Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the South African Veterinary Association
versão On-line ISSN 2224-9435
versão impressa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.94 no.1 Pretoria 2023
http://dx.doi.org/10.36303/jsava.553
ORIGINAL RESEARCH
Helminth parasites of impalas, Aepyceros melampus (Lichtenstein) (Ruminantia: Bovidae), in the Kruger National Park, South Africa: infection patterns from birth to adulthood
IG HorakI; K JunkerII; GJ GallivanIII
IDepartment of Veterinary Tropical Diseases, Faculty ofVeterinary Science, University of Pretoria, South Africa
IINational Collection of Animal Helminths, Epidemiology, Parasites and Vectors Programme, ARC-Onderstepoort Veterinary Institute, South Africa
III43 Leeming Drive, Canada
ABSTRACT
There is limited information on the development of helminth burdens of wild ungulates. This study examined the development of helminth burdens of impalas from birth to adulthood in the southern Kruger National Park, South Africa, based on systematic monthly collections of helminths from lambs, yearling and adult impalas at two sites over the course of a year. Eighteen species of nematodes, two trematode taxa and three species of cestodes were collected. Six species, Cooperia hungi, Cooperioides hamiltoni, Impalaia tuberculata, Strongyloides papillosus, Trichostrongylus deflexus and Trichostrongylus thomasi, each collected from > 75% of the impalas, accounted for > 90% of adult gastrointestinal nematodes. Infection with adult nematodes occurred in the first month of life and all lambs were infected with adults and L4 larvae by the second month. Intensities of infection of adult nematodes and larvae in lambs increased until November when they were similar to those of yearlings and adults. Adult female impalas had a lower intensity of infection of adult nematodes than males from April to July, and a higher intensity of infection from October to December. Intensity of infection of L4 larvae was higher in adult females than adult males throughout the year. These patterns were seen in the most common nematodes, but were more variable for the less common nematodes, trematodes and cestodes. The ratio of L4 larvae to adult nematodes was lowest in lambs and highest in adult impalas. Our results emphasised the importance of age, sex and season as potential sources of variation in specific parasite burdens.
Keywords: wildlife parasites, helminths, impalas, prevalence, seasonal variation
Introduction
Impalas, Aepyceros melampus (Lichtenstein), are medium-sized, lightly built antelope found in eastern and southern Africa. Studies on the ticks of impala in southern Africa (Gallivan et al. 1995; Horak et al. 2003) have found differences in tick infestations related to age, season and sex. There have been numerous studies of the helminth parasites of impalas in southern Africa. Some studies reported helminths collected incidentally or during culling operations (i.e. Mönnig 1933; Meeser 1952; Young & Wagener 1968), while others were restricted in time and numbers of impalas examined (Boomker et al. 1989a; Van Wyk & Boomker 2011). In 1978, Horak (1978b) reported the helminths of impalas each month from February 1975 to February 1976 in the Nylsvley Nature Reserve (NNR) in Limpopo Province, and in 1983, Anderson reported the helminths of impalas each month from March 1973 to February 1975 in the Nyala Game Ranch (NGR) in north-eastern KwaZulu-Natal (KZN). Both studies collected helminths in a systematic manner, but only two to three impalas were examined each month and the age-sex distribution of the impalas examined each month was variable, potentially confounding the effects of season with those of age and sex.
As a component of the surveys of the parasites of wild animals in South Africa, impalas were collected monthly in the southern Kruger National Park (KNP) from January 1980 to December 1980. The collections at Skukuza and Biyamiti always included a lamb, a yearling male and an adult at each site, while only adult males were collected at Crocodile Bridge. An analysis of the adult nematode infections from these impalas was previously published by Negovetich et al. (2006), who examined the burdens of adult nematodes in relation to age and sex. However, they did not consider the impact of season and included impalas collected during the 1982 drought that had substantially higher nematode burdens than those collected in 1980 (Horak unpubl. obs.). The collection of adult males only at Crocodile Bridge also potentially skewed the interpretation of differences between the sexes. In the present paper we re-examine the impact of season, age and sex on the helminth burdens, including L4 nematode larvae, of impalas at Skukuza and Biyamiti.
Materials and methods
Study areas
The town Skukuza (24o 58'S, 31o 36'E; Alt. 262 m) is situated in the south-western region of the KNP in a landscape zone described as Thickets of the Sabie and Crocodile Rivers (Gertenbach 1983). The impalas examined in this region were shot within a 25 km radius of the Skukuza rest camp. The average annual rainfall is approximately 600 mm; from July 1979 to June 1981 it was 619 mm.
The Biyamiti region (25o 06'-25o 28'S, 31o 25'-31o 39'E; Alt. 200350 m) is located in the southern central region of the KNP in a landscape zone described as Mixed Bushwillow Woodlands (Gertenbach 1983). The collection area extended from north of the Malelane entrance gate to the KNP to north of the Biyamiti River. While rainfall data for the study period are not available, annual rainfall averages 600-700 mm in the KNP.
Both study areas fall within the Lowveld vegetation zone (Acocks 1988) in the summer rainfall region; winters are typically dry and frost-free.
Survey animals
Three to seven impalas were shot at each site monthly from January to December 1980. This formed part of the KNP's parasite diversity studies under approval of the then National Parks Board (currently known as SANParks). The collections always included a lamb (< 12 months of age), a yearling male (12-23 months) and an adult (> 24 months). Lambs of either sex were collected until April and in December, and males only from May to November. Only adult males were collected in January and February 1980 at Skukuza, and from January to March at Biyamiti, thereafter adult males and females were collected at both sites. Because parturition in impalas in southern Africa is generally confined to November to January (Skinner & Chimimba 2005), it is possible to visually age impalas fairly accurately until the age of two years, particularly the males because of the age-associated changes in the shape of their horns (Bothma 1989).
Parasite collection
The gastrointestinal tract, lungs and liver were processed for helminth recovery. The oesophagus was cut open lengthwise and macroscopically examined for parasites. After collecting the ingesta, the mucosae of the abomasum, small intestine and large intestine were removed using the blade of a sharp knife. The mucosae of the three organs were combined and digested overnight at room temperature in pepsin and HCl (10 g of pepsin powder and 35 ml of technical hydrochloric acid per litre of normal saline). The digested material was sieved (0.038 mm apertures) the following morning. The liver was cut into approximately 1 cm wide slices and incubated overnight at room temperature in normal saline. The liver slices were thoroughly washed and the washings and incubation fluid sieved the following morning. The gall bladder was not checked separately for parasites.
