Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.94 n.1 Pretoria 2023
http://dx.doi.org/10.36303/jsava.573
ORIGINAL RESEARCH
African horse sickness vaccination status correlated with disease outcome in South Africa
ML GenisI; JE CraffordI; CT WeyerII; D PollardIII; JD GrewarII; AJ GuthrieIV
IVectors and Vector-borne Research Programme, Department of Veterinary Tropical Diseases, Faculty of Veterinary Science, University of Pretoria, South Africa
IISouth African Equine Health and Protocols, South Africa
IIIThe British Horse Society, United Kingdom
IVVeterinary Genetics Laboratory, Equine Research Centre, University of Pretoria, South Africa
ABSTRACT
African horse sickness (AHS) is one of the economically most important equid diseases in southern Africa, contributing significantly to equine morbidity and mortality. Annual vaccination with the Onderstepoort Biological Products polyvalent live attenuated vaccine has been the mainstay of prevention in South Africa. The study objectives were to determine if there is a significant relationship between multiple variables (vaccination status, number of AHSV [African horse sickness virus] serotypes contracted, clinical presentation, order of vaccine administration, age, sex and mean Ct value) and case outcome. The study population consisted of samples of AHS cases from South Africa submitted to the Veterinary Genetics Laboratory, University of Pretoria, that were confirmed positive by real-time RT-qPCR from 1 September 2017 to 30 June 2019 with a definitive disease outcome. At a univariable level, unvaccinated horses were 8.7 times more likely to die compared with horses that were vaccinated annually. Vaccination status was not statistically significant at a multivariable level, possibly due to insufficient sample size. Annual vaccination was shown to be protective. The pulmonary form of the disease and a lower Ct value had an increased likelihood of non-survival. Vaccination order was significant at a multivariable level (AHS2 vaccine administered first had a higher likelihood of survival). The study confirmed that increased case fatality was not due to vaccine failure but instead due to multiple variables, with an increased population of unvaccinated horses being one of these.
Keywords: African horse sickness virus, live attenuated vaccination, vaccination status, case fatality rate
Introduction
African horse sickness (AHS) is one of sub-Saharan Africa's most devastating equine diseases (Zientara et al. 2015). Though endemic to this area, it has spread outside these borders occasionally, causing outbreaks in naïve populations (Lubroth 1988, 1992; Zientara et al. 2015). AHS is caused by African horse sickness virus (AHSV), genus Orbivirus, family Reoviridae (Coetzer & Guthrie 2004; Mellor & Hamblin 2004). Nine (numbered 1-9) AHSV serotypes have been reported (Howell 1962). The spread of its insect vector, midges of the Culicoides spp, mainly C. imicola and C. bolitinos in southern Africa, primarily determines disease occurrence and spread (Du Toit 1944; Venter et al. 2000; Meiswinkel & Paweska 2003). AHS is a controlled disease in South Africa and a World Organization for Animal Health (WOAH) notifiable disease due to the high mortality rates (up to 90%) and potential to swiftly spread to naïve populations (South African Government Gazette 2018; Mellor & Hamblin 2004; OIE 2015; Zientara et al. 2015).
