpenicillin (long-acting) formulation as part of their standard perioperative protocol. From published pharmacokinetic information for this particular long-acting formulation, penicillin concentration only peaks one to four hours after the recommended intramuscular administration (MSD-Animal-Health 2014). Administering the formulation either directly prior to the start of the procedure or immediately postoperatively, as evident in this study, is unlikely to be effective. The long-acting benzyl-penicillin portion of the formulation, which may last up to four weeks, furthermore does not comply with current best use guidelines which conclude that prolonging surgical antimicrobial prophylaxis beyond doses given intraoperatively, does not further decrease the SSI rate (WHO 2016). Overall, considering that it is currently recommended that short (< 90 minute), clean, non-orthopaedic procedures carried out on veterinary patients classified as ASA 1 or 2 (i.e. low risk) do not require antimicrobial prophylaxis (Spohrc et al. 2012; Nelson 2011), emphasis should be placed on correct environmental management before antibiotics are employed.
Conclusion
This study provided a glimpse into the factors that may contribute to the bioaerosol load within a veterinary theatre. The multitude of contributing factors has created a dynamic reservoir of bacteria that, if not carefully managed, could contribute to the incidence of SSI and consequent increase in antibiotic use. Emphasis should therefore be placed on optimising environmental management before antibiotic use is considered.
Acknowledgements
The authors wish to thank the veterinary facilities that participated in this study, as well as the DVTD Bacteriology Laboratory which forms part of the department of Veterinary Tropical Diseases at Onderstepoort and the Potchefstroom Veterinary Laboratory for the processing of samples.
Conflict of interest
The authors declare that they have no financial or personal relationships which may have inappropriately influenced them in writing this paper.
Funding
This study was supported by the University of Pretoria.
Ethics
Ethical approval was obtained from the Faculty of Veterinary Science Research Ethics Committee (V049-18) as well as from the University's centralised Animal Ethics Committee (REC036-18).
ORCID
C van der Merwe https://orcid.org/0000-0003-2628-6548
V Naidoo https://orcid.org/0000-0003-2740-5983
References
Al-Waked, R., 2010, Effect of ventilation strategies on infection control inside operating theatres, Engineering Applications of Computational Fluid Mechanics 4, 1-16. https://doi.org/10.1080/19942060.2010.11015295. [ Links ]
Badia, J., Casey, A., Petrosillo, N., et al., 2017, Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries, Journal of Hospital Infection 96, 1-15. https://doi.org/10.1016/j.jhin.2017.03.004. [ Links ]
Chauveaux, D., 2015, Preventing surgical-site infections: measures other than antibiotics, Orthopaedics & Traumatology: Surgery & Research, 101, S77-S83. https://doi.org/10.1016/j.otsr.2014.07.028. [ Links ]
Clinical and Laboratory Standards Institute (CLSI) 2020. Performance Standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute, 30 ed CLSI supplement M100. [ Links ]
Darouiche, R., 2016, Surgical site infections, Hospital Infection Control 9, 12-15. [ Links ]
Eugster, S., Schawalder, P., Gaschen, F., et al., 2004, A prospective study of postoperative surgical site infections in dogs and cats, Veterinary Surgery 33, 542-550. https://doi.org/10.1111/j.1532-950X.2004.04076.x. [ Links ]
Gawande, A., Baker, P., Barraclough, B., et al., 2009, WHO Guidelines for safe surgery 2009, Safe surgery saves lives. In: Gawande, A. & Weiser, T. (eds.). Geneva: World Health Organization. [ Links ]
Hoffman, P., Williams, J., Stacey, A., et al., 2002, Microbiological commissioning and monitoring of operating theatre suites, Journal of Hospital Infection 52, 1-28. https://doi.org/10.1053/jhin.2002.1237. [ Links ]
Lina, D., Kharate, A., Awati, B., 2019, Estimation of microbial air contamination of livestock farms and hospitals in veterinary college Bidar, India, Journal of Entomology and Zoololgy Studies 7(2). [ Links ]
Mangram, A.J., Horan, T.C., Pearson, M.L., et al., 1999, Guideline for prevention of surgical site infection, 1999, Infection Control & Hospital Epidemiology 20, 247-280. https://doi.org/10.1086/501620. [ Links ]
Mckenzie, B., 2010, Evaluating the benefits and risks of neutering dogs and cats, CAB Reviews 5, 1-18. https://doi.org/10.1079/PAVSNNR20105045. [ Links ]
MSD-Animal-Health 2014. Duplocillin, IVS Desk Reference In: Carrington, C., Plessis, A.D. & Naidoo, V. (eds.) IVS Desk Referece 12 ed.: MIMS. [ Links ]
Najotra, D.K., Malhotra, A.S., Slathia, P., et al., 2017, Microbiological surveillance of operation theatres: Five year retrospective analysis from a Tertiary Care Hospital in North India, International Journal of Applied and Basic Medical Research 7, 165. https://doi.org/10.4103/ijabmr.IJABMR_281_16. [ Links ]
Nelson, L.L., 2011, Surgical site infections in small animal surgery, Veterinary Clinics of North America: Small Animal Practice 41, 1041-56. https://doi.org/10.1016/j.cvsm.2011.05.010. [ Links ]
Owens, C. & Stoessel, K., 2008, Surgical site infections: epidemiology, microbiology and prevention, Journal of Hospital Infection 70, 3-10. https://doi.org/10.1016/S0195-6701(08)60017-1. [ Links ]
Parker, M.T., 1978, Hospital-acquired infections: guidelines to laboratory methods, World Health Organization. Regional Office for Europe 28-32. [ Links ]
Roy, M.C., 2018, Guide to infection control in the healthcare setting, International Society for Infectious Diseases, 2. [ Links ]
Sadrizadeh, S. & Holmberg, S., 2015, Effect of a portable ultra-clean exponential airflow unit on the particle distribution in an operating room, Particuology 18, 170-178. https://doi.org/10.1016/j.partic.2014.06.002. [ Links ]
Salton, M.R.J. & Kwang-Shin, K., 1996, Chapter 2 Structure. In: S, B. (ed.) Medical Microbiology. Galveston (TX): University of Texas Medical Branch. [ Links ]
Shaw, L.F., Chen, I.H., Chen, C.S., et al., 2018, Factors influencing microbial colonies in the air of operating rooms, BMC Infectious Diseases 18, 4. https://doi.org/10.1186/s12879-017-2928-1. [ Links ]
Siegel, J.D., Rhinehart, E., Jackson, M., et al., 2007, Management of multidrug-resistant organisms in health care settings, 2006, American Journal of Infection Control 10, S165-S93. https://doi.org/10.1016/j.ajic.2007.10.006. [ Links ]
Spohrc, A., Schj0thd, B., Wiinberga, B., et al., 2012, Antibiotic Use Guidelines for Companion Animal Practice, Available from: https://www.alimenti-salute.it/sites/default/files/animali%20da%20compagnia%20Antibiotic%20Guidelines%20Danimarca.pdf. Accessed 15 May 2019. [ Links ]