The trachea and the bronchi of the right lung were incised, the lung was cut into blocks and incubated overnight in normal saline. Because the lungworm, Pneumostrongylus calcaratus, is enmeshed in the pulmonary tissue, only small pieces of this nematode were collected and the number of parasites could not be determined accurately. The trachea, bronchi and the chunks of lung were thoroughly washed the following morning and the washings and saline solution in which they had been incubated sieved. The residue remaining in the sieve was collected and the volume measured. The number of eggs of P. calcaratus in a 1 ml aliquot of the residue were counted under a stereoscopic microscope, then the total number of eggs in the residue was calculated.
The processed material from the gastrointestinal tracts, liver and lungs of the impalas was transported to the Parasitology Laboratory of the Faculty of Veterinary Science at Onderstepoort. Two 1/50th aliquots of the total volume of the ingesta of each of the abomasum, the small intestine and the large intestine were examined under a stereoscopic microscope for helminth collection. The remainder of the ingesta of the small intestine and of the caecum and colon were separately poured into a large flat-bottomed tray and adult hookworms, Oesophagostomum spp. and Trichuris spp. were collected and counted. A single 1/10th aliquot of the sieved digested material was examined under a stereoscopic microscope, and a single 1/5th aliquot of the liver washings was similarly examined. Helminths were identified and counted under a compound microscope equipped with differential interference contrast, based on their original descriptions or, if applicable, the latest taxonomic revisions; in the case of cestodes, only specimens with a scolex were included in the counts. Members of the Paramphistominae reported from impalas in Africa belong to the genera Calicophoron (five species) and Cotylophoron (two species) (Pfukenyi & Mukaratirwa 2018). Due to time constraints, specimens were only identified to subfamily level.
Statistical methods
Factors of interest in the analysis of the data were the effects of site, season, age class, and sex of the adult impalas. However, because the sample sizes in each age class were limited and sex was not balanced across the age classes, it was not possible to analyse the data in a single multivariate analysis. Therefore, the data were analysed using exploratory data analysis techniques to describe patterns and trends, with extensive use of graphical analysis to assess differences. The data were subdivided to compare the differences among age classes by month and site, and differences between the sexes in adult impalas by month.
Differences in prevalence were analysed using contingency tables with a x2 and logistic regression. For differences in the intensity of infection, the parasite count data were logarithmically transformed to reduce the inequality of variance created by overdispersion (Petney et al. 1990). The data were then analysed using one-way and multivariate analysis of variance. When factors were significant, a t-test was used to test between two groups and a Student-Newman-Keuls multiple range test was used to test among multiple groups. The prevalence and intensity of infection of L4 larvae and adult nematode burdens of individual impalas were compared using McNemar's x2 and paired t-tests.
Parasitological descriptors such as prevalence, intensity of infection and abundance were used in accordance with Bush et al. (1997).
Results
The collections included 18 species of nematodes, two trematode taxa and three species of cestodes (Table I). With exception of the lungworm P. calcaratus, and the trematode Schistosoma mattheei, which occurs in the blood vessels, the helminths occurred in the gastrointestinal tract. The helminth species were similar at the two sites. The prevalence of the L4 larvae of Oesophagostomum columbianum and Trichostrongylus spp., and of adult Trichuris globulosa, was significantly higher at Skukuza than at Biyamiti (p < 0.05) as were the intensities of infection of the L4 larvae of Cooperia/Cooperioides spp., Haemonchus spp. and Longistrongylus sabie, and the adults of Trichostrongylus deflexus and T. globulosa. The prevalence and intensity of infection of Paramphistominae and the intensity of infection of Moniezia benedeni were significantly higher at Biyamiti (p < 0.05).
Gastrointestinal nematodes
All of the impalas except one newborn lamb at Skukuza were infected with gastrointestinal nematodes. The prevalence of the adults of 10 nematode species, Cooperia hungi, Cooperioides hamiltoni, Cooperioides hepaticae, Gaigeria pachyscelis, Impalaia tuberculata, L. sabie, O. columbianum, Strongyloides papillosus, T. deflexus and Trichostrongylus thomasi was > 75%. The prevalence of Haemonchus krugeri was 57.1% at Skukuza and 70.2% at Biyamiti, while the prevalence of T. globulosa was 57.1% at Skukuza but only 8.5% at Biyamiti (Table I). The prevalence of the remaining species was < 15%.
The most numerous adult gastrointestinal nematodes were C. hungi, C. hamiltoni, I. tuberculata, S. papillosus, T. deflexus and T. thomasi. These six species accounted for > 90% of the adult nematodes. The other common species with a prevalence > 75%, C. hepaticae, G. pachyscelis, L. sabie and O. columbianum, each accounted for < 2.5% of the adult nematodes, while the remaining species accounted for < 1.4% each.
Gastrointestinal nematodes: adult worms
Infection with adult nematodes occurred in the first month of life and all of the lambs were infected by the second month of life. By February (Table II), lambs were infected with adults of 13 of the 17 gastrointestinal nematode species; one species, Haemonchus vegliai, was not collected from lambs, while Haemonchus bedfordi was not collected until March, Trichostrongylus falculatus was not collected until April and T. globulosa was not collected until August.
With the exception of C. hamiltoni, C. hepaticae, O. columbianum, T. thomasi and T. globulosa, the prevalence of adult nematodes did not differ significantly (p < 0.10) among the age classes (Table II). The prevalence of C. hamiltoni and T. thomasi was lower in lambs than in yearling and adult impalas (p = 0.01 and p < 0.001 respectively). No C. hamiltoni were collected from lambs until February (Table II), but the prevalence did not differ among age classes thereafter. No T. thomasi were collected from lambs until February and the prevalence was lower than in yearling and adult impalas until September. The prevalences of C. hepaticae and O. columbianum were higher in yearlings and lambs than in adult impalas (p = 0.01), while the prevalence of T. globulosa was higher in yearlings than in lambs and adults (p = 0.01). In adult impalas, the prevalences of C. hepaticae and S. papillosus were higher in males than in females (88.5% versus 54.5% (p = 0.01) and 92.3% versus 68.2% (p = 0.04), respectively), but the prevalences of the other species did not differ between the sexes.