Disease diagnosis is often presumptively made based on history, clinical signs, and macroscopic pathology. Currently, routine diagnostics in South Africa are performed using the group-specific (GS) real-time reverse-transcriptase quantitative polymerase chain reaction (RT-qPCR) developed by Guthrie et al. (2013) and the serotype-specific (TS) RT-qPCR developed by Weyer et al. (2015). This RT-qPCR has high analytical sensitivity and specificity and the ability to obtain results quickly in an outbreak situation (Guthrie et al. 2013). AHS control consists of a multimodal approach, including protecting AHSV-free countries from the import of infected equids, management (mainly vector control) and vaccination. Annual vaccination with the Onderstepoort Biological Products (OBP) AHS live attenuated vaccine (LAV) is currently the primary means of controlling the disease in endemic areas (Erasmus 1978). According to the Animal Diseases Act 35 of 1984, all equines, except horses that reside in the AHS free and surveillance zones, require compulsory yearly vaccination (Animal Diseases Act 1984). The vaccine is made up of two components: component 1 (AHS1) and component 2 (AHS2), which are administered at least three weeks apart (von Teichman & Smit 2008). AHS1 is trivalent and consists of serotypes 1, 3 and 4; AHS2 is tetravalent and consists of serotypes 2, 6, 7 and 8. AHS serotype 9 is not included in the LAV as it has, until recently, caused minimal disease in southern Africa, and cross-protection is provided from vaccination against AHS serotype 6 (von Teichman & Smit 2008). AHS serotype 5 was included in AHS1 in the past but was removed in October 1993 following suspected reassortment leading to a virulent reassortant strain causing infection and consequent disease in vaccinated horses (von Teichman & Smit 2008; Molini et al. 2015). Von Teichman et al. (2010) showed that AHS serotype 8 gives cross-protection against serotype 5. Immunity to AHSV is serotype-specific. Therefore, horses need immunity to all nine serotypes to be completely protected against the disease (Koekemoer 2008). Molini et al. (2015) showed that most horses only develop neutralising antibodies against all AHSV serotypes after multiple vaccination courses. In the same study, Molini et al. (2015) noted that these neutralising antibodies only seem to reduce the severity of the clinical disease; as vaccinated animals can still contract field infections, and the immune response differs significantly between different horses and to the different AHS serotypes.
There have been alleged cases of OBP AHS-LAV failure (von Teichman & Smit 2008). Von Teichman & Smit (2008) evaluated the vaccine to determine whether or not the reassortment of AHS vaccine strains could result in reassortants and reversion to virulence and therefore cause AHS in susceptible horses. However, no clinical symptoms typical of AHS were observed in vaccinated horses, and all horses showed a good immune response (von Teichman & Smit 2008). In contrast, Weyer et al. (2016) confirmed that the AHS-LAV does have the potential for reassortment and reversion to virulence, which could lead to the emergence and spread of virulent viruses to susceptible equines. No study has looked at the claims of vaccine failure and whether vaccinated horses are less likely to die of AHSV than unvaccinated horses. This study filled the gap by evaluating the correlation between AHS vaccination status and disease outcome. The primary aim of this study was to correlate the AHS vaccination status of horses that tested positive for AHSV with real-time RT-qPCR with the disease outcome (death or survival). The secondary aims were to evaluate the vaccination frequency, disease-causing AHSV serotype prevalence, clinical symptoms related to disease outcome and laboratory results Ct (cycle threshold value) related to disease outcome. Tertiary aims included the age group in which disease was most prevalent, sex related to disease outcome and the order in which vaccine components were administered. The study objectives were to determine if there was a significant relationship between these factors (vaccination status, number of AHSV serotypes contracted, clinical presentation, order of vaccine administration, age, and sex) and disease outcome; and to describe the difference in mean Ct values relating to disease outcome.
Materials and methods
Study population and study period
The study population consisted of horses resident in South Africa, of which samples were submitted to the Veterinary Genetics Laboratory (VGL) at the University of Pretoria (UP) for suspected clinical AHS diagnosis, and who then tested positive with realtime RT-qPCR. Samples submitted between 1 September 2017 and 30 June 2019 were included in the study population.
Study design and data collection
A retrospective descriptive study design was used. Recruitment criteria were as follows: a) only horses with samples submitted from South Africa, b) with a clinical suspicion of AHS, c) submitted to the VGL for diagnostic purposes, d) that tested positive with real-time RT-qPCR and e) had a definitive disease outcome were included in this study. Sample types included whole blood in EDTA, organs or body fluids (fresh or frozen) and serum. Sample processing was performed by the VGL using the GS real-time RT-qPCR (Guthrie et al. 2013) accredited by the WOAH for AHSV diagnostics (World Organisation for Animal Health, 2023). AHSV-serotype determination was done with the AHSV TS real-time RT-qPCR test developed by Weyer et al. (2015). A structured questionnaire was designed for data collection of epidemiological interests that were not obtainable from the VGL submission forms and sample reports. This questionnaire was sent via Google Forms to each veterinarian or owner of a study case, with a follow-up phone conversation if required. The following data were collected for each case: age, sex, AHS episode date and outcome, clinical signs and complications, geographical location at the time of disease and movement prior to the episode, last AHS vaccination administered to the horse, the brand of this vaccine, the order in which the two vaccinations were administered and any vaccine reactions, the frequency of vaccination for AHS over the horse's lifetime. No sample size calculations were performed, as the study recruitment parameters predetermined the sample size (Figure 1).