Stauning, M., Bediako-Bowan, A., Andersen, L., et al., 2018, Traffic flow and microbial air contamination in operating rooms at a major teaching hospital in Ghana, Journal of Hospital Infection 99, 263-270. https://doi.org/10.1016/j.jhin.2017.12.010. [ Links ]
Thompson, K.-A., Bennett, A., Walker, J., 2011, Aerosol survival of Staphylococcus epidermidis, Journal of Hospital Infection 78, 216-220. https://doi.org/10.1016/j.jhin.2010.12.009. [ Links ]
Tolabi, Z., Alimohammadi, M., Hassanvand, M.S., et al., 2019, The investigation of type and concentration of bio-aerosols in the air of surgical rooms: A case study in Shariati Hospital, Karaj, MethodsX 6, 641-650. https://doi.org/10.1016/j.mex.2019.03.016. [ Links ]
Trš an, M., Seme, K., Srčič, S., 2019, The environmental monitoring in hospital pharmacy cleanroom and microbiota catalogue preparation, Saudi Pharmaceutical Journal 27, 455-462. https://doi.org/10.1016/j.jsps.2019.01.007. [ Links ]
Verwilghen, D. & Singh, A., 2015, Fighting surgical site infections in small animals: are we getting anywhere? Veterinary Clinics: Small Animal Practice 45, 243-276. https://doi.org/10.1016/j.cvsm.2014.11.001. [ Links ]
WHO 2016. Global Guidelines for the Prevention of Surgical Site Infection, Geneva, World Health Organization. [ Links ]
Whyte, W., Hodgson, R., Tinkler, J., 1982, The importance of airborne bacterial contamination of wounds, Journal of Hospital Infection 3, 123-135. https://doi.org/10.1016/0195-6701(82)90004-4. [ Links ]
Wood, A., 2016, Guidelines for environmental cleaning. Association of PeriOperative Registered Nurses. [ Links ]
WRHA 2017. Guidelines for routine environmental cleaning of the operating room. Winnipeg-Regional-Health-Authority. [ Links ]/
 Correspondence:
Correspondence:
C van der Merwe
Email: courtz.vdm@gmail.com
Appendix A - List of references used to determine the commensal status of isolated organisms
Acinetobacter
Infectious disease advisor (2019). Acinetobacter species.[Online]. Available from: https://www.infectiousdiseaseadvisor.com/home/decision-support-in-medicine/infectious-diseases/acinetobacter-species/. Accessed 10 October 2019. [ Links ]
Regalado, N.G., MartiN, G., Antony, S.J., 2009, Acinetobacter lwoffii: bacteremia associated with acute gastroenteritis, Travel Med Infect Dis 7, 316-7. [ Links ]
Van der Kolk, J.H., Endimiani, A., Graubner, C., et al., 2019, Acinetobacter in veterinary medicine, with an emphasis on Acinetobacter baumannii, Journal of global antimicrobial resistance 16, 59-71. [ Links ]
Vetbact,, 2018, Actinetobacter Iwoffii. [online]. Available from: https://www.Vetbact,.orq/index.php?artid=141. Accessed 02 February 2020. [ Links ]
Aerococcus
Irizarry, R., Amadi, V., Brathwaite-Sylvester, E., Nicholas-Thomas, R., et al, 2016, Update on urinary tract infections in doqs in a tropical is-land and antimicrobial susceptibility of Escherichia coli isolates for the period 2010-2016, Vet Med Open J 1(3), 56-61. [ Links ]
Martin, V., Vela, A., Gilbert, M., et al., 2007, Characterization of Aerococcus viridans isolates from swine clinical specimens, Journal of Clinical Microbiology 45, 3053-3057. [ Links ]
Parrey, A. H., Sofi, F., Ahmad, M., et al., 2016, Aerococcus viridans infection presenting as cutaneous vasculitis in an immunocompetent patient, Reumatologia 54, 318-320. [ Links ]
Rasmussen, M., 2013, Aerococci and aerococcal infections J Infect, 66, 467-74. [ Links ]
Rasmussen, M., 2016, Aerococcus: an increasingly acknowledged human pathogen, Clinical Microbiology and Infection 22(1), 22-27. [ Links ]
Bacillus
Andrade, N., Schmiedt, C.W., Cornell, K., et al., 2016, Survey of intraoperative bacterial contamination in dogs undergoing elective orthopedic surgery, Veterinary Surgery 45, 214-222. [ Links ]
Antimicrobe. Bacillus species. [Online]. Available from: http://www.antimicrobe.org/new/b82.asp. Accessed 12 October 2019. [ Links ]
Boucher, C., Henton, M.M., Becker, P.J., et al., 2018, Comparative efficacy of three antiseptics as surgical skin preparations in dogs, Veterinary Surgery 47(6), pp.792-801. [ Links ]
Glasset, B., Herbin, S., Granier, S.A., et al., 2018, Bacillus cereus, a serious cause of nosocomial infections: Epidemiologic and genetic survey, PloS one 13, e0194346. [ Links ]
Kim, M., Yoon, H., Lee, M., et al., 2018, Canine pyometra associated with Bacillus species: a case report, Veterinární medicina 63, 143-149. [ Links ]
Kuroki, R., Kawakami, K., Qin, L., et al., 2009, Nosocomial bacteremia caused by biofilm-forming Bacillus cereus and Bacillus thuringiensis Internal medicine 48, 791-796 [ Links ]
Logan, N. ,1988, Bacillus species of medical and veterinary importance Journal of medical microbiology 25, 157-165. [ Links ]
Mcdowell, R.H., Sands, E.M., Friedman, H., 2022, Bacillus cereus, StatPearls [Internet]. StatPearls Publishing. [ Links ]
Mullaney, E.G., 2017, Bacillus thuringiensis. Encyclopedia Britannica. [Online] Available from: https://www.britannica.com/science/Bacillus-thuringiensis. Accessed 15 October 2019. [ Links ]
Rolston, K.V., Nesher, L., Tarrand, J.T., 2014, Current microbiology of surgical site infections in patients with cancer: a retrospective review, Infectious diseases and therapy 3, 245-256. [ Links ]
Vetbact,, 2015, Bacillus thuringiensis. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=1870. Accessed 24 February 2020. [ Links ]
Vetbact,, 2017, Bacillus cereus. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=21&vbsearchstring=bacillus%20cereus. Accessed 03 February 2020. [ Links ]
Chryseobacterium
Isaiah, A., Hoffmann, A.R., Kelley, R., et al., 2017 Characterization of the nasal and oral microbiota of detection dogs, PloS one 12, e0184899. [ Links ]
Mukerji, R., Kakarala, R., Smith, S.J., et al. 2016, Chryseobacterium indologenes: an emerging infection in the USA, BMJ Case Reports bcr2016214486. [ Links ]
Vishnu, T., Soniyamby, A., William, A., et al., 2014, A mini review of an opportunistic pathogen-Chryseobacterium sp. World J Pharm Pharma Sci 3, 599-605. [ Links ]
Doublet, B., Robin, F., Casin, I., et al., 2010 Molecular and biochemical characterization of the natural chromosome-encoded class A β-lactamase from Pseudomonas luteola, Antimicrobial Agents and Chemotherap, 54, 45-51. [ Links ]
Ramana, K.V., Kareem, M.A., Sarada, C.V., et al., 2010, Chryseomonas luteola bacteremia in a patient with left pyocele testis with Fournier's scrotal gangrene, Indian Journal of Pathology and Microbiology 53(3), 568. [ Links ]
Roberts, W., Roessler, C., Francis, P.J., et al., 2018, Post-surgical gangrene with Pseudomonas luteola resulting in limb amputation: a case review, Cureus 10. [ Links ]
Chryseomonas
Chihab, W., Alaoui, A.S., Amar, M., 2004, Chryseomonas luteola identified as the source of serious infections in a Moroccan University Hospital, Journal of Clinical Microbiology 42, 1837-1839. [ Links ]
Comamonas
Abraham, J.M. & Simon, G.L., 2007, Comamonas testosteroni bacteremia: a case report and review of the literature, Infectious Diseases in Clinical Practice 15, 272-273. [ Links ]