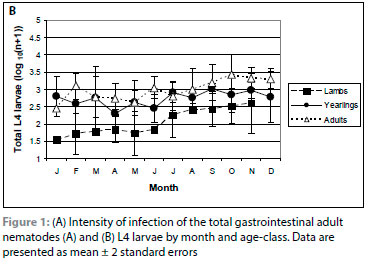
The overall intensity of infection of adult nematodes was significantly lower in lambs than in yearling and adult impalas (p < 0.001) because of lower intensities of infection of C. fuelleborni, C. hungi, C. hamiltoni, T. deflexus and T. thomasi (Table II). The intensity of infection of adult nematodes in lambs increased rapidly from January to March, and then more slowly until November (Figure 1A) when the intensities were similar to those in yearlings and adult impalas. The rapid increase to March or April was seen in C. hungi, I. tuberculata, L. sabie and T. thomasi, while the intensity of infection of C. hamiltoni and T. deflexus increased progressively throughout the year, accounting for the progressive increase in the intensity of infection of adult nematodes in lambs from March to November (data not shown). These six species accounted for approximately 70% of the adult nematodes in lambs.
Except for C. hepaticae, the intensity of infection of adult nematodes did not differ between adult and yearling impalas. The intensity of infection of C. hepaticae was significantly higher in yearlings than in lambs and adult impalas (Table II), and higher in lambs than in adult impalas (p < 0.001). The intensity of infection of C. hepaticae in lambs increased until May, and then was similar to that in yearlings.
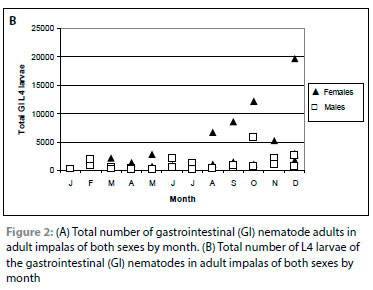
There was no seasonal pattern in the overall intensity of infection of adult nematodes in yearlings (Figure 1A). In adult impalas, the intensity of infection increased from January to December (p = 0.001). There was a significant month-by-sex interaction (p = 0.004), with adult females having lower burdens than adult males from April to July and higher burdens from October to December (Figure 2A). All of the burdens of >10 000 adult nematodes were collected from October to December. During this period, six of nine adult female impalas had >10 000 adult nematodes, with four of the six in December, the only month in which the intensity of infection in all adult females was higher than in adult males. In comparison, only one of eight adult male impalas had >10 000 adult nematodes in the same months (p = 0.05).
This pattern was seen in C. hungi, C. hamiltoni, I. tuberculata, L. sabie, T. deflexus and T. thomasi, and accounted for the progressive increase in the intensity of infection in adult impalas over the year. Amongst the individual nematode species, only the intensity of infection of O. columbianum adults was higher in male impalas than in females (p = 0.02).
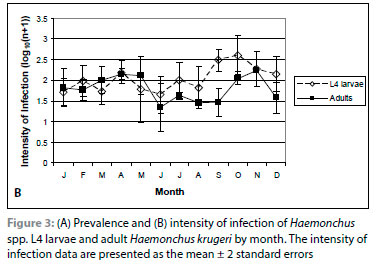
Haemonchus krugeri, which accounted for 95% of the Haemonchus spp. adults, was the only nematode to exhibit a distinct seasonal pattern. The prevalence and intensity of infection of H. krugeri did not differ among the age classes. The prevalence was 100% in February and March, declined to 12.5% in August, and was 100% in October, while the intensity of infection was highest from March to May and lowest from June to September (Figure 3).
Gastrointestinal nematodes: L4 larvae
L4 larvae were collected from lambs in January and all were infected in February. The overall prevalence and intensity of infection of larvae in lambs (Table II) were significantly less than in yearling and adult impalas (p < 0.002). This pattern was consistent in all of the nematode genera. The intensity of infection of larvae increased throughout the year in lambs (Figure 1B), and was similar to the intensity in yearlings in November. Only the larvae of Cooperia/Cooperioides spp. exhibited a progressive increase in intensity of infection over the year in lambs (data not shown), and no O. columbianum larvae were collected from February until June.
The intensity of infection of L4 larvae was higher in adult impalas than in yearlings (p = 0.01; Table II). As with adult nematodes, there was no clear seasonal pattern in yearlings (Figure 1B). The intensity of infection of larvae was significantly higher in adult female than male impalas throughout the year (p < 0.001; Figure 2B), with a marked increase in females from August to December. This accounted for the significant increase in the intensity of infection of larvae in adult impalas from January to October (p = 0.001). This pattern was seen in Cooperia/Cooperioides spp., Haemonchus spp. and I. tuberculata.
The intensity of infection of Haemonchus spp. larvae was highest in September and October in all age classes (Figure 3).
Gastrointestinal nematodes: L4 larvae to adult nematode ratios
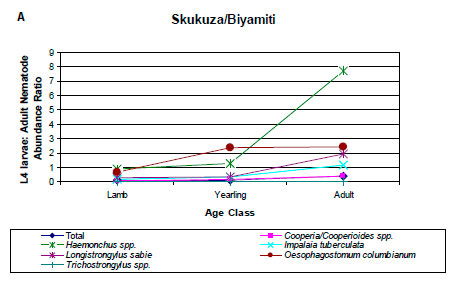
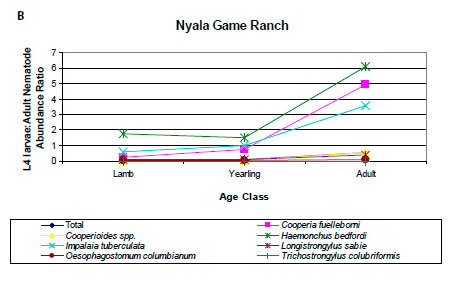
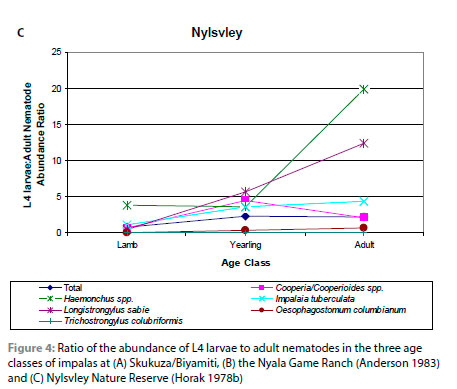
With the exception of Trichostrongylus spp., the prevalences and intensities of infection of L4 larvae relative to adults of all nematodes and the individual nematode genera were lowest in lambs and highest in adult impalas. This is shown in the ratios of the abundance, a measure that incorporates both prevalence and intensity of infection, of L4 larvae and adult nematodes (Figure 4A).
In lambs, the prevalence of the total larvae and the larvae of the individual nematode genera was lower than those of adult nematodes (Table II). In yearling and adult impalas, only the prevalence of Trichostrongylus spp. larvae was significantly lower than the prevalence of the adult nematodes (p < 0.001). In yearlings and adult male impalas, the prevalence of the larvae and adults of the other nematode genera did not differ, while in adult female impalas, the prevalence of Haemonchus spp. and I. tuberculata larvae was significantly higher than those of the adult nematodes (p < 0.05).