Statistical analysis
Data were collated, stored in a comma-separated file, and imported into Stata (IC v.13.0)1 for coding and statistical analyses. A binary outcome variable was created to represent death (1) or recovery (0) following AHS diagnosis. The last vaccination date was captured in the original dataset and converted to days between the last vaccination and the AHS case date. These data were further categorised into two-month intervals up until 12 months between the last vaccination and the AHS case; all cases vaccinated after 12 months were included in a single group. Vaccination status and frequency were considered closely related to warrant separation, and vaccination frequency was retained, with unvaccinated animals being classified as such in the vaccination status variable. Categorical variables, including AHS outcome, AHS serotype class, presence of Equine Encephalosis Virus (EEV), vaccination frequency, vaccination order, sex, clinical presentation and time since last vaccination were described as proportions (%) with corresponding 95% confidence intervals (CI). Age was described as a median (interquartile [IQR] range and range) and cycle threshold (Ct) value as a mean (± standard deviation). Cases were classified into three categories for AHS serotype; testing positive for one, two or no AHS serotypes. Vaccination frequency was divided into four categories; once off, annual, every two years or more, unvaccinated or unknown. Clinical signs were divided into common categories; cardiac (dikkop, subacute), fever, pulmonary (dunkop, peracute), mixed (cardiac and pulmonary signs) and unknown.
Initial relationships between the outcome and the explanatory variables were assessed using a Chi-squared (x2) or Fisher's exact test (the latter where cells in the contingency table had less than five observations) for categorical variables. Age was assessed using the Mann-Whitney U test, and the Ct value was assessed using the independent student's t-test.
Univariable logistic regression models were used to estimate odds ratios (OR) and 95% CIs for an AHS case outcome of death. Variables with p-values of < 0.25 were screened for inclusion in multivariable logistic regression modelling (Hosmer 2013). Continuous variables were tested for evidence against linearity (likelihood ratio statistic [LRS] p-value < 0.05), and where relationships did not appear to be linear, variables were re-coded into biologically plausible or quartile categories. Reference categories were selected based on the lowest expected risk and a sufficient number of observations (Klein 2001). Missing data for categorical variables were re-coded into an "unknown" but otherwise remained missing.
The final multivariable logistic regression model was built using manual, stepwise, and forward selection using the LRS test to compare nested models and establish whether the added variable significantly improved model fit. Variables with the lowest Wald p-values from univariable modelling were added to the model first. Variables with LRS test p-values of < 0.05 were retained in the final model. Variables not retained in the final model were individually forced back into the model at the end to ensure that interactions or confounding variables were not omitted.
Post-fit diagnostic tests, including Pearson's x2 and Hosmer-Lemeshow goodness-of-fit test statistics, were used to evaluate the fit of the model to the data (Hosmer 2013).
Results
Descriptive data
Data were initially available for 233 horses with AHS diagnoses between October 2017 and June 2019. Reasons for exclusion of AHS PCR positive horses (455) included, but were not limited to, samples from outside South Africa, horses sampled for reasons other than clinical suspicion of AHS, samples from other equid species and loss to follow-up. A summary of the categorical variables is presented in Table I. The median age of the horses (n = 194) was seven years (IQR 3, 12 years; range 0.3 to 27 years), and there were slightly more females (51.5%) than males (43.4%). The Ct value was available for 224 horses, and the mean Ct value was 25.4 (± 4.3). The case fatality rate was 67.8%, with only 32.2% of cases recovering. The majority of cases tested positive for only a single AHS serotype (85.5%). Vaccination order was recorded for 59 of the 78 vaccinated horses. AHS1 vaccine was preferentially administered first (66.1% of vaccinated cases). The median time range since the last AHS vaccination (n = 71) was eight months (IQR 6, 10 months; range less than 2 to more than 12 months). Most horses displayed pulmonary clinical signs (38.2%), with cardiac and mixed clinical signs following (27.9% and 20.6%, respectively). The majority of cases (95.3%) did not have concomitant EEV.