Corynebacterium
Achermann, Y., Trampuz, A., Moro, F., et al., 2009, Corynebacterium bovis shoulder prosthetic joint infection: the first reported case, Diagnostic Microbiology and Infectious Disease 64, 213-215. [ Links ]
Bailiff, N.L., Westropp, J.L., Jang, S.S., et al., 2005, Corynebacterium urealyticum urinary tract infection in dogs and cats: 7 cases (1996-2003), Journal of the American Veterinary Medical Association 226, 1676-1680 [ Links ]
Bernard, K., 2012, The genus Corynebacterium and other medically relevant coryneform-like bacteria, Journal of Clinical Microbiology 50, 3152-3158. [ Links ]
Boucher, C., Henton, M.M., Becker, P.J., et al., 2018, Comparative efficacy of three antiseptics as surgical skin preparations in dogs, Veterinary Surgery 47(6), 792-801. [ Links ]
Chow, S.K., Bui, U., Clarridge, J.E., 2015, Corynebacterium bovis eye infections, Washington, USA, 2013, Emerging Infectious Diseases 21, 1687. [ Links ]
Dorella, F., Pacheco, L.G.C., Oliveira, S., et al., 2006, Corynebacterium pseudotuberculosis: microbiology, biochemical properties, pathogenesis and molecular studies of virulence, Veterinary Research 37(2), 201-218. [ Links ]
Oliveira, A., Oliveira, L.C., Aburjaile, F., et al., 2017, Insight of genus Corynebacterium: ascertaining the role of pathogenic and non-pathogenic species, Frontiers in Microbiology 8, 1937. [ Links ]
Rolston, K.V., Nesher, L., Tarrand, J.T., 2014, Current microbiology of surgical site infections in patients with cancer: a retrospective review, Infectious Diseases and Therapy 3, 245-256. [ Links ]
Vetbact,, 2019, Corynebacterium bovis. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=3. Accessed 24 February 2020. [ Links ]
Vetbact,, 2017. Corynebacterium pseudotuberculosis. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=3. Accessed 24 February 2020. [ Links ]
Elizabethkingia
Bordelo, J., Viegas, C., Coelho, C., et al., 2016, First report of bacteremia caused by Elizabethkingia meningoseptica in a dog, The Canadian Veterinary Journal 57, 994. [ Links ]
Govindaswamy, A., Bajpai, V., Trikha, V., et al., 2018, Multidrug resistant Elizabethkingia meningoseptica bacteremia-Experience from a level 1 trauma centre in India, Intractable & Rare Diseases Research 7, 172-176. [ Links ]
Empedobacter
Basani, L. & Aepala, R., 2018, Empedobacter brevis causing early onset sepsis and pneumonia in a neonate: case report and review of literature, Int J Contemp Pediatr 5, 654-6. [ Links ]
Parte, A., Krieg, N.R., Ludwig, W., et al., 2011, Bergey's Manual of Systematic Bacteriology: Volume 4: The Bacteroidetes, Spirochaetes, Tenericutes (Mollicutes), Acidobacteria, Fibrobacteres, Fusobacteria, Dictyoglomi, Gemmatimonadetes, Lentisphaerae, Verrucomicrobia, Chlamydiae, and Planctomycetes (Vol. 4). Springer Science & Business Media. [ Links ]
Enterobacter
Antimicrobe. Entoerobacter. [Online]. Available from: http://www.antimicrobe.org/b97.aspp. Accessed 12 October 2019. [ Links ]
Davin-Regli, A. & Pagès, J.M., 2015, Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment, Frontiers in Microbiology 6, 392 [ Links ]
Fraser, S.L., 2019, Enterobacter Infections. [Online]. Available from: https://emedicine.medscape.com/article/216845-overview. Accessed 12 October 2019. [ Links ]
Gibson, J.S., Morton, J.M., Cobbold, R.N., et al., 2008 Multidrug-resistant E. coli and Enterobacter extraintestinal infection in 37 dogs, Journal of Veterinary Internal Medicine 22, 844-850. [ Links ]
Rogers, K., 2017, Enterobacter. Encyclopedia Britannica. [Online] Available from: https://www.britannica.com/science/Enterobacter. Accessed 14 February 2020. [ Links ]
Rolston, K.V., Nesher, L., Tarrand, J.T., 2014, Current microbiology of surgical site infections in patients with cancer: a retrospective review, Infectious Diseases and Therapy 3, 245-256. [ Links ]
Sanders Jr, W.E. & Sanders, C.C., 1997, Enterobacter spp.: pathogens poised to flourish at the turn of the century, Clinical Microbiology Reviews 10, 220-241. [ Links ]
Weese, J.S., 2008, Investigation of Enterobacter cloacae Infections at a Small Animal Veterinary Teaching Hospital. ACVIM 2008. [Online]. Available from: https://www.vin.com/apputil/content/defaultadv1.aspx?pId=11262&id=3865712. Accessed 14 February 2020. [ Links ]
Enterococcus
Andrade, N., Schmiedt, C.W., Cornell, K., et al., 2016, Survey of intraoperative bacterial contamination in dogs undergoing elective orthopedic surgery, Veterinary Surgery 45, 214-222. [ Links ]
Arias, M.V.B., Padilha, F.N., Perugini, M.R., 2017, Deep tissue culture and hemoculture in dogs with wounds and sepsis, Pesquisa Veterinária Brasileira 37, 1483-1490. [ Links ]
Chong, K.K.L., Tay, W.H., Janela, B., et al., 2017, Enterococcus faecalis modulates immune activation and slows healing during wound infection, The Journal of Infectious Diseases 216(12), 1644-1654. [ Links ]
Fraser, S.L., 2018, Enterococcal Infections. [Online]. Available from: https://emedicine.medscape.com/article/216993-overview. Accessed 15 October 2019. [ Links ]
Ghosh, A., Dowd, S.E., Zurek, L., 2011, Dogs leaving the ICU carry a very large multi-drug resistant enterococcal population with capacity for biofilm formation and horizontal gene transfer, PLoS One 6(7), e22451. [ Links ]
Goh, H.S., Yong, M.A., Chong, K.K.L., et al., 2017, Model systems for the study of Enterococcal colonization and infection, Virulence, 8(8), 1525-1562. [ Links ]
Guardado, R., Asensi, V., Torres, J., et al., 2006, Post-surgical enterococcal meningitis: clinical and epidemiological study of 20 cases, Scandinavian Journal of Infectious Diseases 38, 584-588. [ Links ]
Infectious Disease Advisor, 2019, Gram positive bacteria - Enterococcus. [Online]. Available from: https://www.infectiousdiseaseadvisor.com/home/decision-support-in-medicine/hospital-infection-control/gram-positive-bacteria-enterococcus/. Accessed 18 February 2020. [ Links ]
Jo, H.J., Chae, H.S., Kim, H.J., et al., 2012. High prevalence of Enterococcus spp. From dogs with otitis externa,한국가축위생학회지 (KOJVS) 35, 99-104. [ Links ]
Kataoka, Y., Umino, Y., Ochi, H., et al., 2014, Antimicrobial susceptibility of enterococcal species isolated from antibiotic-treated dogs and cats, Journal of Veterinary Medical Science 76, 1399-1402. [ Links ]
Kau, A.L., Martin, S.M., Lyon, W., et al., 2005, Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice, Infection and Immunity 73, 2461-2468. [ Links ]
Layton, B.A., Walters, S.P., Lam, L.H., et al., 2010, Enterococcus species distribution among human and animal hosts using multiplex PCR, Journal of Applied Microbiology 109(2), 539-547. [ Links ]
Pochhammer, J., Kramer, A., Schäffer, M., 2017, Enterococci and surgical site infections: Causal agent or harmless commensals? Der Chirurg 88, 377-384. [ Links ]