In lambs and yearlings, the intensity of infection of L4 larvae was less than the intensity of infection of adult nematodes throughout the year (Figure 1A, B), reflecting the lower intensities of infection of Cooperia/Cooperioides spp., I. tuberculata, L. sabie and Trichostrongylus spp. larvae relative to adult nematodes (Table II). In adult males, the intensity of infection of larvae was less than that of adult nematodes throughout the year (Figure 2A, B) (p < 0.001), particularly in April and May. In adult females, the intensity of infection of adult nematodes and larvae did not differ from March to September, but the intensity of infection of adult nematodes was higher than that of larvae from October to December. In both sexes, the intensities of infection of Cooperia/Cooperioides spp. and Trichostrongylus spp. L4 larvae were lower than that of adult nematodes. The intensity of infection of I. tuberculata and L. sabie larvae and adults did not differ in adult males, but the intensity of infection of the larvae of both species was higher than that of the adults in adult females (p < 0.05) in August and September (data not shown).
The prevalence of Haemonchus spp. L4 larvae was higher than that of the adults of H. krugeri from July to September and the intensity of infection of L4 larvae was higher than that of the adults from July to October (Figure 3A, B), primarily because of the decline in the prevalence and intensity of infection of H. krugeri adults from July to September. In adult female impalas, the intensity of infection of Haemonchus spp. larvae was higher than that of H. krugeri adults from May to December, and no H. krugeri adults were collected from adult females from May to August.
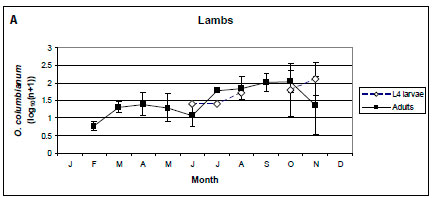
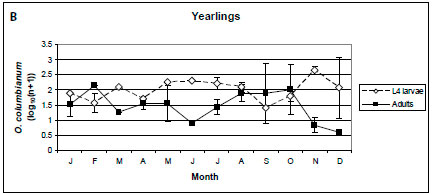
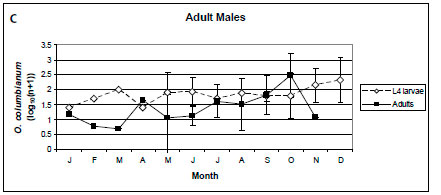
In lambs, only O. columbianum adults were collected from February to May, and the intensities of infection of L4 larvae and adults were similar from July to October (Figure 5A). In yearlings and adult male impalas, the intensity of infection of O. columbianum larvae was marginally higher than that of the adults (p < 0.1), with the most pronounced difference in November and December (Figure 5B, C). In adult females, the intensity of infection of O. columbianum L4 larvae was significantly higher than that of adult nematodes (p < 0.001), except in July and August; no O. columbianum adults were collected from March to June (Figure 5D). Only 40 of the 7 857 O. columbianum L4 larvae were collected from the mucosal scrapings of three of the 96 impalas.
Pneumostrongylus calcaratus
The overall prevalence of P. calcaratus was 83.3% (Table I). The prevalence did not differ between sites, but the number of eggs per infected host was higher at Skukuza (p = 0.005). The prevalence of eggs was significantly lower in lambs (46%) than in yearlings and adults (96% for both) (p = 0.001) (Table II), and the number of eggs per host was also lower in lambs than yearlings and adults (p < 0.001). Eggs were recovered from lambs in February and March, then none were collected until July, but there was no seasonal pattern in yearling and adult impalas. The prevalence and number of eggs per host did not differ between the sexes in adult impalas (p = 0.43).
Trematodes
Two trematode taxa were collected, Paramphistominae and S. mattheei. The prevalence of adult paramphistomines in the rumen of lambs (Table I) was significantly lower than in yearling and adult impalas (p < 0.001). Lambs were first infected in April, with infections until October. No paramphistomines were collected from yearlings from December to February, but the prevalence was 100% from August to October. There was no seasonal pattern in prevalence in adult impalas. The intensity of infection increased with age at Biyamiti (p = 0.01), but not at Skukuza (p = 0.70). There was no seasonal pattern in the intensity of infection. The prevalence and intensity of infection did not differ between the sexes in adult impalas.
Immature paramphistomines were collected from the intestines of one (2%) impala at Skukuza and four (8.5%) at Biyamiti. Only adult impalas were infected, and all had adult paramphistomines in their rumen.
The prevalence of S. mattheei was 6.4% at Biyamiti and 12.2% at Skukuza. None were collected from lambs, and the prevalence and intensity of infection did not differ between yearling and adult impalas. The prevalence tended to be higher in adult female impalas (22.7%) than adult males (4.2%; p < 0.1), but the intensity of infection did not differ.
Cestodes
Three species of cestodes were collected, M. benedeni, Moniezia expansa and Stilesia hepatica. The prevalences were < 10% (Table I). The prevalence of M. benedeni did not differ statistically among the age classes (p = 0.17). Lambs were first infected in September (Table II), and all the infections occurred from September to December. One M. expansa was collected from one lamb at Skukuza (prevalence = 2.0%).
No S. hepatica were collected from lambs (Table II). The prevalence did not differ between adult and yearling impalas, nor between the sexes in adult impalas. There was no seasonal pattern. The mean intensity of infection did not differ between the sites, by age, nor between the sexes. There was no association between the liver parasites S. hepatica and C. hepaticae (Kappa = -0.002; p = 0.94).
Discussion
The present data provide insights into the development of the helminth burdens of impalas from birth to adulthood at two sites in the southern KNP, Skukuza and Biyamiti. These collections differ from previously published data on systematic collections of the helminths from the three age classes (Horak 1978b; Anderson 1983) in that all three age classes were collected each month at both sites. This provides an opportunity to examine the changes in prevalence and intensity of infection by age and sex over the course of a year.