Univariable logistic regression modelling
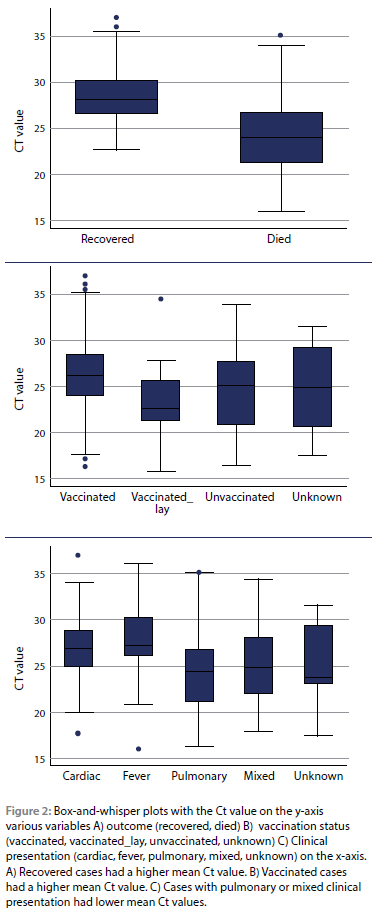
Categories with unknown sex (n = 12) and clinical presentation (n = 6) were temporarily excluded from the analysis as none recovered. A summary of the univariable logistic regression modelling of the 215 remaining cases is presented in Table II. Females were more likely to die (OR 1.6, 95% CI 0.9-2.8), with horses three years old or younger being approximately five times less likely to survive (OR 5.4, 95% CI 2.0-14.4) than those older than 12 years (reference value). Being positive for two AHS serotypes had a higher likelihood of non-survival (OR1.3, 95% CI 0.1-12.3). Ct value was highly correlated with outcome, as seen in Figure 2, with a Wald p-value of < 0.0. Annual vaccination was used as the reference value, with all other categories of vaccination frequency having a higher likelihood of non-survival. Vaccination order do not appear to affect the likelihood of survival (1then2 as the reference; 2then1 OR 0.3, 95% CI 0.1-1.1). Time since the last AHS vaccination did not have a statistically significant impact on survival, with the Wald p-value being > 0.05 for all categories. Pulmonary clinical signs had a markedly increased likelihood of non-survival (OR 116.3, 95% CI 15.3886.3) compared to cardiac clinical signs (reference). Coinfection with EEV was not significantly related to outcome (Wald p-value > 0.05).
Multivariable logistic regression modelling
After excluding variables with missing Ct values and unknown clinical presentation, a full dataset of 218 horses was available to develop the multivariable logistic regression (Table III).
The Ct value of the screening PCR was significantly associated with the odds of death, where for a one-unit increase in Ct (i.e. a decrease in viral load), the odds of death versus recovery increased by a factor of 0.6, which is equivalent to an approximate halving of the odds of death. Compared to the cardiac form of the disease, the pulmonary form was also significantly associated with an increase in the odds of death versus recovery by a factor of 496.6 times. The mixed form also increased the odds of death compared to the cardiac form by a factor of 4.0 times, with the fever form of the disease likely to decrease the odds of death compared to the cardiac form. Vaccinating against AHS with bottle two first and then bottle one decreased the odds of death in future cases by 0.04 compared to first giving bottle one and then bottle two. Horses aged between 12 and 27 years had the highest odds of death compared to younger horses aged between three and seven years. All of the above statements account for the influence of the other variables included in the model.
The Hosmer-Lemeshow (1.65, p = 0.99) and the Pearson x2 (p = 0.99) statistics suggested that the model was an adequate fit for the data.