Rolston, K.V., Nesher, L., Tarrand, J.T., 2014, Current microbiology of surgical site infections in patients with cancer: a retrospective review, Infectious Diseases and Therapy 3, 245-256. [ Links ]
Singer, D.A., Jochimsen, E.M., Gielerak, P. et al., 1996, Pseudo-outbreak of Enterococcus durans infections and colonization associated with introduction of an automated identification system software update, Journal of Clinical Microbiology 34, 2685-2687. [ Links ]
Singer, D.A., Jochimsen, E.M., Gielerak, P., et al., 1996., Pseudo-outbreak of Enterococcus durans infections and colonization associated with introduction of an automated identification system software update, Journal of Clinical Microbiology 34, 2685-2687. [ Links ]
Vetbact,, 2017. Enterococcus fecalis. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=124. Accessed 18 February 2020. [ Links ]
Vijayakrishnan, R. & Rapose, A., 2012, Fatal Enterococcus durans aortic valve endocarditis: a case report and review of the literature, BMC Case Reports 2012, bcr0220125855. [ Links ]
Kocuria
Kandi, V., Palange, P., Vaish, R., et al., 2016, Emerging bacterial infection: identification and clinical significance of Kocuria species, Cureus, 8(8). [ Links ]
Lee, M.K., Choi, S.H., Ryu, D.W., 2013, Descending necrotizing Mediastinitis caused by Kocuria rosea: a case report, BMC Infectious Diseases 13(1), 1-4. [ Links ]
Paul, M., Gupta, R., Khush, S., et al., 2015, Kocuria rosea: An emerging pathogen in acute bacterial meningitis-Case report, J Microbiol Antimicrob Agents 1(1), 4-7. [ Links ]
Purty, S., Saranathan, R., Prashanth, K., et al., 2013, The expanding spectrum of human infections caused by Kocuria species: a case report and literature review, Emerging Microbes & Infections 2(1), pp.1-8. [ Links ]
Kytococcus
Amaraneni, A., Malik, D., Jasra, S., et al., 2015, Kytococcus schroeteri bacteremia in a patient with hairy cell leukemia: a case report and review of the literature, Case Reports in Infectious Diseases 2015. [ Links ]
Chan, J.F.W., Wong, S.S.Y., Leung, S.S.M., et al., 2012, First report of chronic implant-related septic arthritis and osteomyelitis due to Kytococcus schroeteri and a review of human K. schroeteri infections, Infection 40, 567-573. [ Links ]
Frederiksen, W., Magee, J.T., Ursing, J., 1999, Proposed new bacterial taxa and proposed changes of bacterial names published during 1997 and considered to be of interest to medical or veterinary bacteriology. [ Links ]
Jacquier, H., Allard, A., Richette, P., et al., 2010, Postoperative spondylodiscitis due to Kytococcus schroeteri in a diabetic woman. Journal of Medical Microbiology 59(1), 127-129. [ Links ]
Micrococcus
Andrade, N., Schmiedt, C.W., Cornell, K., et al., 2016, Survey of intraoperative bacterial contamination in dogs undergoing elective orthopedic surgery, Veterinary Surgery 45, 214-222. [ Links ]
Boucher, C., Henton, M.M., Becker, P.J., et al., 2018, Comparative efficacy of three antiseptics as surgical skin preparations in dogs, Veterinary Surgery 47(6), pp.792-801. [ Links ]
Environment and Climate Change Canada, Health Canada, 2018, Final Screening Assessment of Micrococcus luteus strain ATCC 4698 [Online]. Available from https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/screening-assessment-micrococcus-luteus.html. Accessed 25 November 2020. [ Links ]
Kalhoro, D.H., Ansari, M.I., Abro, S.H., et al., 2019, 39. Prevalence and antimicrobial sensitivity of bacterial isolates from canine wound infection in Tandojam, Sindh, Pure and Applied Biology 8(1), 372-379. [ Links ]
Kloos, W.E., Tornabene, T.G., Schleifer, K.H., 1974, Isolation and characterization of micrococci from human skin, including two new species: Micrococcus lylae and Micrococcus kristinae, International Journal of Systematic and Evolutionary Microbiology 24(1), 79-101. [ Links ]
Kogure, Y., Nakamura, F., Nukina, A., et al., 201, Catheter-related septic shock by Micrococcus in an autologous hematopoietic stem cell transplantation recipient. American Journal of Infection Control 42(1), 87. [ Links ]
Kooken, J.M., Fox, K.F., Fox, A., 2012, Characterization of Micrococcus strains isolated from indoor air, Molecular and Cellular Probes 26(1), 1-5. [ Links ]
Miltiadous, G. and Elisaf, M., 2011, Native valve endocarditis due to Micrococcus luteus: a case report and review of the literature, Journal of Medical Case Reports 5, 1-3. [ Links ]
MSDS, 2019, Micrococcus spp. Fee Safety Data Sheet Index [Online]. Available from: https://www.msdsonline.com/resources/sds-resources/free-safety-data-sheet-index/micrococcus-spp/. Accessed 25 February 2020. [ Links ]
Murray, E., 2017, Micrococcus luteus in a Veterinary Clinical Setting, Journal of Microbiology. [ Links ]
Vetbact,, 2017, Micrococcus luteus. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=223. Accessed 25 February 2020. [ Links ]
Wickham Microl, 2018, Fact Sheet: Micrococcus luteus. [Online]. Available from https://wickhamlabs.co.uk/technical-resource-centre/fact-sheet-micrococcus-luteus/. Accessed 12 October 2019. [ Links ]
Moraxella
Bush, L.M. Peres, M.T., 2018, Moraxella catarrhalis Infection. [Online]. Available from: https://www.msdmanuals.com/professional/infectious-diseases/gram-negative-cocci-and-coccobacilli/moraxella-catarrhalis-infection. Accessed 14 October 2019. [ Links ]
Shah, S.S., Ruth, A., Coffin, S.E., 2000, Infection due to Moraxella osloensis: case report and review of the literature, Clinical Infectious Diseases 30(1), 179-181. [ Links ]
Verduin, C.M., Hol, C., Fleer, A., et al., 2002, Moraxella catarrhalis: from emerging to established pathogen, Clinical Microbiology Reviews 15(1), 125-144. [ Links ]
Vetbact, 2018, Moreaxella osloensis, [Online]. Available from: https://www.Vetbact,.org/index.php?artid=201. Accessed 19 February 2020. [ Links ]
Davis, M., Dalton, K., Johnson, Z., et al., 2018, 2331. Household pets and recovery of moraxella catarrhalis and other respiratory pathogens from children with asthma, Open Forum Infectious Diseases 5, S692-S693. [ Links ]
Panotea
Büyükcam, A., Tuncer, Ö., Gür, D., et al., 2018, Clinical and microbiological characteristics of Pantoea agglomerans infection in children, Journal of Infection and Public Health 11(3), 304-309. [ Links ]
Cruz, A.T., Cazacu, A.C., Allen, C.H., 2007, Pantoea agglomerans, a plant pathogen causing human disease, Journal of Clinical Microbiology 45(6), 1989-1992. [ Links ]
Mackiewicz, B., Lemieszek, M.K., Golec, M., et al., 2016, Pantoea agglomerans: a mysterious bacterium of evil and good. P. 4. Beneficial effects, Annals of Agricultural and Environmental Medicine 23(2). [ Links ]
Pasteurella
Chun, M.L., Buekers, T.E., Sood, A.K., et al., 2003, Postoperative wound infection with Pasteurella multocida from a pet cat, American Journal of Obstetrics and Gynecology, 188(4), 1115-1116. [ Links ]