The helminth species were similar at the two sites. The prevalences and/or intensities of infection of the L4 larvae of Cooperia/Cooperioides spp., Haemonchus spp., L. sabie, O. columbianum and Trichostrongylus spp., and of the adults of T. deflexus and T. globulosa were significantly higher at Skukuza than at Biyamiti. All of these nematode genera are directly transmitted (Reinecke 1983), and the Thickets of the Sabie and Crocodile Rivers vegetation zone probably has the largest impala population in the KNP (Gertenbach 1983). Several studies have shown a positive relationship between host population densities and transmission success of directly transmitted parasites (Arneberg et al. 1998; Altizer et al. 2003; Calvete et al. 2004). The prevalence and intensity of infection of paramphistomines and the intensity of infection of the cestode, M. benedeni, were higher at Biyamiti. Both of these helminths are indirectly transmitted and the observed differences between the two sites may reflect local variations in the availability of the intermediate hosts (Calvete et al. 2004); freshwater snails of the genus Bulinus O.F. Müller in the case of Paramphistominae (Pfukenyi & Mukaratirwa 2018) and oribatid mites in the case of Moniezia spp. (Schuster et al. 2000).
Gastrointestinal nematodes
The dominant species of gastrointestinal nematodes were C. hungi, C. hamiltoni, I. tuberculata, S. papillosus, T. deflexus and T. thomasi. These six species accounted for > 90% of the adult nematodes, and were collected from > 75% of the impalas. The other common species with a prevalence > 75%, C. hepaticae, G. pachyscelis, L. sabie and O. columbianum, each accounted for < 2.5% of the adult nematodes. The prevalence of C. connochaeti, C. fuelleborni, H. bedfordi, H. vegliai and T. falculatus was < 15%, and each accounted for < 1% of the adult nematodes. These latter species appear to be incidental infections of impalas in the KNP. Cooperia connochaeti and H. bedfordi are parasites of blue wildebeest, Connochaetes taurinus (Burchell), while H. vegliai is a parasite of kudus, Tragelaphus strepsiceros Pallas, and T. falculatus is common in scrub hares, Lepus saxatilis Cuvier (Horak et al. 2021). Cooperia fuelleborni has a strong association with African buffaloes, Syncerus caffer Sparrman, and was found in 71% of buffaloes examined in the KNP (Taylor et al. 2013).
Lambs were infected with nematodes in the first month of life, and by February were infected with 14 of the 18 nematode species. For the majority of the nematode species, the prepatent period is 21-28 days (Reinecke 1983), indicating that infection is occurring as early as late November. The timing of infection was generally consistent with that at NNR (Horak 1978b), with the exception of O. columbianum, which was collected in February in the KNP, but not until September in lambs at NNR, and T. falculatus, which was collected in January at NNR and April in the KNP.
The rapid increase in the intensity of infection of adult gastrointestinal nematodes in lambs until March indicates a rapid acquisition of parasites, and reflects the pattern seen in the common species, C. hungi, I. tuberculata and T. thomasi. The intensity of infection increased more slowly from March to November, reflecting the progressive increase in intensity of infection of C. hamiltoni and T. deflexus. By November, the burdens in lambs were similar to those of yearling and adult impalas.
Negovetich et al. (2006) suggested that female lambs had lower burdens than male lambs, but this may be an artefact as the newborn lambs collected in December were both females and no female lambs were collected after April. The increase in intensity of infection from December to April appeared to be similar in both sexes of lambs in the KNP and at NNR, and the intensity of infection in female lambs collected up until August at NNR (Horak 1978b) and Pafuri in the northern KNP (Horak unpubl. obs.) was within the range of male lambs collected in the same time periods.
Negovetich et al. (2006) also suggested that adult female impalas had higher intensities of infection of adult nematodes than adult and juvenile male impalas, and that C. hamiltoni, I. tuberculata, L. sabie, T. deflexus and T. thomasi were indicator species for adult female impalas. However, the intensity of infection did not differ between adult male and female impalas (p > 0.9) in the present analysis. The intensity of infection in adult females was lower than the intensity of infection in adult and juvenile male impalas from April to July and only exceeded that of adult male impalas in December when all of the females harboured > 10 000 worms. The increase in the intensity of infection in adult female impalas from July to October/December reflected increases in the burdens of C. hungi, C. hamiltoni, I. tuberculata, L. sabie, T. deflexus and T. thomasi. Negovetich et al. (2006) did not include seasonal variation in their analysis, and also included impalas from the 1982 drought. All of the 1982 impalas were collected from October to December, and eight of nine adult female impalas had > 10 000 adult nematodes compared with three of nine adult males (p = 0.05).
Horak (1978b) suggested a periparturient relaxation of resistance in female impalas that would release immunologically inhibited larvae around the time of lambing. Thus, the apparent increase in the intensity of infection in adult female impalas from October to December may have a biological basis. We suspect the apparent effect observed by Negovetich et al. (2006) was inflated by the inclusion of impalas from the 1982 drought that were sampled in the periparturient period.
The prevalences and intensities of infection of most adult nematodes did not differ between adult and yearling impalas. The exceptions were C. hepaticae, in which the prevalence and intensity of infection were significantly higher in yearlings than in lambs and adult impalas, and significantly higher in lambs than in adult impalas, and T. globulosa in which the prevalence was significantly higher in yearlings than in lambs and adults.
In north-eastern Eswatini, Gallivan et al. (1996) reported first infection of C. hepaticae in lambs in January, and a higher prevalence in lambs and yearlings than in adult impalas. At NNR (Horak 1978b), lambs were not infected until April. The prevalence did not differ among age classes, but the intensity of infection was highest in lambs and lowest in adult impalas. A similar pattern was seen in the abundance among the age classes at the NGR (Anderson 1983). Pletcher et al. (1988) interpreted the decrease in the intensity of infection of C. hepaticae in adult impalas as evidence for an acquired immunity. The decrease in prevalence in adult impalas in north-eastern Eswatini (Gallivan et al. 1996) and intensity of infection at NNR and abundance at the NGR support this hypothesis.
The prevalence and intensity of infection of T. globulosa were higher at Skukuza than at Biyamiti. The prevalence at the NGR was 76.1%, but the prevalence at other localities in South Africa was < 10% (Horak unpubl. obs.). At Skukuza, the prevalence was significantly higher in yearlings than in lambs or adults, but there was no difference in intensity of infection among the age classes. The cause or causes of the differences in prevalence and intensity of infection among localities is unknown.
The seasonal patterns in intensities of infection of adult nematodes and L4 larvae in lambs and yearlings in the KNP were similar to those at NNR (Horak 1978b), but there was no apparent seasonal pattern in the intensities of infection in adult impalas at NNR. The intensity of infection of adult nematodes was similar among the age classes at NNR and in the KNP, but the intensity of infection of larvae was higher at NNR where the intensity of infection of larvae was higher than the intensity of infection of adult nematodes in adult impalas throughout the year, and in lambs and yearling impalas during the winter months.