Discussion
The purpose of this study was to evaluate the case fatality of clinically suspected AHS cases that tested real-time RT-qPCR positive when correlated to vaccination status. Previous studies evaluating immunity and the AHSV-LAV vaccine looked at some of the following matters: antibody titres to the nine known AHSV serotypes in horses that had undergone regular vaccination with the AHSV-LAV vaccine and the passive transfer and rate of decay of maternal antibody to the individual serotypes in foals of mares who had had multiple vaccinations with the AHSV-LAV vaccine (Crafford et al. 2013); AHSV-specific immune response in multiple age groups with different numbers of vaccination courses and disease severity related to vaccination status and frequency of vaccination (Molini et al. 2015); the occurrence of vaccine-induced disease (von Teichman & Smit 2008) and reversion-to-virulence mutants and reassortants from viruses within the polyvalent AHSV-LAV formula (Weyer et al., 2016). In Zimbabwe, Gordon et al. (2013) looked at the practice prevalence of AHS in a limited population of horses and the effect of vaccination status on case fatality. This study is a first of its kind in South Africa, statistically evaluating the correlation between AHS vaccination status and disease outcome.
Limitations to note include that due to a large number of censored cases, this may not be a true population representative; that the VGL is not the only laboratory to which samples are submitted for AHSV diagnosis, and that many cases are clinically diagnosed in the field; therefore, this data set does not represent all the suspected AHS cases in South Africa. There was no control group for comparison of the study group findings.
On a univariable level, the current study confirmed that increased case fatality rates are most likely due to a higher number of unvaccinated horses in certain populations. Unvaccinated horses are 8.7 times more likely to die compared with horses that are vaccinated annually. This data corroborates the results obtained by Gordon et al. (2013) in Zimbabwe, where the odds of vaccinated horses dying from AHS were 0.12 times less likely than unvaccinated horses. The vaccination status was not statistically significant when other variables were considered (multivariable analysis). This may be due to insufficient sample size.
The relationship between vaccination frequency and case outcome supported the findings of Molini et al. 2015, showing that annual vaccination is the most protective.
The Ct value result makes biological sense, where one would expect that an increase in viral load is likely to increase the likelihood of death in cases.
The pulmonary form and its impact on the probability of survival are very high compared to the cardiac form. This number is probably unrealistic, although it makes biological sense and agrees with anecdotal clinical experience (Zientara et al. 2015). The fever form on the other hand, is often referred to as difficult to identify or even subclinical, which is supported by the findings of this study.
All age categories outside the reference of three to seven years increased the odds of death. The number of vaccinations received by an individual horse, immune status, and comorbidities are all confounding factors and were unknown in the study population. There does appear to be a relationship between age, vaccination frequency and clinical presentation, as shown in Figure 3. Therefore, no conclusions will be made based on our data and further study in this area is recommended.
While statistically significant at the multivariable level, vaccination order may be influenced by the number of unknown and unvaccinated cases in this dataset. The results are similar to the anecdotal field information the author's received from participating veterinarians, many of whom now vaccinate with AHS2 first to decrease the likelihood of vaccine reactions rather than future death (although these concepts may be linked biologically).
Further investigation is recommended for the following topics: the role subclinical cases play in disease (Weyer et al. 2013), changes in AHS seasonality related to weather patterns over the different areas of South Africa, prevalent AHSV serotypes in the different South African provinces (Mellor & Boorman 1995; Mellor & Leake 2000), the effect of virus phylogeny and genotype on virulence (De Sá et al. 1994), and the effect of viral serotype, individual horse age and sex on disease outcome (Coetzer & Guthrie 2004). A more controlled study evaluating Ct value, clinical signs, age, vaccination frequency and disease outcome will allow the evaluation of the correlation between laboratory results and the clinical picture.
The results of this study have the potential to influence practice by reiterating the importance of vaccination according to government regulations as a method of protection against AHSV in endemic areas. Even though there is the possibility of vaccine-associated disease, vaccination was shown to protect from death due to disease. Study results could assist in making policy more enforced, as outbreaks will be less severe and communities less affected if all horses are vaccinated annually, providing better herd immunity.
The study has shown a definite relationship between vaccination status and case outcome, with vaccinated horses less likely to die. This suggests that the increased fatality was likely due to multiple variables, of which an unvaccinated status is one, and not due to vaccine failure per se. This study further showed that factors such as the frequency of AHS vaccination and age could influence case outcomes. However, the limited data obtained did not allow for extensive analysis and further areas for study were highlighted.
Acknowledgements
The authors would like to acknowledge the cooperation and assistance of all the private veterinarians and horse owners for data collection in this study. Our thanks to the Department of Veterinary Tropical Diseases and the Veterinary Genetics Laboratory, University of Pretoria, for providing the disease and test data.