Cross, S.L., 2019, Pasteurella Multocida Infection. [Online]. Available from: https://emedicine.medscape.com/article/224920-overview. Accessed 25 February 2020. [ Links ]
Giordano, A., Dincman, T., Clyburn, B.E., et al., 2015, Clinical features and outcomes of Pasteurella multocida infection, Medicine 94(36). [ Links ]
Tun, A.E., Benedicenti, L., Galban, E.M., 2018, Pasteurella Multocida meningoencephalomyelitis in a dog secondary to severe periodontal disease, Clinical Case Reports 6(6), 1137. [ Links ]
Vetbact, 2018, Pasteurella multocida. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=56. Accessed 19 February 2020. [ Links ]
Weber, DJ., Kaplan, S.L., 2018, Pasteurella infections. [Online]. Available from https://www.uptodate.com/contents/pasteurella-infections#:~:text=This%20topic%20last%20updated%3A%20Jan,or%20dog%20bites%20or%20licks. Accessed 27 October 2019. [ Links ]
Pseudomonas
Andrade, N., Schmiedt, C.W., Cornell, K., et al., 2016, Survey of intraoperative bacterial contamination in dogs undergoing elective orthopedic surgery, Veterinary Surgery 45, 214-222. [ Links ]
Arias, M.V.B., Padilha, F.N., Perugini, M.R., 2017, Deep tissue culture and hemoculture in dogs with wounds and sepsis, Pesquisa Veterinária Brasileira 37, 1483-1490. [ Links ]
Bernal-Rosas, Y., Osorio-Muñoz, K., Torres-García, O., 2015, Pseudomonas aeruginosa: an emerging nosocomial trouble in veterinary, Revista MVZ Córdoba 20, 4937-4946. [ Links ]
Cafasso, J., 2019, Pseudomonas infections. [Online]. Available from https://www.healthline.com/health/pseudomonas-infections#symptoms. Accessed 27 February 2020. [ Links ]
Iglewski, B.H., Pseudomonas. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 27. [ Links ]
Kang, C.I., Kim, S.H., Kim, H.B., et al., 2003, Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome, Clinical Infectious Diseases 37(6), 745-751. [ Links ]
Lin, D., Foley, S.L., Qi, Y., et al., 2012, Characterization of antimicrobial resistance of Pseudomonas aeruginosa isolated from canine infections, Journal of Applied Microbiology 113(1), 16-23. [ Links ]
Masaadeh, H.A., Jaran, A.S., 2009, Incident of Pseudomonas aeruginosa in post-operative wound infection, Am J Infect Dis 5(1), 1-6. [ Links ]
McNeil, S.A., Nordstrom-Lerner, L., Malani, P.N., et al., 2001, Outbreak of sternal surgical site infections due to Pseudomonas aeruginosa traced to a scrub nurse with onychomycosis, Clinical Infectious Diseases 33(3), 317-323. [ Links ]
Moradali, M.F., Ghods, S., Rehm, B.H., 2017, Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence, Frontiers in Cellular and Infection Microbiology 7, 39. [ Links ]
Moremi, N., Claus, H., Vogel, U., et al, 2017, Surveillance of surgical site infections by Pseudomonas aeruginosa and strain characterization in Tanzanian hospitals does not provide proof for a role of hospital water plumbing systems in transmission, Antimicrobial Resistance & Infection Control 6(1), 1-8. [ Links ]
Newman, J.W., Floyd, R.V., Fothergill, J.L., 2017, The contribution of Pseudomonas aeruginosa virulence factors and host factors in the establishment of urinary tract infections, FEMS Microbiology Letters 364(15), p.fnx124. [ Links ]
Obritsch, M.D., Fish, D.N., MacLaren, R., et al., 2005, Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: epidemiology and treatment options, Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 25(10), 1353-1364. [ Links ]
Oguntibeju, O.O. and Rau, N., 2004, Occurrence of Pseudomonas aeruginosa in post-operative wound infection, Pakistan Journal of Medical Sciences 20, 187-192. [ Links ]
Ranjan, K.P., Ranjan, N., Bansal, S.K., et al., 2010, Prevalence of Pseudomonas aeruginosa in post-operative wound infection in a referral hospital in Haryana, India, Journal of Laboratory Physicians 2(02), 074-077. [ Links ]
Rolston, K.V., Nesher, L., Tarrand, J.T., 2014, Current microbiology of surgical site infections in patients with cancer: a retrospective review, Infectious Diseases and Therapy 3, 245-256. [ Links ]
Todar, K., Pseudomonas aueroginosa. [Online]. Available from http://textbookofbacteriology.net/pseudomonas.html. Accessed 20 May 2020. [ Links ]
Vetbact, 2018, Pseudomonas aeruginosa. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=65. Accessed 27 February 2020. [ Links ]
Walker, R., Pseudomonas infections. [Online]. Available from https://www.vetstream.com/treat/canis/bug/pseudomonas. Accessed 27 February 2020. [ Links ]
Rhodococcus
Bryan, L.K., Clark, S.D., Díaz-Delgado, J., et al., 2017, Rhodococcus equi infections in dogs, Veterinary Pathology 54(1), 159-163. [ Links ]
Kedlaya, I., 2018, Rhodococcus equi Infection. [Online]. Available from: https://emedicine.medscape.com/article/235466-overview. Accessed 27 October 2019. [ Links ]
Paasche, S.R., 2009, Rhodococcus equi infection in a surgical wound, American Society for Clinical Laboratory Science 22(3), 141-145. [ Links ]
Ribeiro, M.G., 2018, Overview of Rhodococcosisn. [Online]. Available from: https://www.msdvetmanual.com/generalized-conditions/rhodococcus-infection/rhodococcus-infection-in-animals?query=Overview%20of%20Rhodococcosis. Accessed 27 October 2019. [ Links ]
Takai, S., Martens, R.J., Julian, A., et al., 2003, Virulence of Rhodococcus equi isolated from cats and dogs, Journal of Clinical Microbiology 41(9), 4468-4470. [ Links ]
Riemerella
Christensen, J.P., 2018, Overview of Riemerella anatipestifer Infection in Poultry. [Online]. Available from https://www.msdvetmanual.com/poultry/riemerella-anatipestifer-infection/riemerella-anatipestifer-infection-in-poultry?query=Overview%20of%20Riemerella%20anatipestifer%20Infection%20in%20Poultry#v55602647. Accessed 14 October 2019. [ Links ]
Vetbact, 2017, Riemerella anatipestifer. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=56. Accessed 19 February 2020. [ Links ]
Sphingomonas
Bayram, N., Devrim, i., Apa, H., et al., 2013, Sphingomonas paucimobilis infections in children: 24 case reports, Mediterranean Journal of Hematology and Infectious Diseases, 5(1). [ Links ]
Ryan, M.P. & Adley, C.C., 2010, Sphingomonas paucimobilis: a persistent Gram-negative nosocomial infectious organism, Journal of Hospital Infection 75(3), 153-157. [ Links ]
Staphyloccocus
Akhaddar, A., Elouennass, M., Naama, O. et al., 2010, Staphylococcus xylosus isolated from an otogenic brain abscess in an adolescent, Surgical Infections 11(6), 559-561. [ Links ]
Andrade, N., Schmiedt, C. W., Cornell, K., et al., 2016, Survey of intraoperative bacterial contamination in dogs undergoing elective orthopedic surgery, Veterinary Surgery 45, 214-222. [ Links ]
Arias, M.V.B., Padilha, F.N., Perugini, M.R., 2017, Deep tissue culture and hemoculture in dogs with wounds and sepsis, Pesquisa Veterinária Brasileira 37, 1483-1490. [ Links ]
Barigye, R., Schaan, L., Gibbs, P.S., et al., 2007, Diagnostic evidence of Staphylococcus warneri as a possible cause of bovine abortion, Journal of Veterinary Diagnostic Investigation 19(6), 694-696. [ Links ]