Horak (1978b) interpreted the higher intensity of infection of L4 larvae than adult nematodes as indicating arrested development in L4 stages during the winter months, particularly in Cooperia/ Cooperioides spp., Haemonchus placei, I. tuberculata and L. sabie. Anderson (1983) also suggested arrested development during the winter months in C. fuelleborni, H. bedfordiand I. tuberculata at the NGR. In the KNP, the only nematode species for which the intensity of infection of larvae exceeded that of the adults in all age classes in the winter months was H. krugeri, while the intensity of infection of I. tuberculata larvae exceeded that of I. tuberculata adults in adult female impalas in August and September. At Pafuri in the northern KNP, no Haemonchus spp. adults were collected in July and August, only Haemonchus spp. larvae, and the intensity of infection of I. tuberculata larvae was also higher than that of the adults (Horak unpubl. obs.).
Interrupted development appears to be common in Haemonchus species in impalas. Horak (1978a) also recorded interrupted development of Haemonchus contortus in blesbok, Damaliscus pygargus phillipsi Harper, in the northern Transvaal (now Limpopo Province), but neither H. vegliai nor H. bedfordi exhibited interrupted development based on L4 larvae to adult ratios in kudus and blue wildebeest in the southern and central KNP respectively (Boomker et al. 1989b; Horak et al. 1983). Impalaia tuberculata also appears to exhibit arrested development in impalas, but not in kudus (Boomker et al. 1989b). Cooperia/Cooperioides spp. only exhibited increased L4 to adult ratios in impalas during the winter months at NNR and the NGR. The reasons for the differences among the localities in impalas are unclear. Arrested development is thought to be a response to inhospitable external conditions (Anderson 2000), but while NNR was the coldest and driest during the winter, the NGR was warmest and wettest of the localities.
No O. columbianum L4 larvae were collected from lambs from February until May, after which the intensities of infection of the larvae and adults were similar. In yearling and adult impalas, the intensity of infection of L4 larvae was higher than that of the adults, particularly in November and December after the spring rains. At NNR, no O. columbianum L4 larvae were collected from lambs (Horak 1978b). The prevalence of O. columbianum L4 larvae was lower than that of the adults in yearling and adult impalas. Anderson (1983) also reported much lower abundances of O. columbianum L4 larvae than adults in all three age classes in the NGR. The reasons for the differences among localities are unknown. In sheep, climate, immunity and nutritional status appear to play roles in O. columbianum infections (Reinecke 1983).
Migration of L4 O. columbianum larvae in the mucosa of the colon where they cause a pathological reaction appears to be an integral part of the life cycle in sheep (Reinecke 1983). However, only 40 of 7 857 L4 larvae were collected from the digested mucosa of the gastrointestinal tracts of impalas. It would thus seem that impalas are better hosts than sheep for O. columbianum, and that migration of the larvae in the intestinal mucosa is not a prerequisite for completion of the life cycle in impalas.
One observation in the current analysis was that the prevalence and intensity of infection of L4 larvae relative to adult nematodes increased from lambs to adult impalas. Examination of the data from Anderson (1983) and Horak (1978b) revealed a similar pattern at the NGR and NNR (Figure 4B, C). With the exception of Trichostrongylus spp., there was generally a three-fold increase in the ratio of the abundance of L4 larvae to adults between lambs and adult impalas. In the KNP, the largest increases were seen in Haemonchus spp., I. tuberculata, L. sabie and O. columbianum, while in the NGR the species were C. fuelleborni, Cooperioides spp., H. bedfordi and I. tuberculata, and at NNR they were Haemonchus spp., I. tuberculata and L. sabie. The increasing L4 to adult ratio from lambs to adult impalas may reflect the development of immunity to infection as impalas age. Pletcher et al. (1988) suggested that the decrease in the intensity of infection of C. hepaticae in adult impalas was evidence for an acquired immunity. This may also apply to other gastrointestinal nematode species. Lambs are immunologically naïve and there is a rapid transition from L4 larvae to adults. However, as impalas age, developing immunity could inhibit the transition of L4 larvae to adults.
Another observation was the difference in L4 larvae to adult ratios between the sexes in adult impalas. The ratios were generally lower in adult males (Figure 2A, B), particularly in April and May, the period of the rut. In adult female impalas, the intensities of infection of L4 larvae and adult nematodes were similar until October when the intensity of infection of adult nematodes exceeded that of the L4 larvae. Horak's (1978b) hypothesis that there was a peripaturient relaxation of resistance in adult females may explain the increased adult to L4 larvae ratio from October to December. A similar phenomenon may occur in adult male impalas during the rut, as a testosterone-mediated reduction in the immune-competency of males has been demonstrated for a number of mammal species (Folstad and Karter 1992; Decristophoris et al. 2007).
Pneumostrongylus calcaratus
Based on the presence of lung lesions, Anderson (1983) reported a prevalence of 97.8% at the NGR, and Gallivan et al. (1989) reported a prevalence of 85% in north-eastern Eswatini. The prevalence of eggs at Skukuza/Biyamiti was 46% in lambs, and 96% in yearling and adult impalas. These were similar to the prevalence of lesions in Eswatini. Lambs in the KNP harboured egg-producing females in February and March. In Eswatini, the prevalence of lesions in lambs increased from 17% (1/6) in February to 100% in August. Heinichen-Anderson (1982) proposed that P. calcaratus is transmitted by the yellow slug, Elisolimax flavescens (Keferstein), which occurs on various trees browsed on by impalas, and suggested that the slugs are ingested during feeding. If so, lambs could be infected early in life when they start browsing, but Heinichen-Anderson (1982) was unable to infect impalas with infected slugs, thus the life cycle of P. calcaratus remains unclear.
Trematodes and cestodes
Trematodes and cestodes have indirect life cycles. With the exception of the Parmaphistominae, they were relatively uncommon in the present study. The prevalence of paramphistomines at Biyamiti (78.7%) was significantly higher than that at Skukuza (46.9%) and similar to that at the NGR (89.1%; Anderson 1983), while the prevalence at Skukuza was similar to that at NNR (50.0%; Horak 1978b). As mentioned above, variation in intermediate host densities and availability might explain the observed differences. In the KNP, the prevalence increased with age. A similar pattern was observed at NNR where no paramphistomines were collected from lambs. The intensity of infection did not differ between yearling and adult impalas at NNR, similar to the pattern at Skukuza. In the KNP, no paramphistomines were collected from lambs and yearlings in the summer months. Anderson (1983) lists months in which few or no paramphistomines were collected, but there is no clear pattern and she does not differentiate among age classes. She suggested that the intermediate host of paramphistomines may be Pseudosuccinea columella (Say) (as Lymnea columella Say), which was common around waterholes in the NGR. Although recent studies suggest that Bulinus spp. rather than P. columella are intermediate hosts of paramphistomines in southern Africa (Pfukenyi & Mukaratirwa 2018; Schols et al. 2020), seasonal shifts in habitat use in relation to water and forage availability by impalas (Young, 1972) would influence the transmission patterns.