Conflicts of interest
The authors declare they have no conflicts of interest that are directly or indirectly related to the research.
Source of funding
ML Genis is a postgraduate student who received funding from Sam Cohen Scholarships.
Ethical declaration
The authors declare that this submission is in accordance with the principles laid down by the Responsible Research Publication Position Statements as developed at the 2nd World Conference on Research Integrity in Singapore, 2010. Prior to the commencement of the study, ethical approval was obtained from the following ethical review board: Animal Ethics Committee of the University of Pretoria, V043-18.
ORCID
ML Genis https://orcid.org/0009-0006-1226-5243
J Crafford https://orcid.org/0000-0001-7343-8327
C Weyer https://orcid.org/0000-0001-8805-7551
D Pollard https://orcid.org/0000-0003-2986-1851
J Grewar https://orcid.org/0000-0002-4496-8051
A Guthrie https://orcid.org/0000-0001-7729-9918
References
Animal Diseases Act, 1984, Cape Town, South Africa: Government Gazette. Available at: https://www.gov.za/sites/default/files/gds_document/201503/act-35-1984.pdf. Accessed 6 August 2019. [ Links ]
Coetzer, J. & Guthrie, A.J., 2004, African horse sickness, in J. Coetzer and R.C. Tustin (eds) Infectious Diseases of Livestock. 2nd edn. Oxford University Press, pp. 1231-1246. [ Links ]
Crafford, J.E., Lourens, C.W., Gardner, I.A., et al., 2013, Passive transfer and rate of decay of maternal antibody against African horse sickness virus in South African Thoroughbred foals, Equine Veterinary Journal 45(5), 604-607. https://doi.org/10.1111/evj.12015. [ Links ]
Du Toit, R., 1944, Transmission of horse-sickness and blue-tongue in South Africa, Farming 19, 421-423. [ Links ]
Erasmus, B.J., 1978, A new approach to polyvalent immunization against African horse sickness, Proceedings of the Fourth International Conference on Equine Infectious Diseases 401-403. [ Links ]
Gordon, S., Bolwell, C., Rogers, C., et al., 2013, Descriptive epidemiology of African horse sickness in Zimbabwe, Onderstepoort Journal of Veterinary Research 80(1), 5. https://doi.org/10.4102/ojvr.v80i1.578. [ Links ]
Guthrie, A.J., MacLachlan, N.J., Joone, C., et al., 2013, Diagnostic accuracy of a duplex real-time reverse transcription quantitative PCR assay for detection of African horse sickness virus, Journal of Virological Methods 189(1), 30-35. https://doi.org/10.1016/j.jviromet.2012.12.014. [ Links ]
Hosmer, D.W., Lemeshow, S.J., Sturdivant, R.X., 2013, Model building strategies and methods for logistic regression, in Applied Logistic Regression. 3rd edn. Hoboken, New Jersey: John Wiley & Sons, Incorporated, pp. 89-153. https://doi.org/10.1002/9781118548387. [ Links ]
Howell, P., 1962, The isolation and identification of further antigenic types of African horse sickness virus, Ondertepoort Journal of Veterinary Research 29(2), 139-149. [ Links ]
Klein, J.P., Rizzo, J.D., Zhang, M.J., et al., 2001, Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part I: Unadjusted analysis, Bone Marrow Transplantation 28, 909-915. https://doi.org/10.1038/sj.bmt.1703260. [ Links ]
Koekemoer, J.J.O., 2008, Serotype-specific detection of African horse sickness virus by real-time PCR and the influence of genetic variations, Journal of Virological Methods 154(1-2), 104-110. https://doi.org/10.1016/j.jviromet.2008.08.010. [ Links ]
Lubroth, J., 1988, African horse sickness and the epizootic in Spain 1987, Equine Practice 10(2), 26-33. [ Links ]
Lubroth, J., 1992, The complete epidemiological cycle of African horse sickness: our incomplete knowledge, in Bluetongue, African horse sickness, and related orbiviruses, Proceedings of the Second International Symposium 197-204. [ Links ]