Barros, E.M., Ceotto, H., Bastos, M.C.F., et al., 2012, Staphylococcus haemolyticus as an important hospital pathogen and carrier of methicillin resistance genes, Journal of Clinical Microbiology 50(1), 166-168. [ Links ]
Belo, L., Serrano, I., Cunha, E., et al., 2018, Skin asepsis protocols as a preventive measure of surgical site infections in dogs: chlorhexidine-alcohol versus povidone-iodine, BMC veterinary research 14, 1-6. [ Links ]
Ben Zakour, N.L., Beatson, S.A., van den Broek, A.H., et al., 2012, Comparative genomics of the Staphylococcus intermedius group of animal pathogens, Frontiers in Cellular and Infection Microbiology 2, 44. [ Links ]
Bhardwaj, B., Bhatnagar, U.B., Conaway, D.G., 2016, An unusual presentation of native valve endocarditis caused by Staphylococcus warneri, Reviews in Cardiovascular Medicine 17(3-4), 140-143. [ Links ]
Boucher, C., Henton, M.M., Becker, P.J., et al., 2018, Comparative efficacy of three antiseptics as surgical skin preparations in dogs, Veterinary Surgery 47(6), 792-801. [ Links ]
Bush, L.M. Peres, M.T., 2019, Staphylococcal Infections. [Online]. Available from: https://www.msdmanuals.com/professional/infectious-diseases/gram-positive-cocci/staphylococcal-infections?query=staphylococcal%20infections. Accessed 12 February 2020. [ Links ]
Campoccia, D., Montanaro, L., Visai, L., et al., 2010, Characterization of 26 Staphylococcus warneri isolates from orthopedic infections, The International Journal of Artificial Organs 33(9), 575-581. [ Links ]
Centers for Disease Control and Prevention. Pneumococcal Disease (Streptococcus pneumonia) [Online]. Available from https://wwwnc.cdc.gov/travel/diseases/pneumococcal-disease-streptococcus-pneumoniae. Accessed 28 February 2020. [ Links ]
Centers for Disease Control and Prevention. Streptococcus pneumonia [Online]. Available from https://www.cdc.gov/pneumococcal/clinicians/streptococcus-pneumoniae.html. Accessed 10 February 2020. [ Links ]
Czekaj, T., Ciszewski, M., Szewczyk, E.M., 2015, Staphylococcus haemolyticus-an emerging threat in the twilight of the antibiotics age, Microbiology 161(Pt_11), 2061-2068. [ Links ]
Degener, J.E., Heck, M.E., Van Leeuwen, et al., 1994, Nosocomial infection by Staphylococcus haemolyticus and typing methods for epidemiological study, Journal of Clinical Microbiology 32(9), 2260-2265. [ Links ]
Espino, L., Bermudez, R., Fidalgo, L.E., et al., 2006, Meningoencephalitis associated with Staphylococcus warneri in a dog, Journal of Small Animal Practice 47(10), 598-602. [ Links ]
Foster T. Staphylococcus. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 12. Available from: https://www.ncbi.nlm.nih.gov/books/NBK8448/. [ Links ]
Grandolfo, E., 2018, Looking through Staphylococcus pseudintermedius infections: Could Sp A be considered a possible vaccine target? Virulence 9(1), 703-706. [ Links ]
Humphreys, H., Becker, K., Dohmen, P.M., et al., 2016, Staphylococcus aureus and surgical site infections: benefits of screening and decolonization before surgery, Journal of Hospital Infection 94(3), 295-304. [ Links ]
Ireland, M. Kollmann, L. Scheftel, J, 2018, MRSA in the Veterinary clinic: Management of Pets and People. [Online]. Available from https://todaysveterinarypractice.com/management-strategiesmrsa-veterinary-clinic-management-pets-people/. Accessed 13 February 2020. [ Links ]
Kamath, U., Singer, C., Isenberg, H.D., 1992, Clinical significance of Staphylococcus warneri bacteremia, Journal of Clinical Microbiology 30(2), 261-264. [ Links ]
Keim, L.S., Torres-Filho, S.R., Silva, P.V. et al., 2011, Prevalence, aetiology and antibiotic resistance profiles of coagulase negative staphylococci isolated in a teaching hospital, Brazilian Journal of Microbiology 42, 248-255. [ Links ]
Klein, E., Smith, D.L., Laxminarayan, R., 2007, Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999-2005, Emerging Infectious Diseases 13(12), 1840. [ Links ]
Large Outbreak Caused by Methicillin Resistant Staphylococcus pseudintermedius ST71 in a Finnish Veterinary Teaching Hospital - From Outbreak Control to Outbreak Prevention. [ Links ]
Lilenbaum, W., Veras, M., Blum, E., et al., 2000, Antimicrobial susceptibility of staphylococci isolated from otitis externa in dogs, Letters in Applied Microbiology 31(1), 42-45. [ Links ]
Loncaric, I., Tichy, A., Handler, S., et al., 2019, Prevalence of methicillin-resistant Staphylococcus sp.(MRS) in different companion animals and determination of risk factors for colonization with MRS, Antibiotics 8(2), 36. [ Links ]
Malik, S., Peng, H., Barton, M.D., 2005, Antibiotic resistance in staphylococci associated with cats and dogs, Journal of Applied Microbiology 99(6), 1283-1293. [ Links ]
Mellinghoff, S.C., Vehreschild, J.J., Liss, B.J., et al., 2018, Epidemiology of surgical site infections with Staphylococcus aureus in Europe: protocol for a retrospective, multicenter study, JMIR Research Protocol 7(3), e8177. [ Links ]
Mellinghoff, S.C., Vehreschild, J.J., Liss, B.J. et al., 2018, Epidemiology of surgical site infections with Staphylococcus aureus in Europe: protocol for a retrospective, multicenter study, JMIR Research Protocols 7(3), e8177. [ Links ]
Otto, M., 2009, Staphylococcus epidermidis- the'accidental'pathogen, Nature Reviews Microbiology 7(8), 555-567. [ Links ]
Padhy, A., Mishra, R., Behera, S.S., et al., 2014, Microbial profile of canine persistent wound infections, Veterinary World 7(4). [ Links ]
Pal, S., Sayana, A., Joshi, A. et al., 2019, Staphylococcus aureus: A predominant cause of surgical site infections in a rural healthcare setup of Uttarakhand, Journal of Family Medicine and Primary Care 8(11), 3600. [ Links ]
Piette, A. & Verschraegen, G., 2009, Role of coagulase-negative staphylococci in human disease, Veterinary Microbiology 134(1-2), 45-54. [ Links ]
Pompilio, A., De Nicola, S., Crocetta, V., et al., 2015, New insights in Staphylococcus pseudintermedius pathogenicity: antibiotic-resistant biofilm formation by a human wound-associated strain, BMC Microbiology 15, 1-14. [ Links ]
Rolston, K.V., Nesher, L., Tarrand, J.T., 2014, Current microbiology of surgical site infections in patients with cancer: a retrospective review, Infectious Diseases and Therapy 3, 245-256. [ Links ]
Rossi, C.C., Salgado, B.A.B., Barros, E.M., et al., 2018, Identification of Staphylococcus epidermidis with transferrable mupirocin resistance from canine skin, The Veterinary Journal 235, 70-72. [ Links ]
Ruzauskas, M., Siugzdiniene, R., Klimiene, I., et al., 2014, Prevalence of methicillin-resistant Staphylococcus haemolyticus in companion animals: a cross-sectional study, Annals of Clinical Microbiology and Antimicrobials 13, 1-7. [ Links ]
Schmidt, V.M., Williams, N.J., Pinchbeck, G., Corless, C.E., Shaw, S., McEwan, N., Dawson, S. and Nuttall, et al., 2014, Antimicrobial resistance and characterisation of staphylococci isolated from healthy Labrador retrievers in the United Kingdom, BMC Veterinary Research 10(1), 1-14. [ Links ]