Three species of cestodes were collected, M. benedeni, M. expansa and S. hepatica. The two Moniezia species appear to be mutually exclusive; M. benedeni has been collected from impalas at several localities in southern Africa, while M. expansa has been collected at others (Horak unpubl. obs.); although both species were collected at Skukuza, each infected impala harboured only one of the two species. Jackson et al. (1998) described mutual exclusion in two congeneric monogeneans, suggesting that direct antagonistic interactions or indirect competition via the host immune system could be involved.
The prevalence of M. benedeni did not differ among the age classes in the KNP, and there was no age-related pattern for M. expansa at NNR (Horak 1978b). Both species appear to be most common in the winter and early spring.
The overall prevalence of S. hepatica (6.3%) was slightly lower than that in northeastern Eswatini (10.3%; Gallivan et al. 1996) and the NGR (15.2%; Anderson 1983). None were collected from lambs, and the prevalence did not differ between adult and yearling impalas, nor between the sexes in adult impalas. There was no seasonal pattern. The age distribution and lack of seasonal differences and differences between the sexes are similar to the observations in Eswatini. Sachs et al. (1969) suggested that infection with S. hepatica and C. hepaticae were associated, but there was no association between S. hepatica and C. hepaticae in the current study, nor in Eswatini (Gallivan et al. 1996).
The present study revealed a species-rich helminth community in impalas in the KNP. The most common nematodes are parasites of impalas or infect a range of hosts (Horak et al. 2021), while nematodes with a prevalence < 15% are parasites of other herbivores and most likely incidental infections in impalas. The age and sex related patterns and seasonal variation of the nematode burden largely reflect those of five of the most numerous species, C. hungi, C. hamiltoni, I. tuberculata, T. deflexus and T. thomasi. The age- and sex-related patterns and seasonal variation of the other species of nematodes, as well as of the trematodes and cestodes, were more variable. This study emphasises the importance of age, sex and season as potential sources of variation in parasite burdens and the importance of evaluating individual species rather than taking a pooled approach when studying the drivers of parasite communities.
Note:
The first research article by Prof Ivan Horak was published 62 years ago in Volume 31 of the Journal of the South African Veterinary Medical Association. The current article represents his 60th contribution to this journal either as sole, senior or coauthor.
Acknowledgements
We are indebted to SANParks for placing the animals as well as their staff and facilities in the Kruger National Park at our disposal and to Dr V. de Vos, who facilitated the project in the park. We gratefully acknowledge the assistance of Messrs. C. Cheney, J. Sithole and B.D. de Klerk with processing the carcasses for the recovery of parasites.
Conflict of interest statement
The authors declare they have no conflicts of interest that are directly or indirectly related to the research.
Funding sources
This research was funded by the Faculty of Veterinary Science, University of Pretoria, SANParks, and Bayer Animal Health.
Compliance with ethical guidelines
The authors declare that this submission is in accordance with the principles laid down by the Responsible Research Publication Position Statements as developed at the 2nd World Conference on Research Integrity in Singapore, 2010. All applicable institutional, national and international guidelines for the care and use of animals were followed.
ORCID
IG Horak https://orcid.org/0000-0002-2200-6126
KJunker https://orcid.org/0000-0001-6650-1201
GJ Gallivan https://orcid.org/0000-0002-2096-5657
References
Acocks, J.P.H., 1988, Veld types of South Africa, Memoirs of the Botanical Survey of South Africa no. 57. [ Links ]
Altizer, S., Nunn, C.L., Thrall, P.H., et al., 2003, Social organization and parasite risk in mammals: integrating theory and empirical studies, Ann RevEcol Evol Syst 34(1), 517-547. https://doi.org/10.1146/annurev.ecolsys.34.030102.151725. [ Links ]
Anderson, I.G., 1983, The prevalence of helminths in impala Aepyceros melampus (Lichtenstein, 1812) under game ranching conditions, S Afr J Wildl Res 13(3), 55-70. [ Links ]
Anderson, R.C., 2000, Nematode parasites of vertebrates. Their development and transmission, 2nd edn, CABI Publishing. https://doi.org/10.1079/9780851994215.0000. [ Links ]
Arneberg, P., Skorping, A., Grenfell, B., et al., 1998, Host densities as determinants of abundance in parasite communities, ProcRoy SocLondon Ser B BiolSci 265(1403), 1283-1289. https://doi.org/10.1098/rspb.1998.0431. [ Links ]
Boomker, J., Horak, I.G., Flamand, J.R.B., et al., 1989a, Parasites of South African wildlife. III. Helminths of common reedbuck, Redunca arundinum, in Natal, Onderstepoort J Vet Res 56 (1), 51-57. [ Links ]
Boomker, J., Horak, I.G., De Vos, V., 1989b, Parasites of South African Wildlife. IV. Helminths of kudu, Tragelaphus strepsiceros, in the Kruger National Park, Onderstepoort J Vet Res 56(2), 111-121. [ Links ]
Bothma, J. du P., 1989, Game Ranch Management, Van Schaik Publishers. [ Links ]
Bush, A.O., Lafferty, K.D., Lotz, J.M0., et al., 1997, Parasitology meets ecology on its own terms: Margolis et al., revisited, J Parasitol 83(4), 575-583. https://doi.org/10.2307/3284227. [ Links ]
Calvete, C., Blanco-Aguiar, J.A., Virgós, E., et al., 2004, Spatial variation in helminth community structure in the red-legged partridge (Alectoris rufa L.): effects of definitive host density, Parasitology 129(1), 101-113. https://doi.org/10.1017/S0031182004005165. [ Links ]