Meiswinkel, R. & Paweska, J.T., 2003, Evidence for a new field Culicoides vector of African horse sickness in South Africa, Preventive Veterinary Medicine 60(3), 243-253. https://doi.org/10.1016/S0167-5877(02)00231-3. [ Links ]
Mellor, P.S. & Boorman, J., 1995, The transmission and geographical spread of African horse sickness and bluetongue viruses, Annals of Tropical Medicine and Parasitology 89(1), 1-15. https://doi.org/10.1080/00034983.1995.11812923. [ Links ]
Mellor, P.S. & Hamblin, C., 2004, African horse sickness, Veterinary Research 445-466. https://doi.org/10.1051/vetres:2004021. [ Links ]
Mellor, P.S. & Leake, C.J., 2000, Climatic and geographic influences on arboviral infections and vectors, Revue scientifique et technique (International Office of Epizootics) 19(1), 41-54. https://doi.org/10.20506/rst.19.L1211. [ Links ]
Molini, U., Marucchella, G., Maseke, A., et al., 2015, Immunization of horses with a polyvalent live-attenuated African horse sickness vaccine: Serological response and disease occurrence under field conditions, Trials in Vaccinology 4, 24-28. https://doi.org/10.1016/j.trivac.2015.03.001. [ Links ]
De Sá, R.O., Zellner, M., Grubman, M.J., 1994, Phylogenetic analysis of segment 10 from African horsesickness virus and cognate genes from other orbiviruses, Virus Research 33, 157-165. https://doi.org/10.1016/0168-1702(94)90052-3. [ Links ]
Animal Health Surveillance, 2015, The OIE Terrestrial Animal Health Code, Chapter 1.4 18, 1-11. https://doi.org/10.1024/0036-7281.147.3.143a. [ Links ]
South African Government Gazette, 2018, Important information regarding vaccination against African horse sickness (AHS) in South Africa. [ Links ]
Von Teichman, B.F., Dungu, B., Smit, T.K., 2010, In vivo cross-protection to African horse sickness Serotypes 5 and 9 after vaccination with Serotypes 8 and 6, Vaccine 28(39), 6505-6517. https://doi.org/10.1016/j.vaccine.2010.06.105. [ Links ]
Von Teichman, B.F. & Smit, T.K., 2008, Evaluation of the pathogenicity of African Horsesickness (AHS) isolates in vaccinated animals, Vaccine 26(39), 5014-5021. https://doi.org/10.1016/j.vaccine.2008.07.037. [ Links ]
Venter, G.J., Graham, S.D., Hamblin, C., 2000, African horse sickness epidemiology: Vector competence of South African Culicoides species for virus serotypes 3, 5 and 8, Medical and Veterinary Entomology 14(3), 245-250. https://doi.org/10.1046/j.1365-2915.2000.00245.x. [ Links ]
Weyer, C.T., Quan, M., Joone, C., et al., 2013, African horse sickness in naturally infected, immunised horses, Equine Veterinary Journal 45(1), 117-119. https://doi.org/10.1111/j.2042-3306.2012.00590.x. [ Links ]
Weyer, C.T., Joone, C., Lourens, C.W., et al., 2015, Development of three triplex real-time reverse transcription PCR assays for the qualitative molecular typing of the nine serotypes of African horse sickness virus, Journal of Virological Methods 223, 69-74. https://doi.org/10.10167j.jviromet.2015.07.015. [ Links ]
Weyer, C.T., Grewar, J.D., Burger, P., et al., 2016, African horse sickness caused by genome reassortment and reversion to virulence of live, attenuated vaccine viruses, South Africa, 2004-2014, Emerging Infectious Diseases 22(12), 2087-2096. https://doi.org/10.3201/eid2212.160718 [ Links ]
World Organization for Animal Health, 2023, Manual of diagnostic tests and vaccines for terrestrial animals. 12th edn. Available at: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/A_summry.htm. Accessed: 31 July 2023. [ Links ]
Zientara, S., Weyer, C.T., Lecollinet, S., 2015, African horse sickness, OIE Revue Scientifique et Technique 34(2), 315-327. [ Links ]
 Correspondence:
Correspondence:
ML Genis
Email: louie.genis@gmail.com
1 StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LLC.