Shoen, H.R., Rose, S.J., Ramsey, S.A., et al., 2019, Analysis of Staphylococcus infections in a veterinary teaching hospital from 2012 to 2015, Comparative Immunology, Microbiology and Infectious Diseases 66, 101332. [ Links ]
Siugzdaite, J. & Gabinaitiene, A., 2017, Methicillin-resistant coagulase-negative staphylococci in healthy dogs, Veterinární Medicina 62(9), 479-487. [ Links ]
Somayaji, R., Priyantha, M.A.R., Rubin, J.E. et al., 2016, Human infections due to Staphylococcus pseudintermedius, an emerging zoonosis of canine origin: report of 24 cases, Diagnostic Microbiology and Infectious Disease 85(4), 471-476. [ Links ]
Vetbact, 2018, Staphylococcus aureus subsp. aureus. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=20. Accessed 12 February 2020. [ Links ]
Vetbact, 2017, Staphylococcus dysgalactiae subsp. Equi similis. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=121. Accessed 02 February 2020. [ Links ]
Vetbact, (2018, Staphylococcus epidermidis. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=205. Accessed 17 February 2020. [ Links ]
Weese, S., 2016, Methicillin-resistant staphylococcal infections, Complications in Small Animal Surgery 39-44. [ Links ]
Raz, R., Colodner, R., Kunin, C.M., 2005, Who are you-Staphylococcus saprophyticus? Clin Infect Dis 40(6), 896-8. [ Links ]
Widerström, M., 2016, Commentary: significance of Staphylococcus epidermidis in health care-associated infections, from contaminant to clinically relevant pathogen: this is a wake-up call! Journal of Clinical Microbiology 54(7), 1679-1681. [ Links ]
Windahl, U., Bengtsson, B., Nyman, A.K. et al., 2015, The distribution of pathogens and their antimicrobial susceptibility patterns among canine surgical wound infections in Sweden in relation to different risk factors, Acta Veterinaria Scandinavica 57(1), 1-10. [ Links ]
Streptococcus
Abrahamian, F.M. & Goldstein, E.J., 2011, Microbiology of animal bite wound infections, Clinical Microbiology Reviews 24(2), 231-246. [ Links ]
Antimicrobe. B Streptococcus species (Group G and Group C Streptococci, Viridans Group, Nutritionally Variant Streptococci). [Online]. Available from: http://www.antimicrobe.org/new/b241.asp. Accessed 15 October 2019. [ Links ]
Bereket, W., Hemalatha, K., Getenet, B., et al., 2012, Update on bacterial nosocomial infections, European Review for Medical & Pharmacological Sciences 16(8). [ Links ]
Bush, L.M., Peres, M.T., 2019, Streptococcal Infections. [Online]. Available from: https://www.msdmanuals.com/professional/infectious-diseases/gram-positive-cocci/streptococcal-infections?query=Streptococcal%20 Infections. Accessed 04 March 2020. [ Links ]
Catto, B.A., Jacobs, M.R., Shlaes, D.M., 1987, Streptococcus mitis: a cause of serious infection in adults, Archives of Internal Medicine 147(5), 885888. [ Links ]
Centers for Disease Control and Prevention. Streptococcus pneumonia [Online]. Available from: https://www.cdc.gov/pneumococcal/clinicians/streptococcus-pneumoniae.html. Accessed 10 February 2020. [ Links ]
Chanter, N., 1997, Streptococci and enterococci as animal pathogens, Journal of Applied Microbiology 83(S1), 100S-109S. [ Links ]
Chanter, N., 1997, Streptococci and enterococci as animal pathogens. Journal of Applied Microbiology 83(S1), 100S-109S. [ Links ]
Ciszewski, M., Zegarski, K., Szewczyk, E.M., 2016, Streptococcus dysgalactiae subsp. equisimilis isolated from infections in dogs and humans: are current subspecies identification criteria accurate? Current Microbiology 73, 684-688. [ Links ]
Clapper, W.E., Meade, G.H., 1963, Normal flora of the nose, throat, and lower intestine of dogs Journal of Bacteriology 85(3), 643-648. [ Links ]
Deng, W. & Farricielli, L., 2014, Group G streptococcal sepsis, septic arthritis and myositis in a patient with severe oral ulcerations, Case Reports p.bcr2013200338. [ Links ]
Di Domenico, E.G., Toma, L., Prignano, G., et al., 2015, Misidentification of Streptococcus uberis as a human pathogen: a case report and literature review, International Journal of Infectious Diseases 33, 79-81. [ Links ]
Facklam, R., 2002, What happened to the streptococci: overview of taxonomic and nomenclature changes, Clinical Microbiology Reviews 15(4), 613-630. [ Links ]
Feng, Y., Zhang, H., Wu, Z., et al., 2014, Streptococcus suis infection: an emerging/reemerging challenge of bacterial infectious diseases? Virulence 5(4), 477-497. [ Links ]
Gaschen, F., 2008, Nosocomial Infection: Prevention and Approach. World Small Animal Veterinary Association World Congress Proceedings. [Online]. Available from: https://www.vin.com/apputil/content/defaultadv1.aspx?meta=Generic&pId=11268&id=3866711. Accessed 04 March 2020. [ Links ]
Giacometti, A., Cirioni, O., Schimizzi, A.M., et al., 2000, Epidemiology and microbiology of surgical wound infections, Journal of Clinical Microbiology 38(2), 918-922. [ Links ]
Ginders, M., Leschnik, M., Künzel, F., et al., 2017, Characterization of Streptococcus pneumoniae isolates from Austrian companion animals and horses, Acta Veterinaria Scandinavica 59(1), 1-5. [ Links ]
Gomez, E., Kennedy, C.C., Gottschalk, M., et al., 2014, Streptococcus suis-related prosthetic joint infection and streptococcal toxic shocklike syndrome in a pig farmer in the United States, Journal of Clinical Microbiology 52(6), 2254-2258 [ Links ]
Gottschalk, M., 2018, Streptococcus suis Infection. [Online]. Available from https://www.msdvetmanual.com/generalized-conditions/streptococcal-infections-in-pigs/streptococcus-suis-infection-in-pigs?query=streptococcus%20suis. Accessed 14 October 2019. [ Links ]
Guillet, M., Zahar, J.R., Timsit, M.O., et al., 2012, Horizontal transmission of Streptococcus pneumoniae in the surgical ward: a rare source of nosocomial wound infection, American Journal of Infection Control 40(1), 71-72. [ Links ]
Hughes, J.M., Wilson, M.E., Brandt, C.M., et al., 2009, Human infections due to Streptococcus dysgalactiae subspecies equisimilis, Clinical Infectious Diseases 49(5), 766-772. [ Links ]
Hughes, J.M., Wilson, M.E., Wertheim, H.F., et al., 2009, Streptococcus suis: an emerging human pathogen, Clinical Infectious Diseases 48(5), 617-625. [ Links ]
Iowa State University College of Veterinary Medicine, Veterinary Diagnostic and Production Animal Medicine, Streptococcal Infections [Online]. Available from https://vetmed.iastate.edu/vdpam/FSVD/swine/index-diseases/streptococcal-infection. Accessed 09 February 2020. [ Links ]
Jaalama, M., Palomäki, O., Vuento, R., et al., 2018, Prevalence and clinical significance of Streptococcus dysgalactiae subspecies equisimilis (Groups C or G Streptococci) colonization in pregnant women: a retrospective cohort study, Infectious Diseases in Obstetrics and Gynecology 2018, 2321046. [ Links ]