Decristophoris, P.M., von Hardenberg, A., McElligott, A.G., 2007, Testosterone is positively related to the output of nematode eggs in male Alpine ibex (Capra ibex) faeces, Evol Ecol Res 9, 1277-1292. [ Links ]
Folstad, I. & Karter, AJ., 1992, Parasites, bright males, and the immunocompetence handicap, Am Nat 139(3), 603-622. https://doi.org/10.1086/285346. [ Links ]
Gallivan, G.J., Barker, I.K., Alves, R.M.R., et al., 1989, Observations on the lungworm, Pneumostrongylus calcaratus, in impala (Aepyceros melampus) from Swaziland, J Wildl Dis 25(1), 76-82. https://doi.org/10.7589/0090-3558-25.L76. [ Links ]
Gallivan, G.J., Culverwell, J., Girdwood, R., et al., 1995, Ixodid ticks of impala (Aepyceros melampus) in Swaziland: effect of age class, sex, body condition and management, S Afr J Zool 30 (4), 178-186. https://doi.org/10.1080/02541858.1995.11448385. [ Links ]
Gallivan, G.J., Barker, I.K., Culverwell, J., et al.,1996, Prevalence of hepatic helminths and associated pathology in impala (Aepyceros melampus) in Swaziland, J Wildl Dis 32(1), 137-141. https://doi.org/10.7589/0090-3558-32.L137. [ Links ]
Gertenbach, W.P.D., 1983, Landscapes of the Kruger National Park, Koedoe 26(1), 9-121. https://doi.org/10.4102/koedoe.v26i1.591. [ Links ]
Heinichen-Anderson, I.G., 1982, The life cycle of the lungworm, Pneumostrongylus calcaratus, J S Afr Vet Ass 53(2), 109-114. [ Links ]
Horak, I.G., 1978a, Parasites of domestic and wild animals in South Africa. IX. Helminths in blesbok, Onderstepoort J Vet Res 45, 55-58. [ Links ]
Horak, I.G., 1978b, Parasites of domestic and wild animals in South Africa. X. Helminths in impala, Onderstepoort J Vet Res 45, 221-228. [ Links ]
Horak, I.G., De Vos, V., Brown, M.R.,1983, Parasites of domestic and wild animals in South Africa. XVI. Helminth and arthropod parasites of blue and black wildebeest (Connochaetes taurinus and Connochaetes gnou), Onderstepoort J Vet Res 50, 243-255. [ Links ]
Horak, I.G., Gallivan, G.J., Braack, L.E.O., et al., 2003, Parasites of domestic and wild animals in South Africa. XLI. Arthropod parasites of impalas, Aepyceros melampus, in the Kruger National Park, Onderstepoort J Vet Res 70, 131-163. [ Links ]
Horak, I.G., Boomker, J., Junker, K., et al., 2021, Some gastrointestinal nematodes and ixodid ticks shared by several wildlife species in the Kruger National Park, South Africa, Parasitology 148(6), 140-146. https://doi.org/10.1017/S0031182021000135. [ Links ]
Jackson, J.A., Tinsley, R.C., Hinkel, H.H., 1998, Mutual exclusion of congeneric monogenean species in a space-limited habitat, Parasitology 117(6), 563-569. https://doi.org/10.1017/S0031182098003370. [ Links ]
Meeser, M.J.N., 1952, A preliminary survey of endo- and ectoparasites of impala - Aepyceros melampus, S Afr Vet Med Ass 23(4), 221-223. [ Links ]
Mönnig, H.O., 1933, Wild antelopes as carriers of nematode parasites of domestic ruminants. Part III, Onderstepoort J Vet Sci Anim Ind 1(1), 77-92. [ Links ]
Negovetich, N.J., Fellis, K.J., Esch, G.W., et al., 2006, An examination of the infracommunities and component communities from impala (Aepyceros melampus) in the Kruger National Park, South Africa, J Parasitol 92(6), 1180-1190. https://doi.org/10.1645/GE-934R.1. [ Links ]
Petney, T.N., Van Ark, H., Spickett, A.M., 1990, On sampling tick populations: the problem of overdispersion, Onderstepoort J Vet Res 57, 123-127. [ Links ]
Pfukenyi, D.M. & Mukaratirwa, S., 2018, Amphistome infections in domestic and wild ruminants in East and Southern Africa: A review, Onderstepoort J Vet Res 85(1), 1-13. https://doi.org/10.4102/ojvr.v85i1.1584. [ Links ]
Pletcher, J.M., Horak, I.G., De Vos, V., et al., 1988, Hepatic lesions associated with Cooperioides hepaticae (Nematoda: Trichostrongyloidea) infection in impala (Aepyceros melampus) of the Kruger National Park, J Wildl Dis 24(4), 650-655. https://doi.org/10.7589/0090-3558-24A650. [ Links ]
Reinecke, R.K.,1983, Veterinary Helminthology, Butterworth Publishers, pp. 392. [ Links ]
Sachs, R., Hoffman, H.R., Sorheim, A.O., 1969, Stilesia infestation in East African antelopes, Vet Rec 84(9), 233-234. [ Links ]
Schols, R., Mudavanhu, A., Carolus, H., et al., 2020, Exposing the barcoding void: an integrative approach to study snail-borne parasites in a one health context, Front Vet Sci 7:605280. https://doi.org/10.3389/fvets.2020.605280. [ Links ]
Schuster, R., Coetzee, L., Putterill, J.F., 2000., Oribatid mites (Acari, Oribatida) as intermediate hosts of tapeworms of the family Anoplocephalidae (Cestoda) and the transmission of Moniezia expansa cysticercoids in South Africa, Onderstepoort J Vet Res 67:49-55. [ Links ]
Skinner, J.D. & Chimimba, C.T., 2005, The Mammals of the Southern African Subregion, 3rd edn, Cambridge University Press. https://doi.org/10.1017/CBO9781107340992. [ Links ]
Taylor, W.A., Skinner, J.D., Boomker, J., 2013, Nematodes of the small intestine of African buffaloes, Syncerus caffer, in the Kruger National Park, South Africa, Onderstepoort J Vet Res 80(1), Art.#562. https://doi.org/10.4102/ojvr.v80i1.562. [ Links ]
Van Wyk, I.C., Boomker, J., 2011, Parasites of South African wildlife. XIX. The prevalence of helminths in some common antelopes, warthogs and a bushpig in the Limpopo province, South Africa, Onderstepoort J Vet Res 78(1), Art. #308. https://doi.org/10.4102/ojvr.v78i1.308. [ Links ]
Young, E. & Wagener, L.JJ., 1968, The impala as a source of food and by-products: Data on production potential, parasites and pathology of free-living impalas of the Kruger National Park, J S Afr Vet Med Ass 39(4), 81-86. [ Links ]
Young, E., 1972, Observations on the movement patterns and daily home range size of impala, Aepyceros melampus (Lichtenstein), in the Kruger National Park, Zool Afr 7(1), 187-195. https://doi.org/10.1080/00445096.1972.11447439. [ Links ]
 Correspondence:
Correspondence:
K Junker
Email: junkerk@arc.agric.za