Kłos, M. & Wójkowska-Mach, J., 2017, Pathogenicity of virulent species of group C streptococci in human Canadian Journal of Infectious Diseases and Medical Microbiology 2017, 9509604. [ Links ]
Lamm, C.G., Ferguson, A.C., Lehenbauer, T.W. et al., 2010, Streptococcal infection in dogs: a retrospective study of 393 cases, Veterinary Pathology 47(3), 387-395. [ Links ]
Lu, B., Diao, B., Fang, Y., et al., 2016, First molecular evidence of intrauterine and surgical-site infections caused by Streptococcus dysgalactiae subsp. Equisimilis, The Journal of Infection in Developing Countries 10(06), 673-677. [ Links ]
Mazumder, S.A., 2018, Group D Streptococcus (GDS) Infections (Streptococcus bovis/ Streptococcus gallolyticus) [Online]. Available from: https://emedicine.medscape.com/article/229209-overview. Accessed 10 February 2020. [ Links ]
Milton, A.A.P., Priya, G.B., Aravind, M., et al., 2015, Nosocomial infections and their surveillance in veterinary hospitals, Adv Anim Vet Sci 3(2s), 1-24. [ Links ]
Muckle, A., Giles, J., Lund, L., et al., 2010, Isolation of Streptococcus suis from the urine of a clinically ill dog, The Canadian Veterinary Journal 51(7), 773. [ Links ]
Oteo, J., Avilla, J., Alós, J.I. et al., 1997, Urinary tract infection caused by Streptococcus mitis highly resistant to penicillin, The Pediatric Infectious Disease Journal 16(7), 724-725. [ Links ]
Petersson-Wolfe, C.S. & Currin, J.F., 2012, Streptococcus uberis: A practical summary for controlling mastitis. [ Links ]
Prado, C.A.N., 2018, Pneumococcal Infections (Streptococcus pneumonia) [Online]. Available from: https://emedicine.medscape.com/article/225811-overview. Accessed 10 February 2020. [ Links ]
Rolston, K.V., Nesher, L., Tarrand, J.T., 2014, Current microbiology of surgical site infections in patients with cancer: a retrospective review, Infectious Diseases and Therapy 3, 245-256. [ Links ]
Ruiz del Castillo, J., 1995, Streptococcus bovis in a surgical wound and a colonic neoplasm Gastroenterologia y Hepatologia 18(9), 474-476. [ Links ]
Savini, V., Cecinati, V., Onofrillo, D., et al., 2012, Surgical wound infection by Streptococcus pneumoniae after a cat-scratch disease, The International Journal of Lower Extremity Wounds 11(4), 311-312. [ Links ]
Shanan, S., Gumaa, S.A., Sandström, G., et al., 2011, Significant association of Streptococcus bovis with malignant gastrointestinal diseases, International Journal of Microbiology 2011. [ Links ]
Shelburne, S.A., Sahasrabhojane, P., Saldana, M., et al., 2014, Streptococcus mitis strains causing severe clinical disease in cancer patients, Emerging Infectious Diseases 20(5), 762. [ Links ]
Skyes, J., 2017, Streptococcus Infections in Dogs and Cats - An underestimated Pathogen [Online]. Available from: https://www.vin.com/apputil/content/defaultadv1.aspx?pId=20539&id=8506228. Accessed 04 March 2020. [ Links ]
Swartz, M.N., 1994, Hospital-acquired infections: diseases with increasingly limited therapies, Proceedings of the National Academy of Sciences 91(7), 2420-2427. [ Links ]
Van der Linden, M., Al-Lahham, A., Nicklas, W., et al., 2009, Molecular characterization of pneumococcal isolates from pets and laboratory animals PloS one 4(12), p.e8286. [ Links ]
Vetbact, 2015, Streptococcus pneumonia. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=145. Accessed 10 February 2020. [ Links ]
Vetbact, 2017, Streptococcus uberis. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=19. Accessed 09 February 2020. [ Links ]
Vetbact, 2018, Staphylococcus suis. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=147. Accessed 09 February 2020. [ Links ]
Vetbact, 2019, Staphylococcus dysgalactiae subsp. Dysgalactiae. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=17. Accessed 10 February 2020. [ Links ]
Windahl, U., Bengtsson, B., Nyman, A.K., et al., 2015, The distribution of pathogens and their antimicrobial susceptibility patterns among canine surgical wound infections in Sweden in relation to different risk factors, Acta Veterinaria Scandinavica 57(1), 1-10. [ Links ]
Woo, P.C., Fung, A.M., Lau, S.K., et al., 2001, Group G beta-hemolytic streptococcal bacteremia characterized by 16S ribosomal RNA gene sequencing, Journal of Clinical Microbiology 39(9), 3147-3155. [ Links ]
Woznick, W., Woznick, J., Polenakovik, H., 2018, Lifesaving Streptococcus bovis Surgical Site Infection, Infection Control & Hospital Epidemiology 39(5), 632-633. [ Links ]
Trueperella
Rzewuska, M., Kwiecień, E., Chrobak-Chmiel, D., et al., 2019, Pathogenicity and virulence of Trueperella pyogenes: a review, International Journal of Molecular Sciences 20(11), 2737. [ Links ]
Vetbact, 2019, Trueperella pyogenes. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=1. Accessed 09 February 2020. [ Links ]
Vibrio
Antimicrobe. Vibrio vulnificus. [Online]. Available from: http://www.antimicrobe.org/new/b244.asp. Accessed 12 October 2019. [ Links ]
Heng, S.P., Letchumanan, V., Deng, C.Y., et al., 2017, Vibrio vulnificus: an environmental and clinical burden, Frontiers in Microbiology 8, 997. [ Links ]
Morris, J.G., 2019, Vibrio vulnificus infections. [Online]. Available from https://www.uptodate.com/contents/vibrio-vulnificus-infections. Accessed 01 January 2020. [ Links ]
Vetbact, 2018, Vibrio vulnificus. [Online]. Available from: https://www.Vetbact,.org/index.php?artid=84. Accessed 12 February 2020. [ Links ]
Yersinia
Butler, T., Yersinia pseudotuberculosis. [Online]. Available from http://www.antimicrobe.org/b265.asp. Accessed 12 October 2020. [ Links ]
Haburchak, D.R., 2017, Pseudotuberculosis (Yersinia pseudotuberculosis Infection). [Online]. Available from: https://emedicine.medscape.com/article/226871-overview. Accessed 09 February 2020. [ Links ]
Infectious Disease Advisor, 2019, Other Yersinia species isolated from humans.[Online]. Available from: https://www.infectiousdiseaseadvisor.com/home/decision-support-in-medicine/infectious-diseases/other-yersinia-species-isolated-from-humans/. Accessed 09 February 2020. [ Links ]
Infectious Disease Advisor, 2019, Yersinia enterocolitica. [Online]. Available from: https://www.infectiousdiseaseadvisor.com/home/decision-support-in-medicine/infectious-diseases/yersinia-enterocolitica/. Accessed 09 February 2020. [ Links ]
Infectious Disease Advisor, 2019, Yersinia psudotuberculosis [Online]. Available from: https://www.infectiousdiseaseadvisor.com/home/decision-support-in-medicine/infectious-diseases/yersinia-pseudotuberculosis/. Accessed 12 October 2019. [ Links ]
Merck ManualVeterinary Manual, Common infections of the gastrointestinal tract. [Online]. Available from: https://www.merckvetmanual.com/multimedia/table/common-infections-of-the-gastrointestinal-tract. Accessed 09 February 2020. [ Links ]
Vetbact, 2018, Yersinia pseudotuberculosis. [Online]. Available from: https://swww.Vetbact,.org/index.php?artid=79. Accessed 09 February 2020. [ Links ]














